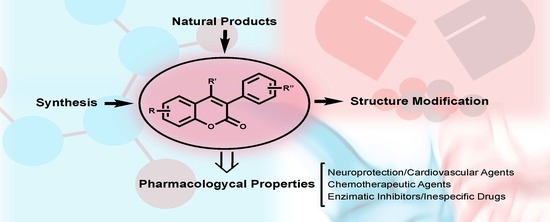
3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade
Abstract
:1. Introduction
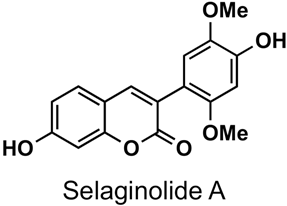
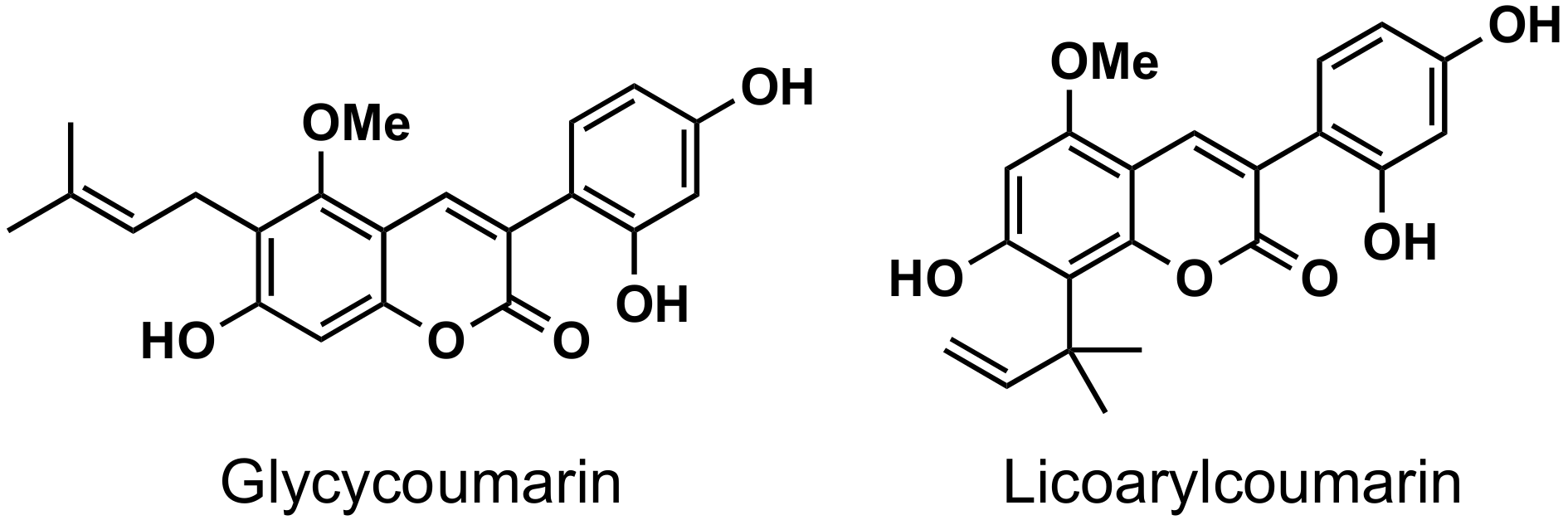
2. Presence of 3-Phenylcoumarins in Nature
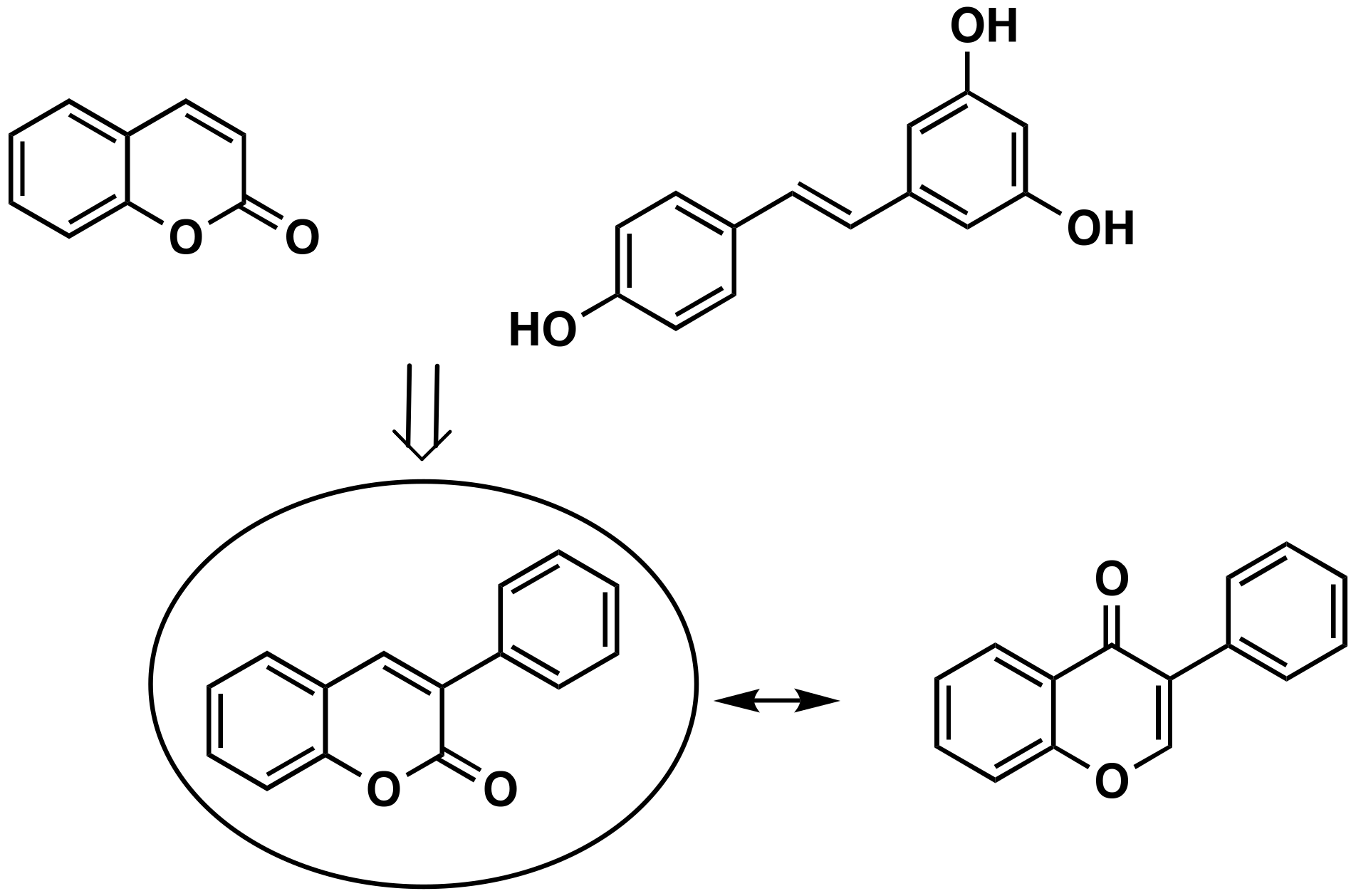
3. Synthesis of the 3-Phenylcoumarin Scaffold
3.1. Coumarin Coupling with the Benzene Ring
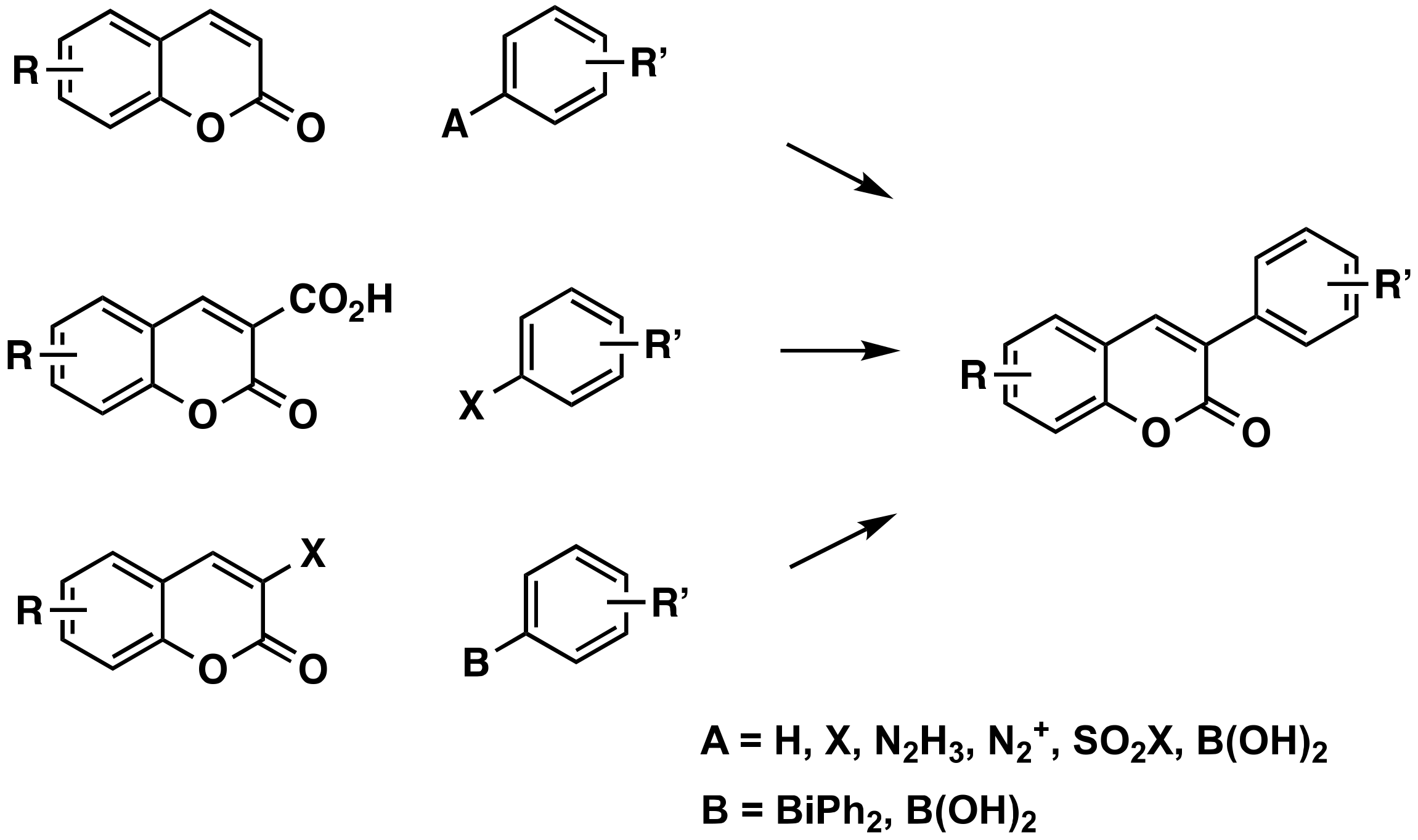
3.1.1. Without Prior Activation of the Coumarin and the Aryl
3.1.2. Using an Activated Arene
3.1.3. From 3-Carboxycoumarins
3.1.4. From 3-Halocoumarins
3.2. Construction of the Coumarin Bicyclic System
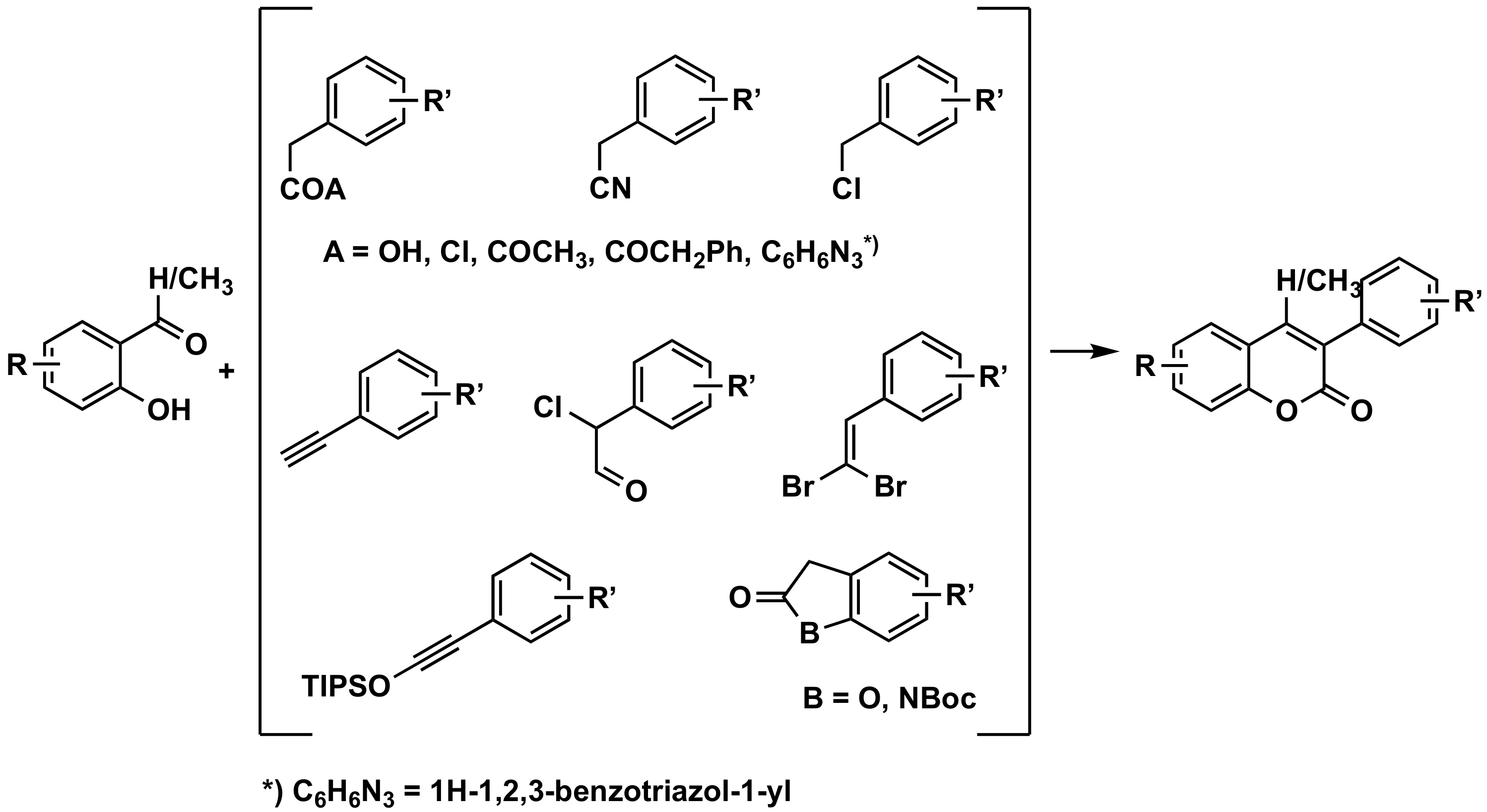
3.2.1. From o-hydroxybenzenecarbonyls
By Reaction with Phenylacetic Acids
By Reaction with Other Synthons
3.2.2. From Phenols

3.2.3. From Phenolic Esters
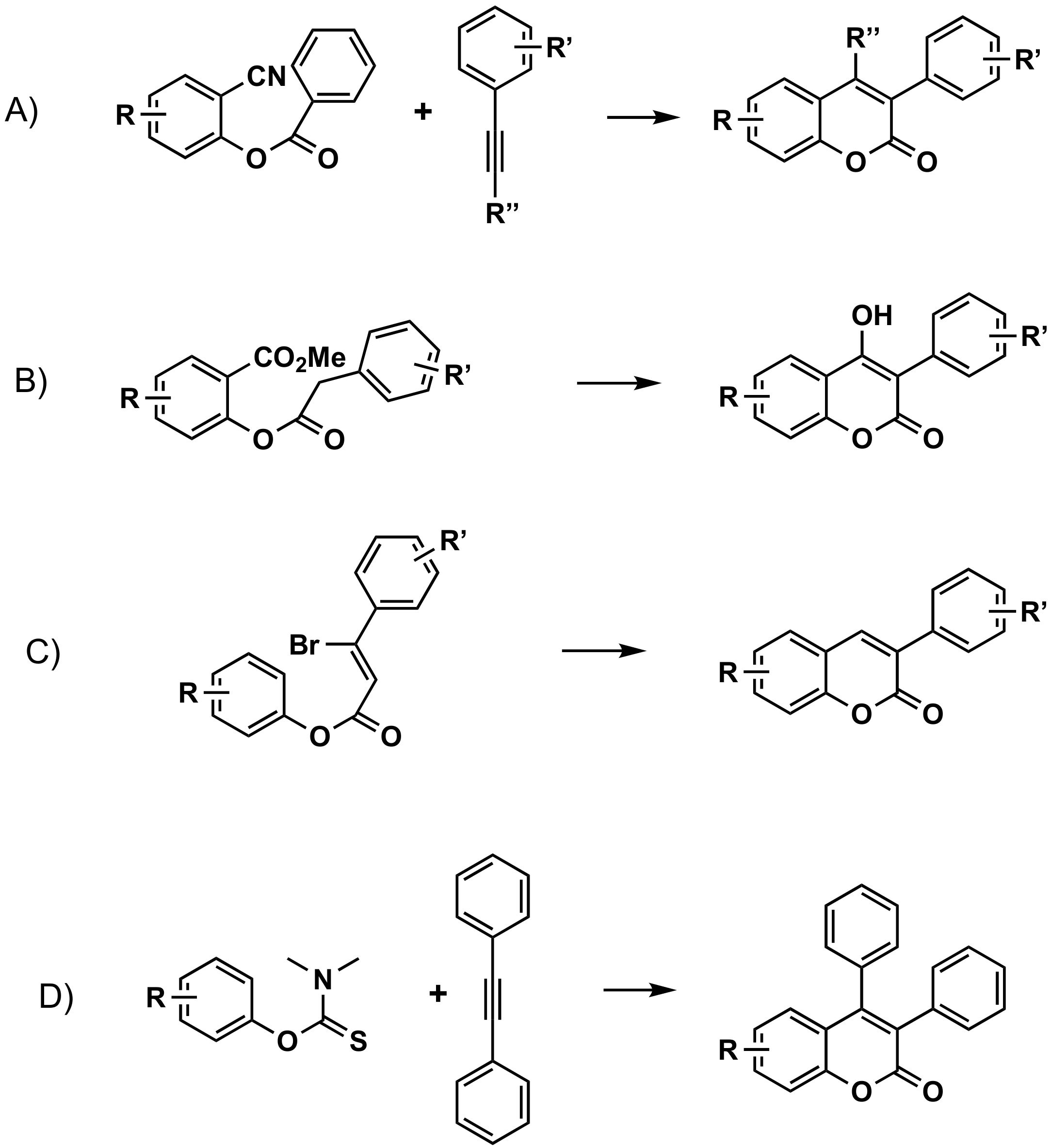
3.2.4. From Cinnamic Acids
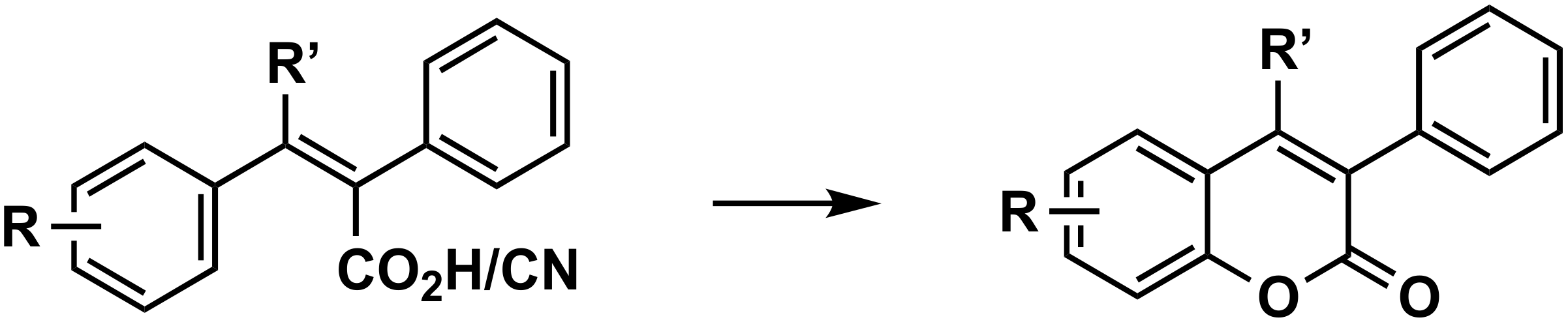
3.2.5. From Other Aromatic Substrates
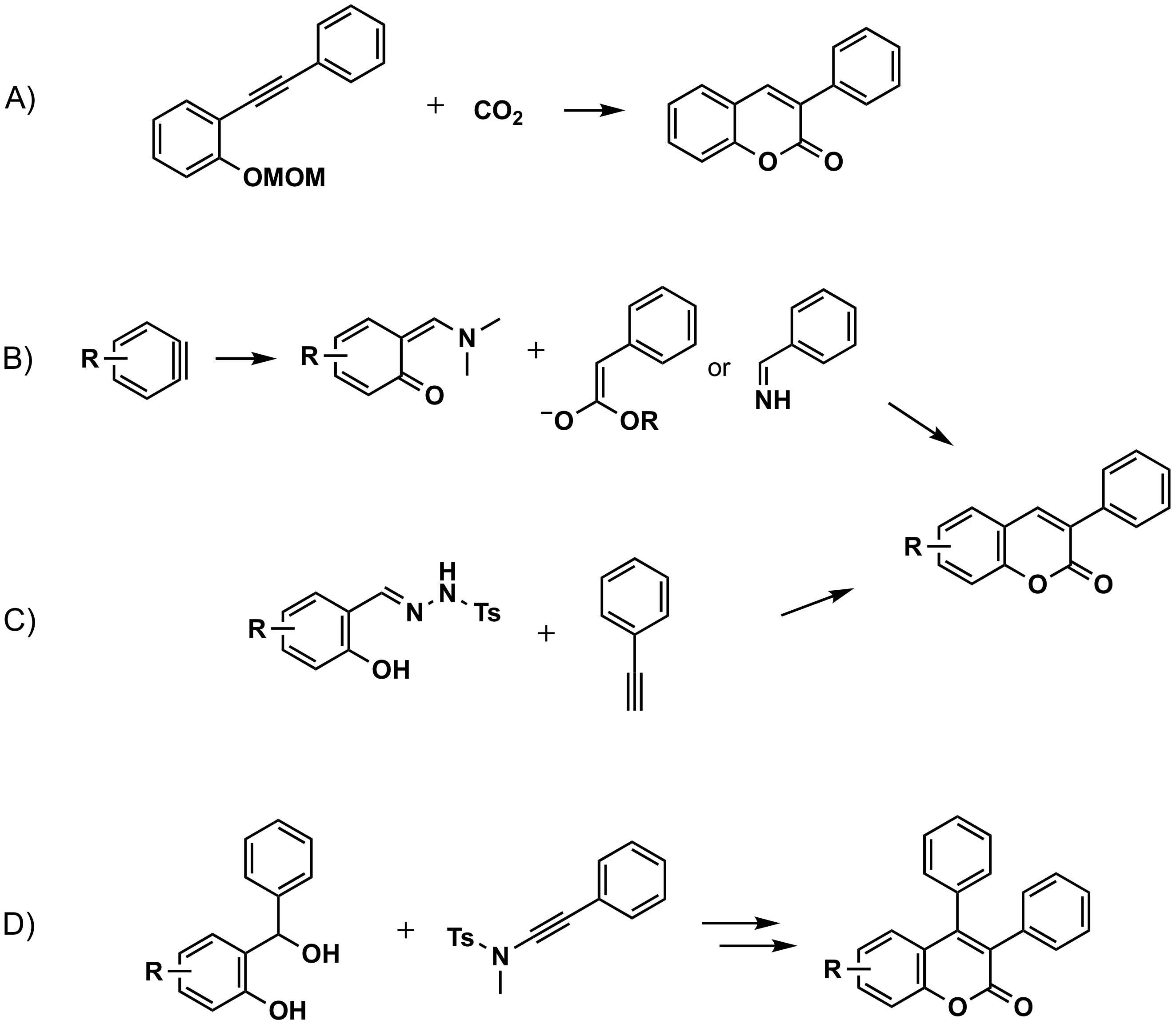
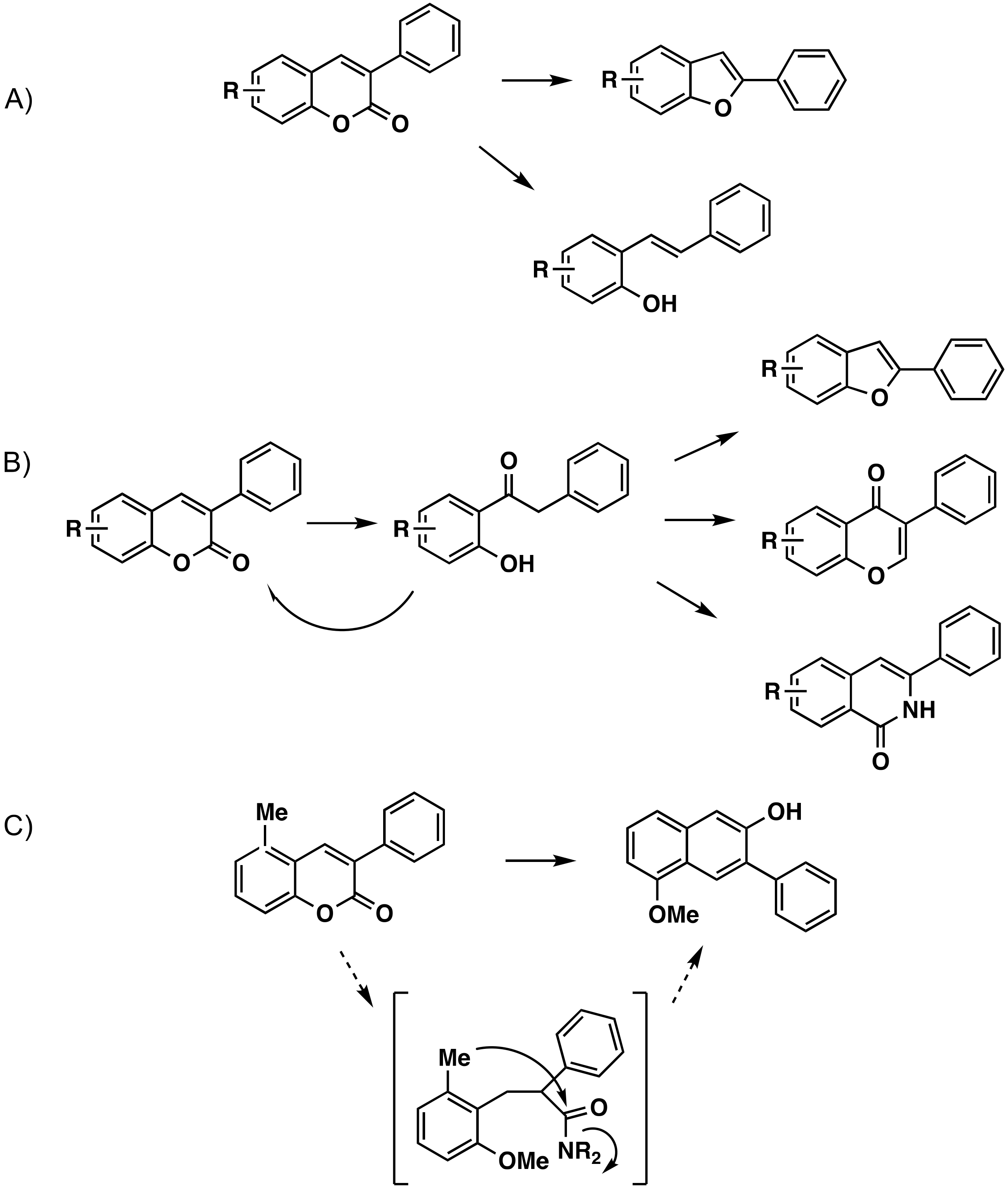
3.3. Construction of the Benzene Ring at Position 3 of the Coumarin

4. Modifications of the 3-Arylcoumarin Scaffold
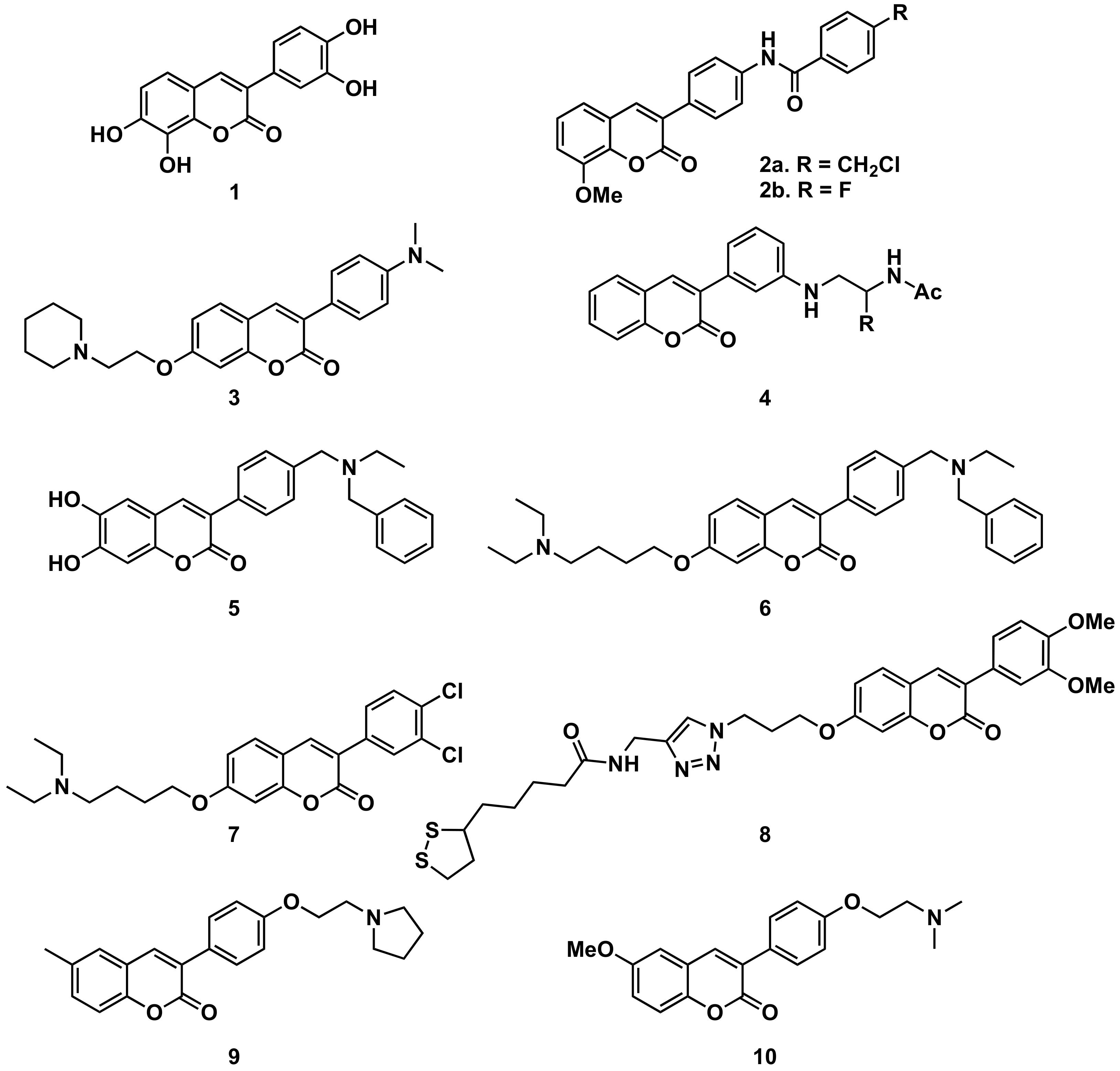
5. Pharmacological Interest of 3-Arylcoumarins
5.1. Neuroprotective Agents
5.1.1. Alzheimer’s Disease and AChE
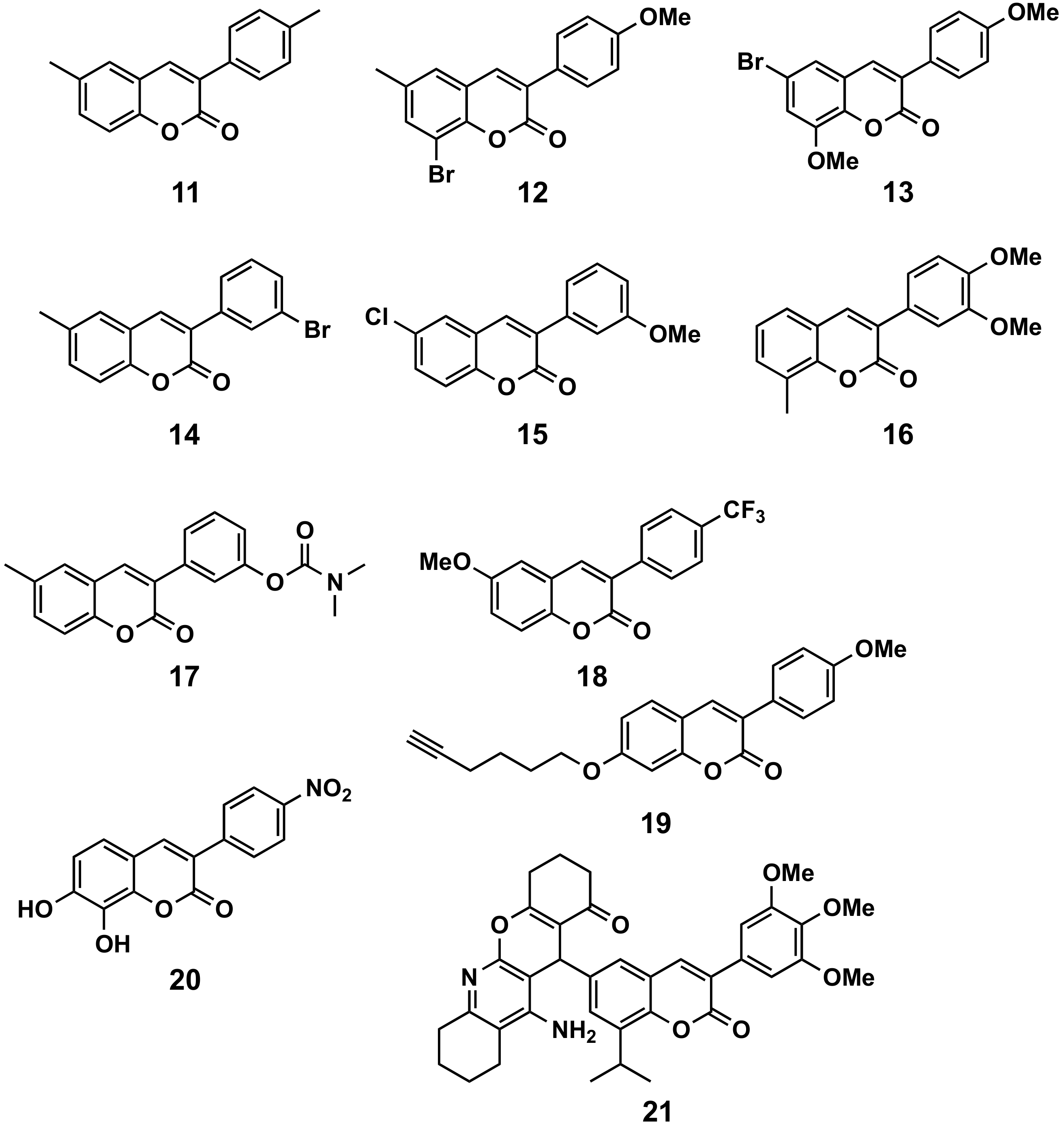
5.1.2. Parkinson’s Disease and MAO-B
5.1.3. Inflammation
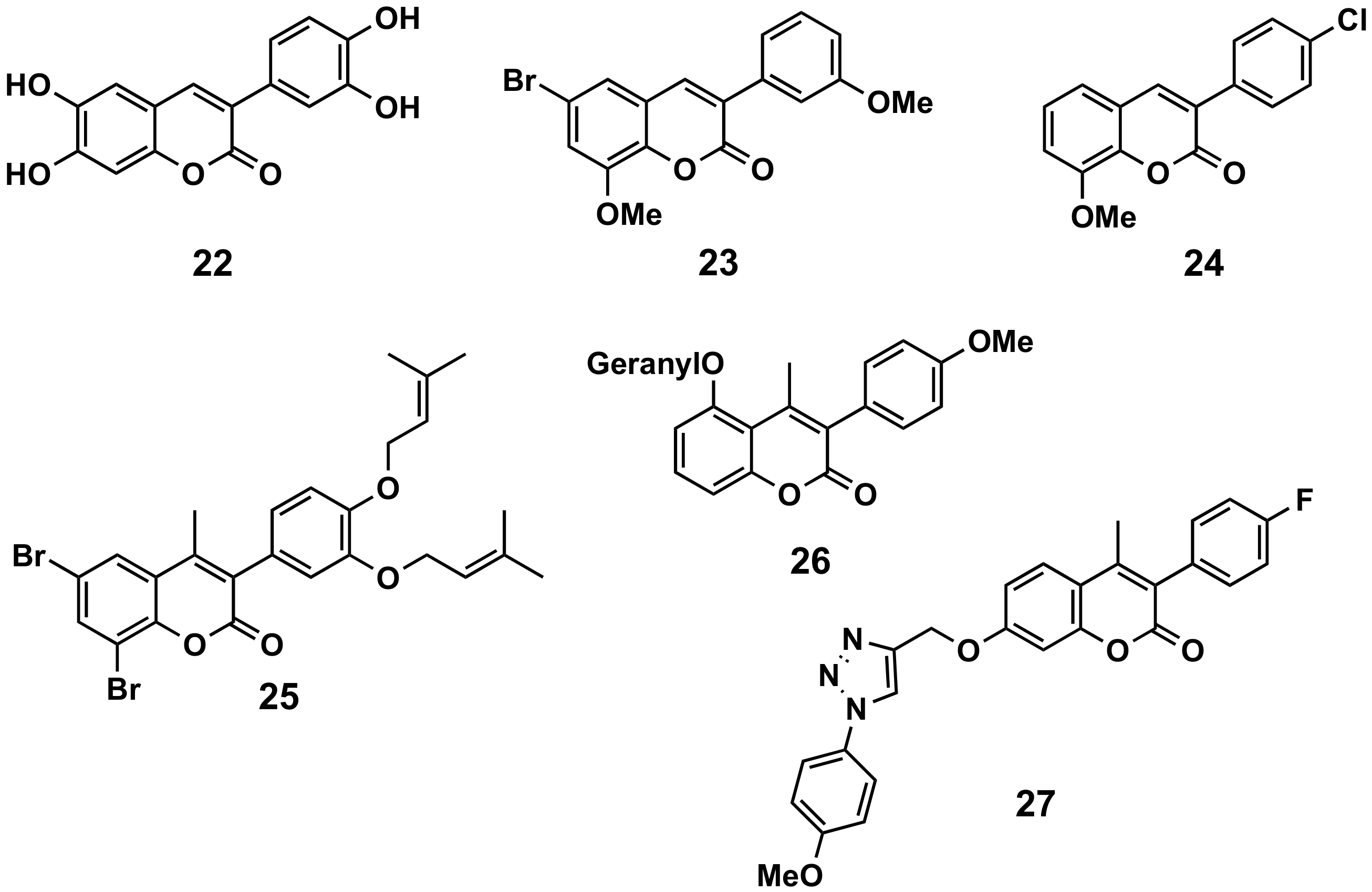
5.1.4. Oxidation
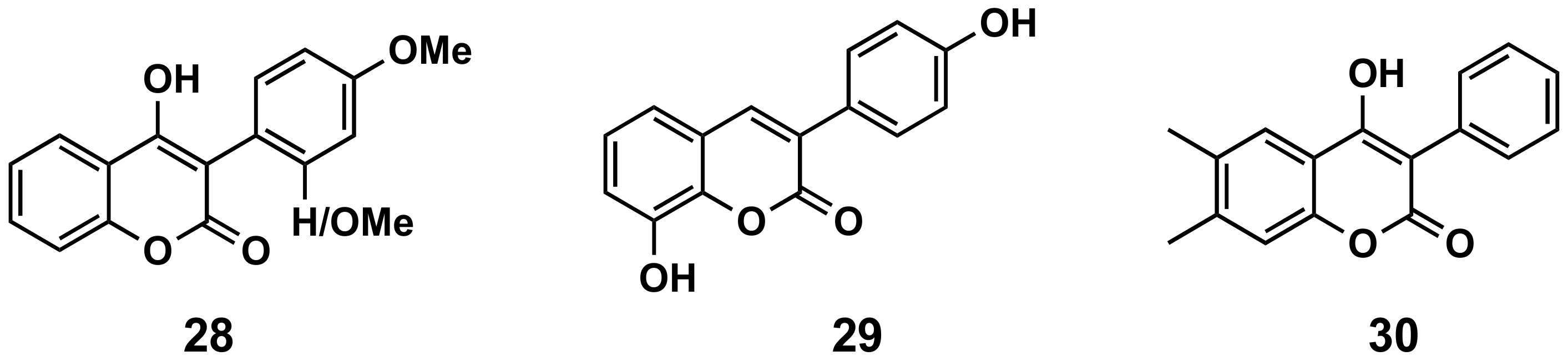
5.1.5. Cardiovascular Diseases

5.2. Chemotherapy
5.2.1. Antimicrobial Agents

5.2.2. Antiviral Agents
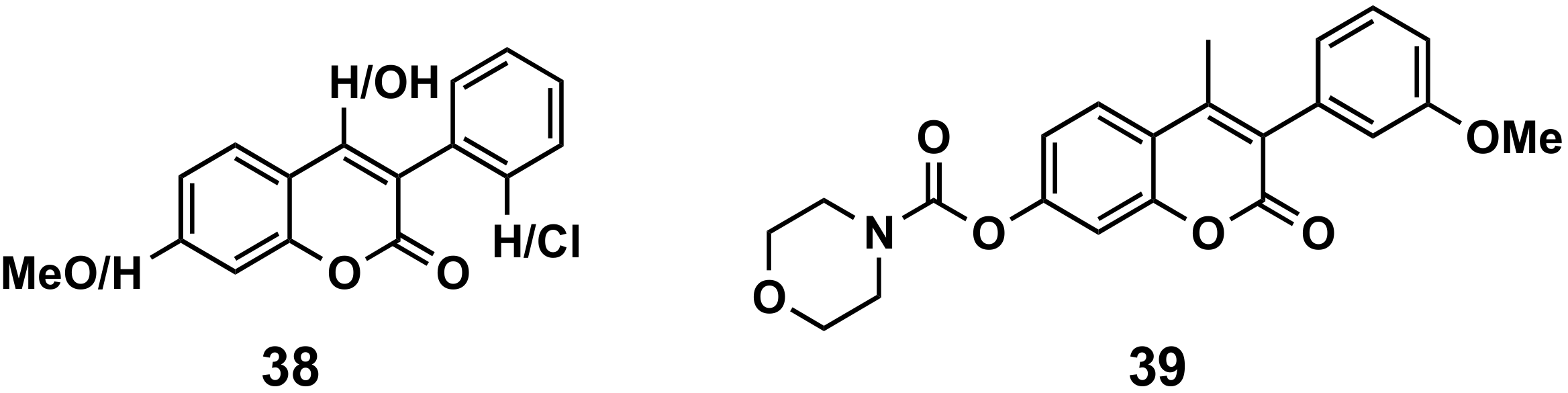
5.2.3. Antiparasitic Agents
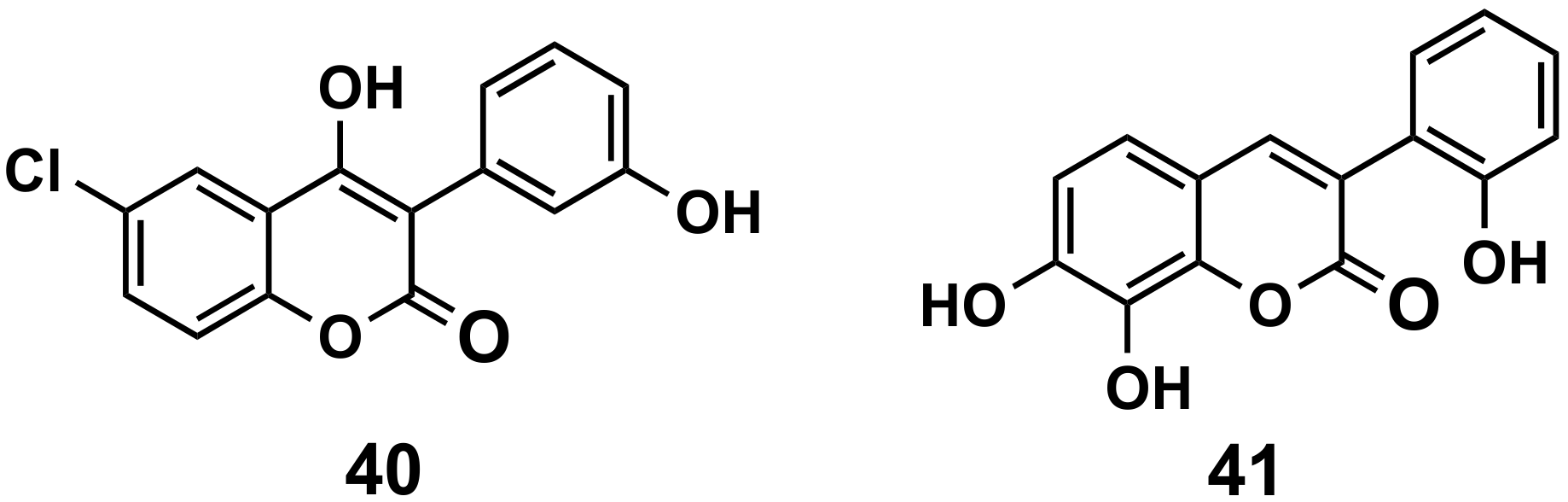
5.2.4. Anticancer Agents
3-Phenylcoumarins with Simple Substitutions
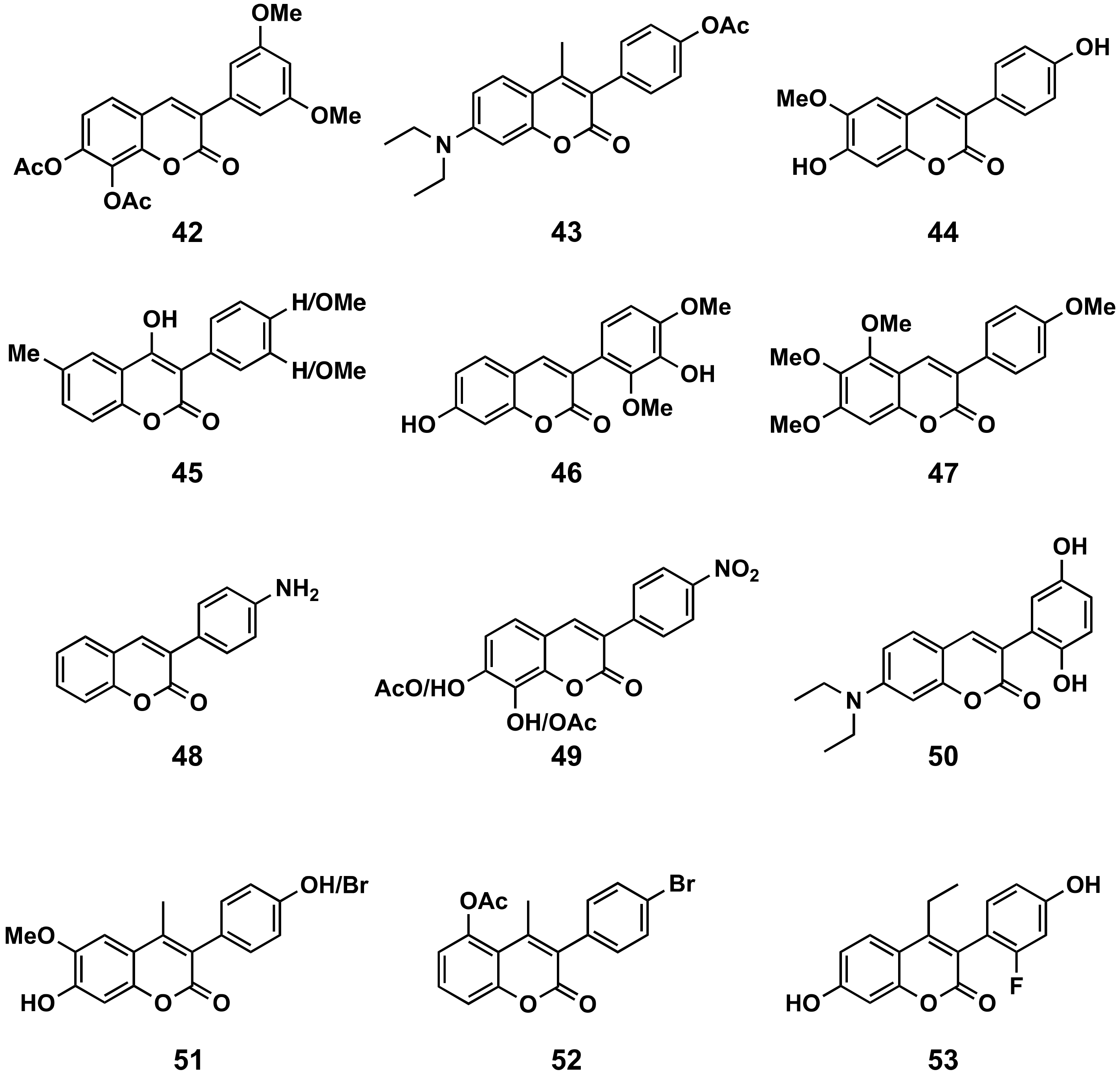
3-Phenylcoumarins with Rings at Position 4

Hybrid 3-Phenylcoumarins
5.3. Other Pharmacological Interests
5.3.1. Enzymatic Inhibitors

5.3.2. Global Pharmacological Effects

6. Other Interests: Fluorescent Probes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gong, T.; Wang, D.X.; Yang, Y.; Liu, P.; Chen, Y.; Yu, D.Q. A novel 3-arylcoumarin and three new 2-arylbenzofurans from Mucuna birdwoodiana. Chem. Pharm. Bull. 2010, 58, 254–256. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.W.; Liu, P.; Yin, W.P.; Jiang, Y.L.; Ren, Y.L. Isolation and identification of antibacterial neo-compounds from the red ants of ChangBai Mountain, Tetramorium sp. Bioorg. Med. Chem. Lett. 2012, 22, 2175–2181. [Google Scholar] [CrossRef]
- Su, Z.; Wang, P.; Yuan, W.; Li, S. Flavonoids and 3-arylcoumarin from Pterocarpus soyauxii. Planta Med. 2013, 79, 487–491. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Deng, Y.; Muñoz-Acuña, U.; Yuan, C.; Lai, H.; Chai, H.; Chagwedera, T.E.; Farnsworth, N.R.; de Blanco, E.J.C.; et al. Bioactive Modified Isoflavonoid Constituents of the Root Bark of Sphenostylis marginata ssp. erecta. J. Org. Chem. 2013, 78, 10166–10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbin, M.X.; Syed, A.M.; Anuradha, V.; Moorthi, M. Identification of bioactive compounds from Rhizophora mucronata methanolic leaf extract by GC-MS analysis. Int. J. Pharm. Sci. Res. 2020, 11, 5268–5272. [Google Scholar]
- Nguyen, D.T.; To, D.C.; Tran, T.T.; Tran, M.H.; Nguyen, P.H. PTP1B and α-glucosidase inhibitors from Selaginella rolandi-principis and their glucose uptake stimulation. J. Nat. Med. 2021, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- El-Saber, B.G.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, E.; Yin, S.; Zhao, S.; Zhao, C.; Yan, M.; Fan, L.; Hu, H. Protective effects of glycycoumarin on liver diseases. Phytother. Res. 2020, 34, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Hazrati, H.; Mohasselyazdi, N.; Khoobi, M.; Shafiee, A. Palladium catalyzed dehydrogenative arylation of coumarins: An unexpected switch in regioselectivity. Chem. Commun. 2013, 49, 10935–10937. [Google Scholar] [CrossRef]
- She, Z.; Shi, Y.; Huang, Y.; Cheng, Y.; Song, F.; You, J. Versatile palladium-catalyzed C-H olefination of (hetero)arenes at room temperature. Chem. Commun. 2014, 50, 13914–13916. [Google Scholar] [CrossRef]
- Martins, S.; Branco, P.S.; de la Torre, M.C.; Sierra, M.A.; Pereira, A. New methodology for the synthesis of 3-substituted coumarins via palladium-catalyzed site-selective cross-coupling reactions. Synlett 2010, 19, 2918–2922. [Google Scholar] [CrossRef]
- Yakushiji, F.; Haramo, M.; Miyadera, Y.; Uchiyama, C.; Takayama, K.; Hayashi, Y. Palladium-catalyzed C3-selective mono-arylation of 4-hydroxycoumarin. Tetrahedron Lett. 2014, 55, 3316–3318. [Google Scholar] [CrossRef]
- Yuan, J.W.; Li, W.J.; Yang, L.R.; Mao, P.; Xiao, Y.M. Regioselective C-3 arylation of coumarins with arylhydrazines via radical oxidation by potassium permanganate. Z. Naturforschung B 2016, 71, 1115–1123. [Google Scholar] [CrossRef]
- Chauhan, P.; Ravi, M.; Singh, S.; Prajapati, P.; Yadav, P.P. Regioselective α-arylation of coumarins and 2-pyridones with phenylhydrazines under transition-metal-free conditions. RSC Adv. 2016, 6, 109–118. [Google Scholar] [CrossRef]
- Skripskaya, O.V.; Feilo, N.O.; Neshchadin, A.O.; Elenich, O.V.; Lytvyn, R.Z.; Obushak, N.D.; Yagodinets, P.I. Synthesis of nitrogen heterocycles underlaid by application of 3-(4-acetylphenyl)-2H-coumarin. Russ. J. Org. Chem. 2013, 49, 1655–1660. [Google Scholar] [CrossRef]
- Masahiro, K.; Kounosuke, O.; Motomu, K. Metal-free C(3)-H arylation of coumarins promoted by catalytic amounts of 5,10,15,20-tetrakis(4-diethylaminophenyl)porphyrin. Chem. Commun. 2015, 51, 9718–9721. [Google Scholar]
- Ali, M.; Farnaz, J. Chlorophyll-catalyzed photochemical regioselective coumarin C–H arylation with diazonium salts. New J. Chem. 2020, 44, 16692–16696. [Google Scholar]
- Jafarpour, F.; Barzegar Amiri Olia, M.; Hazrati, H. Highly Regioselective α-Arylation of Coumarins via Palladium-Catalyzed C-H Activation/Desulfitative Coupling. Adv. Synth. Catal. 2013, 355, 3407–3412. [Google Scholar] [CrossRef]
- Yuan, J.W.; Yang, L.R.; Yin, Q.Y.; Mao, P.; Qu, L.B. KMnO4/AcOH-mediated C3-selective direct arylation of coumarins with arylboronic acids. RSC Adv. 2016, 6, 35936–35944. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Baumgartner, M.T. A different route to 3-aryl-4-hydroxycoumarins. Tetrahedron Lett. 2010, 51, 5322–5324. [Google Scholar] [CrossRef]
- Neha, T.; Rama, K.P. Metal-free direct C-arylation of 1,3-dicarbonyl compounds and ethyl cyanoacetate: A platform to access diverse arrays of meta-functionalized phenols. Chem. Commun. 2018, 54, 11423–11426. [Google Scholar]
- Messaoudi, S.; Brion, J.D.; Alami, M. Palladium-Catalyzed Decarboxylative Coupling of Quinolinone-3-Carboxylic Acids and Related Heterocyclic Carboxylic Acids with (Hetero)aryl Halides. Org. Lett. 2012, 14, 1496–1499. [Google Scholar] [CrossRef]
- Jafarpour, F.; Zarei, S.; Barzegar Amiri Olia, M.; Jalalimanesh, N.; Rahiminejadan, S. Palladium-Catalyzed Decarboxylative Cross-Coupling Reactions: A Route for Regioselective Functionalization of Coumarins. J. Org. Chem. 2013, 78, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Golshani, M.; Khoobi, M.; Jalalimanesh, N.; Jafarpour, F.; Ariafard, A. A transition-metal-free fast track to flavones and 3-arylcoumarins. Chem. Commun. 2017, 53, 10676–10679. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.L.N.; Kumar, A. Pd-catalyzed cross-coupling study of bi-functional 3-bromo-4-trifloxycoumarins with triarylbismuth reagents. Tetrahedron 2015, 71, 5137–5147. [Google Scholar] [CrossRef]
- Degorce, S.L.; Bailey, A.; Callis, R.; De Savi, C.; Ducray, R.; Lamont, G.; MacFaul, P.; Maudet, M.; Scott, M.; Morgentin, R.; et al. Investigation of (E)-3-[4-(2-Oxo-3-aryl-chromen-4-yl)oxyphenyl]acrylic Acids as Oral Selective Estrogen Receptor Down-Regulators. J. Med. Chem. 2015, 58, 3522–3533. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Eugenio, E. Synthesis of 3-arylcoumarins via Suzuki-cross-coupling reactions of 3-chlorocoumarin. Tetrahedron Lett. 2011, 52, 1225–1227. [Google Scholar] [CrossRef]
- Prendergast, A.M.; McGlacken, G.P. Regioselective Chlorination and Suzuki–Miyaura Cross-Coupling of 4-Alkoxycoumarins, 4-Alkoxy-2-pyrones, and Related Heterocycles. Eur. J. Org. Chem. 2017, 32, 4827–4835. [Google Scholar] [CrossRef]
- Matos, M.J.; Delogu, G.; Podda, G.; Santana, L.; Uriarte, E. Regioselective synthesis of bromo-substituted 3-arylcoumarins. Synthesis 2010, 16, 2763–2766. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Gaspar, A.; Borges, F.; Uriarte, E.; Santana, L. Improved Synthesis of 3-(Aminoaryl)coumarins. Org. Prep. Proced. Int. 2012, 44, 522–526. [Google Scholar] [CrossRef]
- Taksande, K.; Borse, D.S.; Lokhande, P. Facile Metal-Free Synthesis of 3-Aryl-4-Substituted Coumarins from O-Hydroxy Carbonyl Compounds. Synth. Commun. 2010, 40, 2284–2290. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Palnati, G.R.; Avula, S.R.; Kumar, A. Efficient and General Synthesis of 3-Aryl Coumarins Using Cyanuric Chloride. Synlett 2012, 23, 611–621. [Google Scholar] [CrossRef]
- Augustine, J.K.; Bombrun, A.; Ramappa, B.; Boodappa, C. An efficient one-pot synthesis of coumarins mediated by propylphosphonic anhydride (T3P) via the Perkin condensation. Tetrahedron Lett. 2012, 53, 4422–4425. [Google Scholar] [CrossRef]
- Rahmani-Nezhad, S.; Khosravani, L.; Saeedi, M.; Divsalar, K.; Firoozpour, L.; Pourshojaei, Y.; Sarrafi, Y.; Nadri, H.; Moradi, A.; Mahdavi, M.; et al. Synthesis and Evaluation of Coumarin-Resveratrol Hybrids as 15-Lipoxygenase Inhibitors. Synth. Commun. 2015, 45, 741–749. [Google Scholar] [CrossRef]
- Phakhodee, W.; Duangkamol, C.; Yamano, D.; Pattarawarapan, M. Ph3P/I2-Mediated Synthesis of 3-Aryl-Substituted and 3,4-Disubstituted Coumarins. Synlett 2017, 28, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, B.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Synthesis and biological evaluation of 3-arylcoumarin derivatives as potential anti-diabetic agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhala, P.; Savanur, H.M.; Sutar, S.M.; Malunavar, S.S.; Kalkhambkar, R.G.; Laali, K.K. Facile one-pot synthetic access to libraries of diversely substituted 3-aryl (alkyl)-coumarins using ionic liquid (IL) or conventional base/solvent, and an IL-mediated approach to novel coumarin-bearing diaryl-ethynes. Tetrahedron Lett. 2020, 61, 151854. [Google Scholar] [CrossRef]
- Hwang, I.-T.; Lee, S.-A.; Hwang, J.-S.; Lee, K.-I. A Facile Synthesis of Highly Functionalized 4-Arylcoumarins via Kostanecki Reactions Mediated by DBU. Molecules 2011, 16, 6313–6321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripathi, S.K.; Logeeswari, K. Synthesis of 3-arylcoumarin derivatives using ultrasound. Int. J. Org. Chem. 2013, 3, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Makrandi, J.K. An efficient one pot synthesis of 3-phenyl and 3-naphthylcoumarins using microwave irradiations. Heterocycl. Lett. 2012, 2, 162–166. [Google Scholar]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wet-osot, S.; Duangkamol, C.; Phakhodee, W.; Pattarawarapan, M. Ultrasound-Assisted Solvent-Free Parallel Synthesis of 3-Arylcoumarins Using N-Acylbenzotriazoles. ACS Comb. Sci. 2016, 18, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, H.; Zhang, H.; Yang, Y.; Wang, F. Synthesis of 2H-chromenones from salicylaldehydes and arylacetonitriles. Molecules 2017, 22, 1197. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.F.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. A general palladium-catalyzed carbonylative synthesis of chromenones from salicylic aldehydes and benzyl chlorides. Chem. Eur. J. 2013, 19, 12245–12248. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Srivastava, V.P.; Yadav, L.D.S. Pd-catalysed carbonylative annulation of salicylaldehydes with benzyl chlorides using N-formylsaccharin as a CO surrogate. New J. Chem. 2018, 42, 16281–16286. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Shi, L.; Liu, M.; Yue, Y.; Li, F.; Zhuo, K. Base-promoted synthesis of coumarins from salicylaldehydes and aryl-substituted 1,1-dibromo-1-alkenes under transition-metal-free conditions. Chem. Commun. 2014, 50, 9887–9890. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, C.J. A Complete Switch of the Directional Selectivity in the Annulation of 2-Hydroxybenzaldehydes with Alkynes. Angew. Chem. Int. Ed. 2014, 53, 13862–13865. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.; Lu, W. Synthesis of 3-arylcoumarins through N-heterocyclic carbene catalyzed condensation and annulation of 2-chloro-2-arylacetaldehydes with salicylaldehydes. Tetrahedron 2013, 69, 3669–3676. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, P.; Jia, Y.; Fang, Z.; Li, P. Organocatalytic condensation–ring opening–annulation cascade reactions between N-Bocindolin-2-ones/benzofuran-2(3H)-ones and salicylaldehydes for synthesis of 3-arylcoumarins. Org. Biomol. Chem. 2017, 15, 7505–7508. [Google Scholar] [CrossRef]
- Pochampalli, J.; Mandala, D.; Doddi, B.; Nalla, U. Synthesis and characterization of some novel coumarin based pyrazoles, isoxazole and pyrimidyl derivatives. J. Pharm. Res. 2012, 5, 1957–1962. [Google Scholar]
- Zhu, F.; Li, Y.; Wang, Z.; Wu, X.F. Iridium-Catalyzed Carbonylative Synthesis of Chromenones from Simple Phenols and Internal Alkynes at Atmospheric Pressure. Angew. Chem. Int. Ed. 2016, 55, 14151–14154. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Ramkumar, V.; Sankararaman, S. Highly Selective and Modular Synthesis of 3-Aryl-4-(arylethynyl)-2H-chromen-2-ones from 2-Iodoaryl 2-Arylacetates through a Carbonylative Sonogashira Coupling–Intramolecular Aldol Cascade Reaction. Eur. J. Org. Chem. 2016, 23, 4041–4049. [Google Scholar] [CrossRef]
- Kim, S.; Kang, D.; Lee, C.H.; Lee, P.H. Synthesis of Substituted Coumarins via Brønsted Acid Mediated Condensation of Allenes with Substituted Phenols or Anisoles. J. Org. Chem. 2012, 77, 6530–6537. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Sun, J. Synthesis of Coumarins via [4 + 2] Cyclization of Siloxy Alkynes and Salicylaldehydes. Synlett 2021, 32, 207–210. [Google Scholar]
- Nakai, K.; Kurahashi, T.; Matsubara, S. Nickel-Catalyzed Cycloaddition of o-Arylcarboxybenzonitriles and Alkynes via Cleavage of Two Carbon-Carbon σ Bonds. J. Am. Chem. Soc. 2011, 133, 11066–11068. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Z.; Yan, G.H.; Chen, W.H.; Yang, X.M. Microwave-assisted efficient synthesis of 2-hydroxydeoxybenzoins from the alkali degradation of readily prepared 3-aryl-4-hydroxycoumarins in water. Chem. Pharm. Bull. 2013, 61, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Dao, P.D.Q.; Ho, S.L.; Lim, H.J.; Cho, C.S. Microwave-Assisted Cyclization under Mildly Basic Conditions: Synthesis of 6H-Benzo[c]chromen-6-ones and Their 7,8,9,10-Tetrahydro Analogues. J. Org. Chem. 2018, 83, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, F.; Yang, L.; Xia, C. Access to coumarins by rhodium-catalyzed oxidative annulation of aryl thiocarbamates with internal alkynes. Org. Lett. 2015, 17, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Synthesis of coumarins via PIDA/I2-mediated oxidative cyclization of substituted phenylacrylic acids. RSC Adv. 2013, 3, 4311–4320. [Google Scholar] [CrossRef]
- Wang, S.; Xi, C. Nickel-Catalyzed Arylative Carboxylation of Alkynes with Arylmagnesium Reagents and Carbon Dioxide Leading to Trisubstituted Acrylic Acids. Org. Lett. 2018, 20, 4131–4134. [Google Scholar] [CrossRef]
- Ge, H.Y.; Fang, Z.J.; Qian, B.H. Synthesis of 3-arylcoumarins by FeCl3-promoted cyclization of ortho-methoxy-substituted (E)-2,3-diphenylpropenoic acids or their methyl esters. Chem. Heterocycl. Compd. 2014, 50, 12–18. [Google Scholar] [CrossRef]
- Sukriye, N.K.E.S.; Ozen, F.; Koran, K.; Yilmaz, I.; Gorgulu, A.O.; Erdemir, S. Coumarin Based Highly Selective “off-on-off” Type Novel Fluorescent Sensor for Cu2+ and S2- in Aqueous Solution. J. Fluoresc. 2017, 27, 463–471. [Google Scholar]
- Jing, H.; Aloysius, E.; Wei, F.; Jin-Hui, X.; Yu, Z.; Jie, W. Visible-Light-Driven Alkyne Hydro-/Carbocarboxylation Using CO2 via Iridium/Cobalt Dual Catalysis for Divergent Heterocycle Synthesis. J. Am. Chem. Soc. 2018, 140, 5257–5263. [Google Scholar]
- Yoshida, H.; Ito, Y.; Ohshita, J. Three-component coupling using arynes and DMF: Straightforward access to coumarins via ortho-quinone methides. Chem. Commun. 2011, 47, 8512–8514. [Google Scholar] [CrossRef]
- Ragupathi, A.; Sagadevan, A.; Charpe, V.P.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Visible-light-driven copper-catalyzed aerobic oxidative cascade cyclization of N-tosylhydrazones and terminal alkynes: Regioselective synthesis of 3-arylcoumarins. Chem. Commun. 2019, 55, 5151–5154. [Google Scholar] [CrossRef]
- Luo, W.F.; Ye, L.W.; Li, L.; Qian, P.C. Regio- and diastereoselective synthesis of trans-3,4-diaryldihydrocoumarins via metal-free [4 + 2] annulation of ynamides with o-hydroxybenzyl alcohols. Chem. Commun. 2021, 57, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Aydıner, B.; Yalçın, E.; Korkmaz, V.; Seferoğlu, Z. Efficient one-pot three-component method for the synthesis of highly fluorescent coumarin-containing 3,5-disubstituted-2,6-dicyanoaniline derivatives under microwave irradiation. Synth. Commun. 2017, 47, 2174–2188. [Google Scholar] [CrossRef]
- Pu, W.C.; Mu, G.M.; Zhang, G.L.; Wang, C. Copper-catalyzed decarboxylative intramolecular C-O coupling: Synthesis of 2-arylbenzofuran from 3-arylcoumarin. RSC Adv. 2014, 4, 903–906. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Sheng, J.; Song, X.; Du, Z.; Li, M.; Zhang, X.; Zou, Y. Decarboxylation of α,β-unsaturated aromatic lactones: Synthesis of E-ortho-hydroxystilbenes from 3-arylcoumarins or isoaurones. Green Chem. 2018, 20, 804–808. [Google Scholar] [CrossRef]
- Patel, J.J.; Laars, M.; Gan, W.; Board, J.; Kitching, M.O.; Snieckus, V. Directed Remote Lateral Metalation: Highly Substituted 2-Naphthols and BINOLs by In Situ Generation of a Directing Group. Angew. Chem. Int. Ed. 2018, 57, 9425–9429. [Google Scholar] [CrossRef] [Green Version]
- Jie, Y.; Pingping, Z.; Yuheng, H.; Teng, L.; Jie, S.; Xiaojing, W. Synthesis and biological evaluation of 3-arylcoumarins as potential anti-Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 651–656. [Google Scholar]
- Hu, Y.H.; Yang, J.; Zhang, Y.; Liu, K.C.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and biological evaluation of 3–(4-aminophenyl)-coumarin derivatives as potential anti-Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 1083–1092. [Google Scholar] [CrossRef]
- Viña, D.; Matos, M.J.; Yáñez, M.; Santana, L.; Uriarte, E. 3-Substituted coumarins as dual inhibitors of AChE and MAO for the treatment of Alzheimer’s disease. Med. Chem. Commun. 2012, 3, 213–218. [Google Scholar] [CrossRef]
- Alves de Souza, G.; John da Silva, S.; Martins Cardoso, C.; Nora Castro, R.; Sant’Anna, C.M.R.; Eugen Kümmerle, A. Discovery of novel dual-active 3-(4-(dimethylamino)phenyl)-7-aminoalcoxy-coumarin as potent and selective acetylcholinesterase inhibitor and antioxidant. J. Enzym. Inhib. Med. Chem. 2019, 34, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Bachiller, M.I.; Horatscheck, A.; Lisurek, M.; Rademann, J. Alzheimer’s Disease: Identification and Development of β-Secretase (BACE-1) Binding Fragments and Inhibitors by Dynamic Ligation Screening (DLS). ChemMedChem 2013, 8, 1041–1056. [Google Scholar] [CrossRef]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, e00023. [Google Scholar] [CrossRef]
- Montanari, S.; Bartolini, M.; Neviani, P.; Belluti, F.; Gobbi, S.; Pruccoli, L.; Tarozzi, A.; Falchi, F.; Andrisano, V.; Miszta, P.; et al. Multitarget Strategy to Address Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and Computational Studies of Coumarin-Based Derivatives. ChemMedChem 2016, 11, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Abdshahzadeh, H.; Golshani, M.; Nadri, H.; Saberi Kia, I.; Abdolahi, Z.; Forootanfar, H.; Ameri, A.; Tueylue, K.T.; Ayazgok, B.; Jalili-Baleh, L.; et al. 3-aryl coumarin derivatives bearing aminoalkoxy moiety as multi-target-directed ligands against Alzheimer’s disease. Chem. Biodivers. 2019, 16, e1800436. [Google Scholar] [CrossRef]
- Jalili-Baleh, L.; Forootanfar, H.; Tüylü Küçükkılınç, T.; Nadri, H.; Abdolahi, Z.; Ameri, A.; Jafari, M.; Ayazgok, B.; Baeeri, M.; Mahban Rahimifard, M.; et al. Design, synthesis and evaluation of novel multi-target-directed ligands for treatment of Alzheimer’s disease based on coumarin and lipoic acid scaffolds. Eur. J. Med. Chem. 2018, 152, 600–614. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550. [Google Scholar] [CrossRef]
- Wang, Z.M.; Li, X.M.; Xue, G.M.; Xu, W.; Wang, X.B.; Kong, L.Y. Synthesis and evaluation of 6-substituted 3-arylcoumarin derivatives as multifunctional acetylcholinesterase/monoamine oxidase B dual inhibitors for the treatment of Alzheimer’s disease. RSC Adv. 2015, 5, 104122–104137. [Google Scholar] [CrossRef]
- Matos, M.J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and Study of a Series of 3-Arylcoumarins as Potent and Selective Monoamine Oxidase B Inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef]
- Viña, D.; Matos, M.J.; Ferino, G.; Cadoni, E.; Laguna, R.; Borges, F.; Uriarte, E.; Santana, L. 8-Substituted 3-Arylcoumarins as Potent and Selective MAO-B Inhibitors: Synthesis, Pharmacological Evaluation, and Docking Studies. ChemMedChem 2012, 7, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vina, D.; Janeiro, P.; Borges, F.; Santana, L.; Uriarte, E. New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 5157–5160. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Viña, D. MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins. Bioorg. Med. Chem. Lett. 2011, 21, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vilar, S.; García-Morales, V.; Tatonetti, N.P.; Uriarte, E.; Santana, L.; Viña, D. Insight into the Functional and Structural Properties of 3-Arylcoumarin as an Interesting Scaffold in Monoamine Oxidase B Inhibition. ChemMedChem 2014, 9, 1488–1500. [Google Scholar] [CrossRef]
- Serra, S.; Ferino, G.; Matos, M.J.; Vázquez-Rodríguez, S.; Delogu, G.; Viña, D.; Cadoni, E.; Santana, L.; Uriarte, E. Hydroxycoumarins as selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 258–261. [Google Scholar] [CrossRef]
- Delogu, G.L.; Serra, S.; Quezada, E.; Uriarte, E.; Vilar, S.; Tatonetti, N.P.; Viña, D. Monoamine Oxidase (MAO) Inhibitory Activity: 3-Phenylcoumarins versus 4-Hydroxy-3-phenylcoumarins. ChemMedChem 2014, 9, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Martins, C.; Fonseca, A.; Nunes, R.; Matos, M.J.; Silva, R.; Otero-Espinar, F.J.; Uriarte, E.; Borges, F. PEGylated PLGA Nanoparticles as a Smart Carrier to Increase the Cellular Uptake of a Coumarin-Based Monoamine Oxidase B Inhibitor. ACS Appl. Mater. Interfaces 2018, 10, 39557–39569. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.S.; Pan, L.F.; Xie, S.S.; Wang, X.B.; Kong, L.Y. Synthesis and evaluation of 6-methylcoumarin derivatives as potent and selective Monoamine Oxidase B inhibitors. Med. Chem. Commun. 2015, 6, 592–600. [Google Scholar] [CrossRef]
- Rauhamaki, S.; Postila, P.A.; Niinivehmas, S.; Kortet, S.; Schildt, E.; Pasanen, M.; Manivannan, E.; Ahinko, M.; Koskimies, P.; Nyberg, N.; et al. Structure-activity relationship analysis of 3-phenylcoumarin-based monoamine oxidase B inhibitors. Front. Chem. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, M.D.; Hinz, S.; Müller, C.E.; Gütschow, M. Alkynyl-coumarinyl ethers as MAO-B inhibitors. Bioorg. Med. Chem. 2014, 22, 1916–1928. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Aghimien, M.O.; Eyunni, S.V.K.; Latinwo, L.M. Identification of 7,8-dihydroxy-3-phenylcoumarin as a reversible monoamine oxidase enzyme inhibitor. J. Biochem. Mol. Toxicol. 2021, 35, e22651. [Google Scholar] [CrossRef]
- Matos, M.J.; Rodriguez-Enriquez, F.; Vilar, S.; Santana, L.; Uriarte, E.; Hripcsak, G.; Estrada, M.; Rodriguez-Franco, M.I.; Vina, D. Potent and selective MAO-B inhibitory activity: Amino- versus nitro-3-arylcoumarin derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sashidhara, K.V.; Modukuri, R.K.; Jadiya, P.; Bhaskara Rao, K.; Sharma, T.; Haque, R.; Kumar Singh, D.; Banerjee, D.; Siddiqi, M.I.; Nazir, A. Discovery of 3-Arylcoumarin-tetracyclic Tacrine Hybrids as Multifunctional Agents against Parkinson’s Disease. ACS Med. Chem. Lett. 2014, 5, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Mella, J.; González, C.; Viña, D.; Uriarte, E.; Matos, M.J. 3-Arylcoumarins as highly potent and selective monoamine oxidase B inhibitors: Which chemical features matter? Bioorg. Chem. 2020, 101, 103964. [Google Scholar] [CrossRef]
- Soufi, W.; Merad, M.; Lebbad, F.; Ghalem, S.; Hacene, F.B. Interaction of monoamine oxidase-B with a series of coumarin by molecular modeling methods. Asian J. Chem. 2016, 28, 634–638. [Google Scholar] [CrossRef]
- Morales Helguera, A.; Pérez-Garrido, A.; Gaspar, A.; Reis, J.; Cagide, F.; Viña, D.; Cordeiro, M.N.; Borges, F. Combining QSAR classification models for predictive modeling of human monoamine oxidase inhibitors. Eur. J. Med. Chem. 2013, 59C, 75–90. [Google Scholar] [CrossRef]
- Giulio, F.; Cadoni, E.; Matos, M.J.; Quezada, E.; Uriarte, E.; Santana, L.; Vilar, S.; Tatonetti, N.P.; Yáñez, M.; Viña, D.; et al. MAO Inhibitory Activity of 2-Arylbenzofurans versus 3-Arylcoumarins: Synthesis, in vitro Study, and Docking Calculations. ChemMedChem 2013, 8, 956–966. [Google Scholar]
- Andrade, M.F.; Kabeya, L.M.; Bortot, L.O.; dos Santos, G.B.; Santos, E.O.L.; Albiero, L.R.; Figueiredo-Rinhel, A.S.G.; Carvalho, C.A.; Azzolini, A.E.C.S.; Caliri, A.; et al. The 3-phenylcoumarin derivative 6,7-dihydroxy-3-[3’,4’-methylenedioxyphenyl]-coumarin down-modulates the FcγR- and CR-mediated oxidative metabolism and elastase release in human neutrophils: Possible mechanisms underlying inhibition of the formation and release of neutrophil extracellular traps. Free Radic. Biol. Med. 2018, 115, 421–435. [Google Scholar]
- Pu, W.; Lin, Y.; Zhang, J.; Wang, F.; Wang, C.; Zhang, G. 3-Arylcoumarins: Synthesis and potent anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 5432–5434. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef] [PubMed]
- Kavetsou, E.; Katopodi, A.; Argyri, L.; Chainoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D.; Chroni, A.; Detsi, A. Novel 3-aryl-5-substituted-coumarin analogues: Synthesis and bioactivity profile. Drug Dev. Res. 2020, 81, 456–469. [Google Scholar] [CrossRef]
- Dharavath, R.; Nagaraju, N.; Reddy, M.R.; Ashok, D.; Sarasija, M.; Vijjulatha, M.; Vani, T.; Jyothi, K.; Prashanthi, G. Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1,2,3-triazoles. RSC Adv. 2020, 10, 11615–11623. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, S.A.; Nazareno, M.A.; Baumgartner, M.T. Effect of different C3-aryl substituents on the Antioxidant activity of 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2011, 19, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Borges, F.; Olea-Azar, C. Remarkable antioxidant properties of a series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem. 2013, 2, 3900–3906. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Mura, F.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E.; Olea-Azar, C. Study of coumarin-resveratrol hybrids as potent antioxidant compounds. Molecules 2015, 20, 3290–3308. [Google Scholar] [CrossRef] [Green Version]
- Nolan, K.A.; Scott, K.A.; Barnes, J.; Doncaster, J.; Whitehead, R.C.; Stratford, I.J. Pharmacological inhibitors of NAD(P)H quinone oxidoreductase, NQO1: Structure/activity relationships and functional activity in tumour cells. Nolan. Biochem. Pharmacol. 2010, 80, 977–981. [Google Scholar] [CrossRef]
- Scott, K.A.; Barnes, J.; Whitehead, R.C.; Stratford, I.J.; Nolan, K.A. Inhibitors of NQO1: Identification of compounds more potent than dicoumarol without associated off-target effects. Biochem. Pharmacol. 2011, 81, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Erzincan, P.; Saçan, M.T.; Yüce-Dursun, B.; Daniş, Ö.; Demir, S.; Erdem, S.S.; Ogan, A. QSAR models for antioxidant activity of new coumarin derivatives. SAR QSAR Environ. Res. 2015, 26, 721–737. [Google Scholar] [CrossRef]
- Quezada, E.; Delogu, G.; Picciau, C.; Santana, L.; Podda, G.; Borges, F.; García-Morales, V.; Viña, D.; Orallo, F. Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins. Molecules 2010, 15, 270–279. [Google Scholar] [CrossRef]
- Singh, L.R.; Kumar, A.; Upadhyay, A.; Gupta, S.; Palanati, G.R.; Sikka, K.; Siddiqi, M.I.; Yadav, P.N.; Sashidhara, K.V. Discovery of coumarin-dihydroquinazolinone analogs as niacin receptor 1 agonist with in-vivo anti-obesity efficacy. Eur. J. Med. Chem. 2018, 152, 208–222. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules 2013, 18, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.K.; Pathak, A.; Malairajan, P.; Chaturvedi, D. Design, synthesis and antimicrobial evaluations of novel 3,7-disubstituted 2H-1-benzopyran-2-ones. Asian J. Chem. 2020, 32, 2397–2402. [Google Scholar] [CrossRef]
- Mandala, D.; Valeru, A.; Pochampalli, J.; Vankadari, S.R.; Gatla, R.; Thampu, R. Synthesis, antimicrobial activity, and molecular modeling of novel 4-(3-(4-benzylpiperazin-1-yl)propoxy)-7-methoxy-3-substituted phenyl-2H-chromen-2-one. Med. Chem. Res. 2013, 22, 5481–5489. [Google Scholar] [CrossRef]
- Giri, R.R.; Lad, H.B.; Bhila, V.G.; Patel, C.V.; Brahmbhatt, D.I. Modified Pyridine-Substituted Coumarins: A New Class of Antimicrobial and Antitubercular Agents. Synth. Commun. 2015, 45, 363–375. [Google Scholar] [CrossRef]
- Olmedo, D.; Sancho, R.; Bedoya, L.M.; Lopez-Perez, J.L.; Del Olmo, E.; Munoz, E.; Alcami, J.; Gupta, M.P.; San Feliciano, A. 3-Phenylcoumarins as Inhibitors of HIV-1 Replication. Molecules 2012, 17, 9245–9257. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.B.B.; Watanabe, N.; Saito, A.; Futamura, Y.; Abd El Galil, K.H.; Koito, A.; Najimudin, N.; Osada, H. Vipirinin, a Coumarin-based HIV-1 Vpr Inhibitor, Interacts with a Hydrophobic Region of VPR. J. Biol. Chem. 2011, 286, 14049–14056. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef]
- Robledo-O’Ryan, N.; Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Moncada-Basualto, M.; Mura, F.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis, antioxidant and antichagasic properties of a selected series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem. 2017, 25, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.F.; Tao, L.Y.; Sun, H.Y.; Wei, W.; Chen, Y.; Fu, L.W.; Zou, Y. Design, synthesis and antitumor activity of a series of novel coumarin-stilbenes hybrids, the 3-arylcoumarins. Chin. Chem. Lett. 2010, 21, 1295–1298. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Cooperwood, J.; Sinclair, A.; Abdullah, A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011, 31, 2017–2022. [Google Scholar] [PubMed]
- Yang, J.; Liu, G.Y.; Dai, F.; Cao, X.Y.; Kang, Y.F.; Hu, L.M.; Tang, J.J.; Li, X.Z.; Li, Y.; Jin, X.L.; et al. Synthesis and biological evaluation of hydroxylated 3-phenylcoumarins as antioxidants and antiproliferative agents. Bioorg. Med. Chem. Lett. 2011, 21, 6420–6425. [Google Scholar] [CrossRef]
- Serra, S.; Chicca, A.; Delogu, G.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Casu, L.; Gertsch, J. Synthesis and cytotoxic activity of non-naturally substituted 4-oxycoumarin derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5791–5794. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, D.V.; Chernysheva, N.B.; Salamandra, L.K.; Konyushkin, L.D.; Atamanenko, O.P.; Semenova, M.N.; Semenov, V.V. Synthesis of Polyalkoxy-3-(4-Methoxyphenyl)Coumarins with Antimitotic Activity from Plant Allylpolyalkoxybenzenes. Mendeleev Commun. 2013, 23, 147–149. [Google Scholar] [CrossRef]
- Sail, V.; Hadden, M.K. Identification of Small Molecule Hes1 Modulators as Potential Anticancer Chemotherapeutics. Chem. Biol. Drug Des. 2013, 81, 334–342. [Google Scholar] [CrossRef]
- Musa, M.A.; Gbadebo, A.J.; Latinwo, L.M.; Badisa, V.L.D. 7,8-Dihydroxy-3-(4-nitrophenyl)coumarin induces cell death via reactive oxygen species-independent S-phase cell arrest. J. Biochem. Mol. Toxicol. 2018, 32, 794–801. [Google Scholar] [CrossRef]
- Musa, M.A.; Latinwo, L.M.; Virgile, C.; Badisa, V.L.D.; Gbadebo, A.J. Synthesis and in vitro evaluation of 3-(4-nitrophenyl)coumarin derivatives in tumor cell lines. Bioorg. Chem. 2015, 58, 96–103. [Google Scholar] [CrossRef]
- LiHong, W.; GuoJing, Q.; YuanDi, G.; Le, S.; Qing, Y.F.J.; BaoXiang, Z.; JunYing, M. A small molecule targeting glutathione activates Nrf2 and inhibits cancer cell growth through promoting Keap-1 S-glutathionylation and inducing apoptosis. RSC Adv. 2018, 8, 792–804. [Google Scholar]
- Zou, H.; Jiang, H.; Zhou, J.Y.; Zhu, Y.; Cao, L.; Xia, P.; Zhang, Q. Synthesis of 3-(4-hydroxyphenyl)-4-methyl-6-methoxy-7-hydroxycoumarin and its analogues as angiogenesis inhibitors. J. Chin. Pharm. Sci. 2010, 19, 136–140. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Z.; Luo, J.; Tang, C.; Zhang, S.; Ning, W.; Dong, C.; Huang, J.; Liu, X.; Zhou, H.B. Dual functional small molecule fluorescent probes for image-guided estrogen receptor-specific targeting coupled potent antiproliferative potency for breast cancer therapy. Bioorg. Med. Chem. 2017, 25, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Waryoba, C.; Ugochukwu, N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010, 30, 4613–4618. [Google Scholar] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F.; Rahman, T. In-vitro antiproliferative activity of benzopyranone derivatives in comparison with standard chemotherapeutic drugs. Arch. Pharm. 2011, 344, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, S.; Yao, Y.; Zhou, L.; Zhao, J.; Gu, Y.; Wang, K.; Li, X. Design, synthesis, and anti-tumor activities of novel triphenylethylene-coumarin hybrids, and their interactions with Ct-DNA. Bioorg. Med. Chem. Lett. 2013, 23, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, Y.; Li, S.; Lv, M.; Chen, H.; Li, X. Cytotoxicity and DNA binding property of triphenylethylene-coumarin hybrids with two amino side chains. Bioorg. Med. Chem. Lett. 2014, 24, 900–904. [Google Scholar] [CrossRef]
- Tan, G.; Yao, Y.; Gu, Y.; Li, S.; Lv, M.; Wang, K.; Chen, H.; Li, X. Cytotoxicity and DNA binding property of the dimers of triphenylethylene-coumarin hybrid with one amino side chain. Bioorg. Med. Chem. Lett. 2014, 24, 2825–2830. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, L.; Yao, Y.; Li, S.; Lv, M.; Wang, K.; Li, X.; Chen, H. Anticancer activity and DNA binding property of the dimers of triphenylethylene-coumarin hybrid with two amino side chains. Med. Chem. Res. 2015, 24, 2314–2324. [Google Scholar] [CrossRef]
- Cui, N.; Lin, D.D.; Shen, Y.; Shi, J.G.; Wang, B.; Zhao, M.Z.; Zheng, L.; Chen, H.; Shi, J.H. Triphenylethylene-Coumarin Hybrid TCH-5c Suppresses Tumorigenic Progression in Breast Cancer Mainly Through the Inhibition of Angiogenesis. Anticancer Agents Med. Chem. 2019, 19, 1253–1261. [Google Scholar] [CrossRef]
- Luo, G.; Chen, M.; Lyu, W.; Zhao, R.; Xu, Q.; You, Q.; Xiang, H. Design, synthesis, biological evaluation and molecular docking studies of novel 3-aryl-4-anilino-2H-chromen-2-one derivatives targeting ERα as anti-breast cancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 2668–2673. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Zang, G.; Wu, C.; Tang, Z.; Liu, L.; You, Q.; Xiang, H. Novel SERMs based on 3-aryl-4-aryloxy-2H-chromen-2-one skeleton—A possible way to dual ERα/VEGFR-2 ligands for treatment of breast cancer. Eur. J. Med. Chem. 2017, 140, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Muyaba, M.; Lyu, W.; Tang, Z.; Zhao, R.; Xu, Q.; You, Q.; Xiang, H. Design, synthesis and biological evaluation of novel 3-substituted 4-anilino-coumarin derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2017, 27, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.K.; Ansari, M.I.; Yadav, N.; Gupta, P.K.; Gupta, A.K.; Saxena, R.; Fatima, I.; Manohar, M.; Kushwaha, P.; Khedgikar, V.; et al. Design and synthesis of ERα/ERβ selective coumarin and chromene derivatives as potential anti-breast cancer and anti-osteoporotic agents. RSC Adv. 2014, 4, 8828–8845. [Google Scholar] [CrossRef]
- Shen, W.; Mao, J.; Sun, J.; Sun, M.; Zhang, C. Synthesis and biological evaluation of resveratrol-coumarin hybrid compounds as potential antitumor agents. Med. Chem. Res. 2013, 22, 1630–1640. [Google Scholar] [CrossRef]
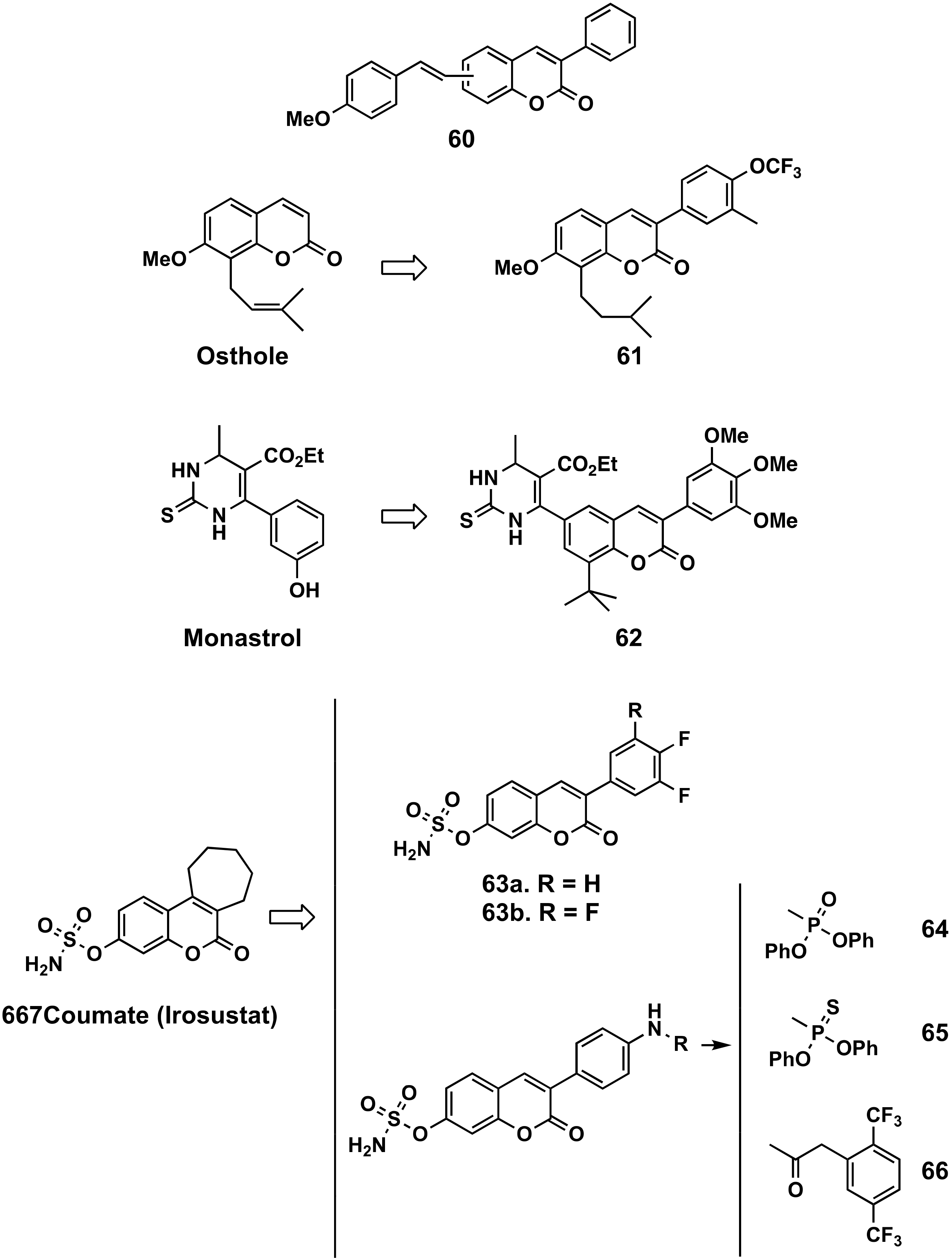
- You, L.; An, R.; Wang, X.; Li, Y. Discovery of novel osthole derivatives as potential anti-breast cancer treatment. Bioorg. Med. Chem. Lett. 2010, 20, 7426–7428. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Avula, S.R.; Sharma, K.; Palnati, G.R.; Bathula, S.R. Discovery of coumarin-monastrol hybrid as potential antibreast tumor-specific agent. Eur. J. Med. Chem. 2013, 60, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Stanway, S.; Purohit, A.; Woo, L.W.L.; Sufi, S.; Vigushin, D.; Ward, R.; Wilson, R.; Stanczyk, F.Z.; Dobbs, N.; Kulinskaya, E.; et al. Phase I study of STX 64 (667 Coumate) in Breast Cancer Patients: The First Study of a Steroid Sulphatase Inhibitor. Clin. Cancer Res. 2006, 12, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Demkowicz, S.; Daśko, M.; Kozak, W.; Krawczyk, K.; Witt, D.; Masłyk, M.; Kubiński, K.; Rachon, J. Synthesis and Biological Evaluation of Fluorinated 3-Phenylcoumarin-7-O-Sulfamate Derivatives as Steroid Sulfatase Inhibitors. Chem. Biol. Drug Des. 2016, 87, 233–238. [Google Scholar] [CrossRef]
- Daśko, M.; Maslyk, M.; Kubiński, K.; Aszyk, J.; Rachon, J.; Demkowicz, S. Synthesis and steroid sulfatase inhibitory activities of N-phosphorylated 3-(4-aminophenyl)-coumarin-7-O-sulfamates. Med. Chem. Commun. 2016, 7, 1146–1150. [Google Scholar] [CrossRef]
- Daśko, M.; Demkowicz, S.; Biernacki, K.; Harrous, A.; Rachon, J.; Kozak, W.; Martyna, A.; Maslyk, M.; Kubiński, K.; Boguszewska-Czubara, A. Novel steroid sulfatase inhibitors based on N-thiophosphorylated 3-(4-aminophenyl)-coumarin-7-O-sulfamates. Drug Dev. Res. 2019, 80, 857–866. [Google Scholar] [CrossRef]
- Daśko, M.; Przybyłowska, M.; Rachon, J.; Masłyk, M.; Kubiński, K.; Misiak, M.; Składanowski, A.; Demkowicz, S. Synthesis and biological evaluation of fluorinated N-benzoyl and N-phenylacetoyl derivatives of 3-(4-aminophenyl)-coumarin-7-O-sulfamate as steroid sulfatase inhibitors. Eur. J. Med. Chem. 2017, 128, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Niinivehmas, S.; Postila, P.A.; Rauhamäki, S.; Manivannan, E.; Kortet, S.; Ahinko, M.; Huuskonen, P.; Nyberg, N.; Koskimies, P.; Lätti, S.; et al. Blocking oestradiol synthesis pathways with potent and selective coumarin derivatives. J. Enzym. Inhib. Med. Chem. 2018, 33, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Santana, L.; Uriarte, E.; Delogu, G.; Corda, M.; Fadda, M.B.; Era, B.; Fais, A. New halogenated phenylcoumarins as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3342–3345. [Google Scholar] [CrossRef]
- Matos, M.J.; Varela, C.; Vilar, S.; Hripcsak, G.; Borges, F.; Santana, L.; Uriarte, E.; Fais, A.; Di Petrillo, A.; Pintus, F.; et al. Design and discovery of tyrosinase inhibitors based on a coumarin scaffold. RSC Adv. 2015, 5, 94227–94235. [Google Scholar] [CrossRef]
- Fais, A.; Era, B.; Asthana, S.; Sogos, V.; Medda, R.; Santana, L.; Uriarte, E.; Matos, M.J.; Delogu, F.; Kumar, A. Coumarin derivatives as promising xanthine oxidase inhibitors. Int. J. Biol. Macromol. 2018, 120, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Era, B.; Delogu, G.L.; Pintus, F.; Fais, A.; Gatto, G.; Uriarte, E.; Borges, F.; Kumar, A.; Matos, M.J. Looking for new xanthine oxidase inhibitors: 3-Phenylcoumarins versus 2-phenylbenzofurans. Int. J. Biol. Macromol. 2020, 162, 774–780. [Google Scholar] [CrossRef]
- Alparslan, M.M.; Danış, O. In Vitro Inhibition of Human Placental Glutathione S-Transferase by 3-Arylcoumarin Derivatives. Arch. Pharm. 2015, 348, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lalehan, O.; Özkan, D.; Basak, Y.D.; Serap, D.; Cihan, G.; Ayse, O. Investigation of HMG-CoA reductase inhibitory and antioxidant effects of various hydroxycoumarin derivatives. Arch. Pharm. 2020, 353, 1900378. [Google Scholar]
- Sashidhara, K.V.; Rao, K.B.; Sonkar, R.; Modukuri, R.K.; Chhonker, Y.S.; Kushwaha, P.; Chandasana, H.; Khanna, A.K.; Bhatta, R.S.; Bhatia, G.; et al. Hybrids of coumarin-indole: Design, synthesis and biological evaluation in Triton WR-1339 and high-fat diet induced hyperlipidemic rat models. Med. Chem. Commun. 2016, 7, 1858–1869. [Google Scholar] [CrossRef]
- Rullo, M.; Niso, M.; Pisani, L.; Carrieri, A.; Altomare, C.D. 1,2,3,4-Tetrahydroisoquinoline/2H-chromen-2-one conjugates as nanomolar P-glycoprotein inhibitors: Molecular determinants for affinity and selectivity over multidrug resistance associated protein 1. Eur. J. Med. Chem. 2019, 161, 433–444. [Google Scholar] [CrossRef]
- Fan, J.; Kuang, Y.; Dong, Z.; Yi, Y.; Zhou, Y.; Li, B.; Qiao, X.; Ye, M. Prenylated Phenolic Compounds from the Aerial Parts of Glycyrrhiza uralensis as PTP1B and α-Glucosidase Inhibitors. J. Nat. Prod. 2020, 83, 814–824. [Google Scholar] [CrossRef]
- Gupta, M.K.; Kumar, S.; Chaudhary, S. Synthesis and Investigation of Antidiabetic Response of New Coumarin Derivatives Against Streptozotocin Induced Diabetes in Experimental Rats. Pharma. Chem. J. 2020, 12, 1122–1127. [Google Scholar] [CrossRef]
- De Souza Santos, M.; Freire de Morais del Lama, M.P.; Deliberto, L.A.; da Silva Emery, F.; Tallarico, P.; Zumstein, M.; Georgetto Naal, R.M. In situ screening of 3-arylcoumarin derivatives reveals new inhibitors of mast cell degranulation. Arch. Pharm. Res. 2013, 36, 731–738. [Google Scholar] [CrossRef]
- Matos, M.J.; Hogger, V.; Gaspar, A.; Kachler, S.; Borges, F.; Uriarte, E.; Santana, L.; Klotz, K.N. Synthesis and adenosine receptors binding affinities of a series of 3-arylcoumarins. J. Pharm. Pharmacol. 2013, 65, 1590–1597. [Google Scholar] [CrossRef]
- Matos, M.J.; Vilar, S.; Kachler, S.; Fonseca, A.; Santana, L.; Uriarte, E.; Borges, F.; Tatonetti, N.P.; Klotz, K.N. Insight into the Interactions between Novel Coumarin Derivatives and Human A3 Adenosine Receptors. ChemMedChem 2014, 9, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vilar, S.; Vazquez-Rodriguez, S.; Kachler, S.; Klotz, K.N.; Buccioni, M.; Delogu, G.; Santana, L.; Uriarte, E.; Borges, F. Structure-Based Optimization of Coumarin hA3 Adenosine Receptor Antagonists. J. Med. Chem. 2020, 63, 2577–2587. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Chatterjee, M.; Kumar Verma, A.; Palit, G. Discovery and synthesis of novel 3-phenylcoumarin derivatives as antidepressant agents. Bioorg. Med. Chem. Lett. 2011, 21, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Rao, K.B.; Singh, S.; Modukuri, R.K.; Teja, G.A.; Chandasana, H.; Shukla, S.; Bhatta, R.S. Synthesis and evaluation of new 3-phenylcoumarin derivatives as potential antidepressant agents. Bioorg. Med. Chem. Lett. 2014, 24, 4876–4880. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Modukuri, R.K.; Bhaskara, R.K.; Gupta, S.; Singh, S.; Aruna, T.G.; Shukla, S. Design and synthesis of new series of coumarin-aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg. Med. Chem. Lett. 2015, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cambray, S.; Bandyopadhyay, A.; Gao, J. Fluorogenic diazaborine formation of semicarbazide with designed coumarin derivatives. Chem. Commun. 2017, 53, 12532–12535. [Google Scholar] [CrossRef]
- Meimetis, L.G.; Carlson, J.C.T.; Giedt, R.J.; Kohler, R.H.; Weissleder, R. Ultrafluorogenic Coumarin-Tetrazine Probes for Real-Time Biological Imaging. Angew. Chem. Int. Ed. 2014, 53, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, L.; Zhou, Y.; Zhang, W.; Wang, Y.; Zhu, Y. Synthesis and activity of a coumarin-based fluorescent probe for hydroxyl radical detection. Luminescence 2020, 35, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.N.; Yuksel, F.; Ozpinar, G.A.; Bulut, M.; Durmus, M. 7-Oxy-3-(3,4,5-trimethoxyphenyl)coumarin substituted phthalonitrile derivatives as fluorescent sensors for detection of Fe3+ ions: Experimental and theoretical study. Sens. Actuators B Chem. 2014, 194, 377–388. [Google Scholar] [CrossRef]
- Elmas, S.N.K.; Sukriye, N.; Ozen, F.; Koran, K.; Gorgulu, A.O.; Sadi, G.; Yilmaz, I.; Erdemir, S. Selective and sensitive fluorescent and colorimetric chemosensor for detection of CO3−2 anions in aqueous solution and living cells. Talanta 2018, 188, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.N.; Bae, S.; Han, K.; Shin, I.S.; Kim, S.K.; Hong, J.I. Electrostatic Modification for Promotion of Flavin-Mediated Oxidation of a Probe for Flavin Detection. Chem. Eur. J. 2017, 23, 16078–16084. [Google Scholar] [CrossRef]
- Lee, D.N.; Kim, E.; Lee, J.H.; Kim, J.S.; Kang, C.; Hong, J.I. Flavin-mediated photo-oxidation for the detection of mitochondrial flavins. Chem. Commun. 2016, 52, 13487–13490. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Zhu, J.; Miller, R.H.; Wang, Y. Design, Synthesis, and Evaluation of Coumarin-Based Molecular Probes for Imaging of Myelination. J. Med. Chem. 2011, 54, 2331–2340. [Google Scholar] [CrossRef] [Green Version]
- Oshikawa, Y.; Furuta, K.; Tanaka, S.; Ojida, A. Cell Surface-Anchored Fluorescent Probe Capable of Real-Time Imaging of Single Mast Cell Degranulation Based on Histamine-Induced Coordination Displacement. Anal. Chem. 2016, 88, 1526–1529. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Lee, J.H.; Park, N.; Suh, M.; Chae, W.S.; Cao, J.; Peng, X.; Jung, H.; Kang, C.; et al. A Self-Calibrating Bipartite Viscosity Sensor for Mitochondria. J. Am. Chem. Soc. 2013, 135, 9181–9185. [Google Scholar] [CrossRef]
- Hanthorn, J.J.; Haidasz, E.; Gebhardt, P.; Pratt, D.A. A versatile fluorescence approach to kinetic studies of hydrocarbon autoxidations and their inhibition by radical-trapping Antioxidants. Chem. Commun. 2012, 48, 10141–10143. [Google Scholar] [CrossRef]
- Ali, M.; Dondaine, L.; Adolle, A.; Sampaio, C.; Chotard, F.; Richard, P.; Denat, F.; Bettaieb, A.; Le Gendre, P.; Laurens, V.; et al. Anticancer Agents: Does a Phosphonium Behave Like a Gold(I) Phosphine Complex? Let a “Smart” Probe Answer. J. Med. Chem. 2015, 58, 4521–4528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Tan, S.; Xu, L.; Qian, X. Rapid and sensitive fluorescent probes for monoamine oxidases B to A at low concentrations. Tetrahedron Lett. 2012, 53, 6881–6884. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Gong, Y.; Yu, T.; Zhao, Y. Synthesis and characterization of SFX-based coumarin derivatives for OLEDs. Dye. Pigm. 2021, 185, 108969. [Google Scholar] [CrossRef]
- Galeta, J.; Dzijak, O.J.; Dracinsky, M.; Vrabel, M. A Systematic Study of Coumarin–Tetrazine Light-Up Probes for Bioorthogonal Fluorescence Imaging. Chem. Eur. J. 2020, 26, 9945–9953. [Google Scholar] [CrossRef]
- Ong, J.X.; Pang, V.Y.T.; Tng, L.M.; Ang, W.H. Pre-Assembled Coumarin-Rhodamine Scaffold for Ratiometric Sensing of Nitric Oxide and Hypochlorite. Chem. Eur. J. 2018, 24, 1870–1876. [Google Scholar] [CrossRef]
- Ye, D.; Wang, L.; Li, H.; Zhou, J.; Cao, D. Synthesis of coumarin-containing conjugated polymer for naked-eye detection of DNA and cellular imaging. Sens. Actuators B Chem. 2013, 181, 234–243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.J.; Uriarte, E.; Santana, L. 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade. Molecules 2021, 26, 6755. https://doi.org/10.3390/molecules26216755
Matos MJ, Uriarte E, Santana L. 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade. Molecules. 2021; 26(21):6755. https://doi.org/10.3390/molecules26216755
Chicago/Turabian StyleMatos, Maria J., Eugenio Uriarte, and Lourdes Santana. 2021. "3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade" Molecules 26, no. 21: 6755. https://doi.org/10.3390/molecules26216755
APA StyleMatos, M. J., Uriarte, E., & Santana, L. (2021). 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade. Molecules, 26(21), 6755. https://doi.org/10.3390/molecules26216755