Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium
Abstract
1. Introduction
2. Results and Discussion
2.1. Production and Purification of MnP
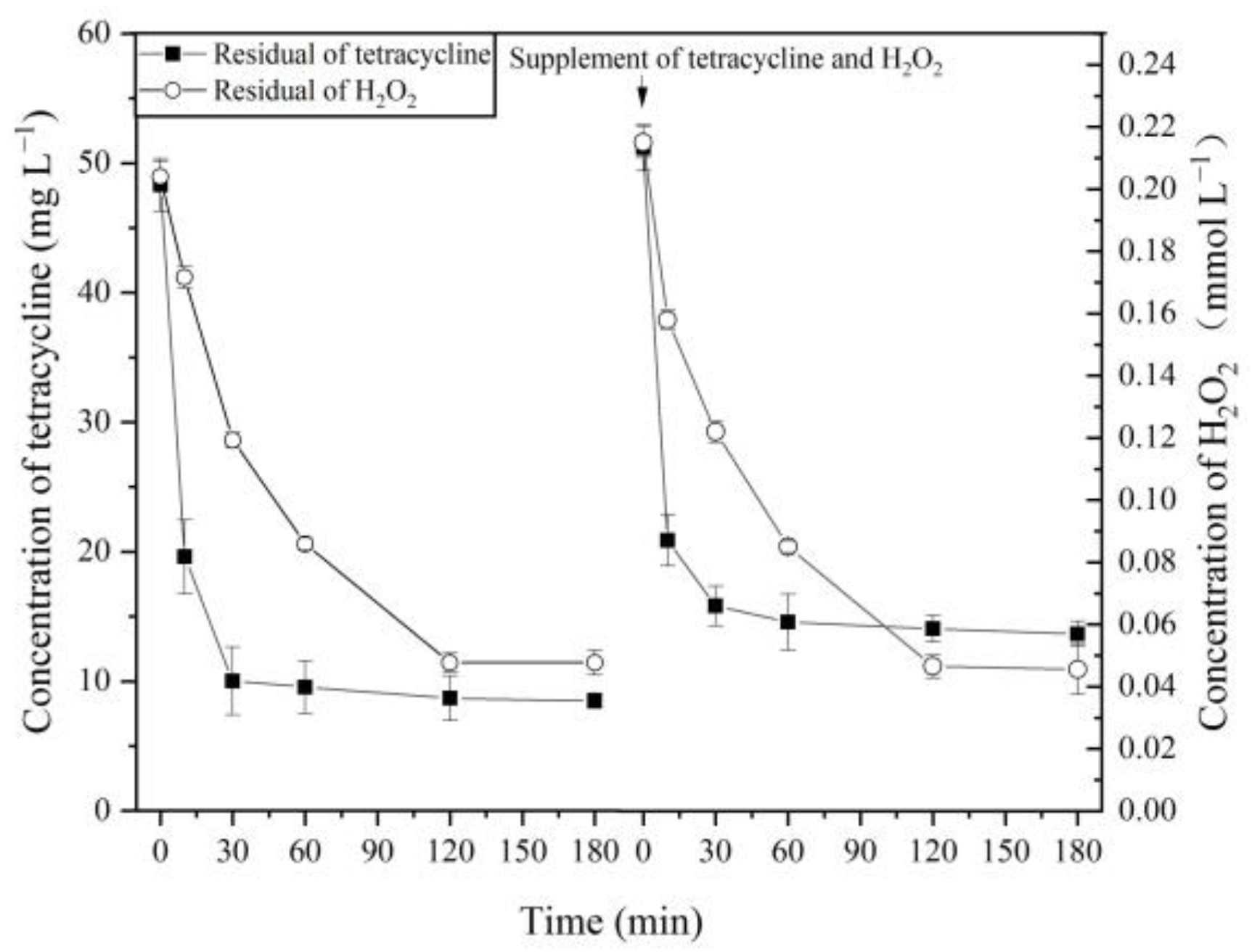
2.2. Degradation of TC by MnP
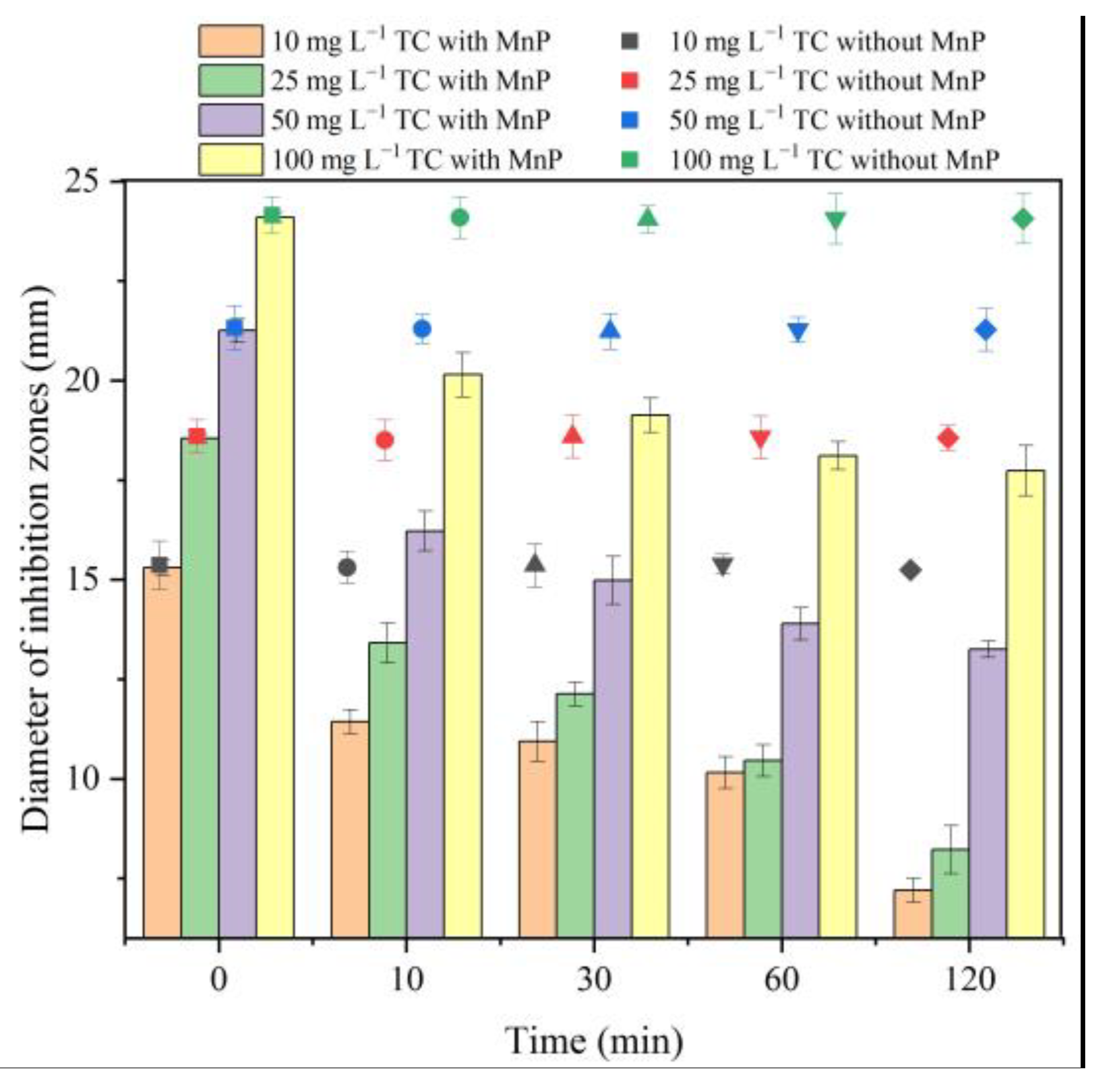
2.3. Antibacterial Potency of Tetracycline Degradation Products
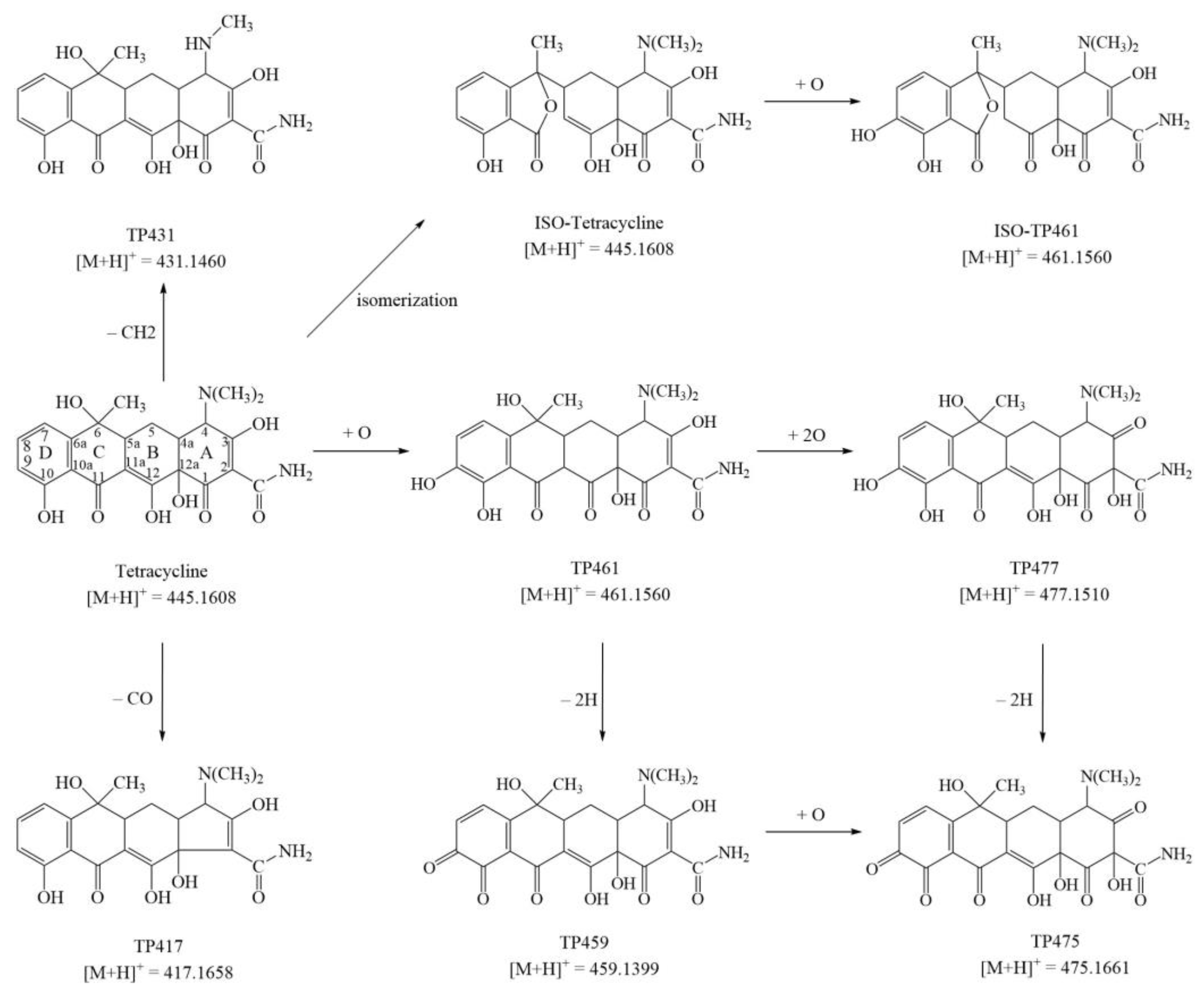
2.4. Transformation Products and a Possible Pathway
3. Materials and Methods
3.1. Chemicals and Microorganism
3.2. Activation and Enzyme Production of Fungal Strain
3.3. Optimization of Enzyme Production Medium for MnP Production
3.4. Purification of MnP
3.5. Enzyme Activity Analysis
3.6. Tetracycline Removal by MnP
3.7. Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leng, Y.; Bao, J.; Chang, G.; Zheng, H.; Li, X.; Du, J.; Snow, D.; Li, X. Biotransformation of tetracycline by a novel bacterial strain Stenotrophomonas maltophilia DT1. J. Hazard. Mater. 2016, 318, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, T.; Bi, J.; Wang, C. Spatiotemporal heterogeneity of antibiotic pollution and ecological risk assessment in Taihu Lake Basin, China. Sci. Total Environ. 2018, 643, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Bao, J.; Xiao, H.; Song, D.; Du, J.; Mohapatra, S.; Werner, D.; Wang, J. Transformation mechanisms of tetracycline by horseradish peroxidase with/without redox mediator ABTS for variable water chemistry. Chemosphere 2020, 258, 127306. [Google Scholar] [CrossRef]
- Leng, Y.; Xiao, H.; Li, Z.; Liu, Y.; Wang, J. Transformation of sulfadiazine in humic acid and polystyrene microplastics solution by horseradish peroxidase coupled with 1-hydroxybenzotriazole. Chemosphere 2020, 269, 128705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of china: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Tang, J.; Wang, S.; Fan, J.; Long, S.; Wang, L.; Tang, C.; Tam, N.F.; Yang, Y. Predicting distribution coefficients for antibiotics in a river water–sediment using quantitative models based on their spatiotemporal variations. Sci. Total Environ. 2019, 655, 1301–1310. [Google Scholar] [CrossRef]
- Peng, Q.; Song, J.; Li, X.; Yuan, H.; Li, N.; Duan, L.; Zhang, Q.; Liang, X. Biogeochemical characteristics and ecological risk assessment of pharmaceutically active compounds (PhACs) in the surface seawaters of Jiaozhou Bay, North China. Environ. Pollut. 2019, 255, 113247. [Google Scholar] [CrossRef]
- Lu, L.; Liu, J.; Li, Z.; Zou, X.; Guo, J.; Liu, Z.; Yang, J.; Zhou, Y. Antibiotic resistance gene abundances associated with heavy metals and antibiotics in the sediments of Changshou Lake in the three Gorges Reservoir area, China. Ecol. Indic. 2020, 113, 106275. [Google Scholar] [CrossRef]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef]
- Leng, Y.; Xiao, H.; Li, Z.; Wang, J. Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in coastal areas of Beibu Gulf, China. Sci. Total Environ. 2020, 714, 136899. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Li, S. Transformation and toxicity evaluation of tetracycline in humic acid solution by laccase coupled with 1-hydroxybenzotriazole. J. Hazard. Mater. 2017, 331, 182–188. [Google Scholar] [CrossRef]
- Duran, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B Environ. 2000, 28, 83–99. [Google Scholar] [CrossRef]
- Park, H.; Choung, Y.-K. Degradation of antibiotics (tetracycline, sulfathiazole, ampicillin) using enzymes of glutathion S-transferase. Hum. Ecol. Risk Assess. Int. J. 2007, 13, 1147–1155. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, J.-F.; Gu, W.-H.; Peng, S.-J.; Wang, L.-C.; Wang, J.-W.; Li, H.-X. Leaching of copper from waste printed circuit boards using Phanerochaete chrysosporium fungi. Hydrometallurgy 2020, 196, 105427. [Google Scholar] [CrossRef]
- Sosa-Martínez, J.; Balagurusamy, N.; Benavente-Valdés, J.R.; Montañez, J.; Morales-Oyervides, L.J.J.o.E.M. Process performance improvement for the simultaneous production of ligninolytic enzymes in solid culture using agricultural wastes through the Taguchi method. J. Environ. Manag. 2021, 293, 112966. [Google Scholar] [CrossRef]
- Kulkarni, A.N.; Kadam, S.K.; Jeon, B.-H.; Govindwar, S.P. Enhanced application of cross-linked enzyme aggregates of lichen Dermatocarpon vellereceum released extracellular enzymes for degradation of textile dyes. Int. Biodeter. Biodegr. 2020, 153, 105044. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium—A white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef]
- Suda, T.; Hata, T.; Kawai, S.; Okamura, H.; Nishida, T. Treatment of tetracycline antibiotics by laccase in the presence of 1-hydroxybenzotriazole. Bioresour. Technol. 2012, 103, 498–501. [Google Scholar] [CrossRef]
- Ji, C.; Nguyen, L.N.; Hou, J.; Hai, F.I.; Chen, V.J.S.; Technology, P. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 178, 215–223. [Google Scholar] [CrossRef]
- Potumarthi, R.; Baadhe, R.R.; Nayak, P.; Jetty, A.J.B.t. Simultaneous pretreatment and sacchariffication of rice husk by Phanerochete chrysosporium for improved production of reducing sugars. Bioresour. Technol. 2013, 128, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Sungusia, M.G.; Sawada, T.; Kuwahara, M. Lignin-degrading enzyme production by Bjerkandera adusta immobilized on polyurethane foam. J. Biosci. Bioeng. 1999, 88, 41–47. [Google Scholar] [CrossRef]
- Fu, S.Y.; Yu, H.S.; Buswell, J.A. Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Pleurotus sajor-caju. FEMS Microbiol. Lett. 1997, 147, 133–137. [Google Scholar] [CrossRef][Green Version]
- Tekere, M.; Zvauya, R.; Read, J.S. Ligninolytic enzyme production in selected sub-tropical white rot fungi under different culture conditions. J. Basic Microb. 2001, 41, 115–129. [Google Scholar] [CrossRef]
- Patrick, F.; Mtui, G.; Mshandete, A.M.; Kivaisi, A. Optimization of laccase and manganese peroxidase production in submerged culture of Pleurotus sajorcaju. Afr. J. Biotechnol. 2011, 10, 10166–10177. [Google Scholar]
- Boominathan, K.; Reddy, C.A. cAMP-mediated differential regulation of lignin peroxidase and manganese-dependent peroxidase production in the white-rot basidiomycete Phanerochaete chrysosporium. Proc. Natl. Acad. Sci. USA 1992, 89, 5586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Ma, Y.-L.; Yang, J.; Wang, L.-Q.; Lv, J.-M.; Ren, C.-J. Aqueous tetracycline degradation by H2O2 alone: Removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Rahul, D.; Aditi, K.; Divyashri, B.; Ali, M.; Amitava, M.; Ram, M.; Pavel, F. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar]
- Ding, D.; Liu, C.; Ji, Y.; Yang, Q.; Chen, L.; Jiang, C.; Cai, T. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem. Eng. J. 2017, 308, 330–339. [Google Scholar] [CrossRef]
- Guo, R.X.; Chen, J.Q. Phytoplankton toxicity of the antibiotic chlortetracycline and its UV light degradation products. Chemosphere 2012, 87, 1254–1259. [Google Scholar] [CrossRef]
- Liu, H.; Qu, J.; Zhang, T.; Ren, M.; Zhang, Z.; Cheng, F.; He, D.; Zhang, Y.-N. Insights into degradation pathways and toxicity changes during electro-catalytic degradation of tetracycline hydrochloride. Environ. Pollut. 2020, 258, 113702. [Google Scholar] [CrossRef]
- He, T.; Bao, J.; Leng, Y.; Snow, D.; Kong, S.; Wang, T.; Li, X. Biotransformation of Doxycycline by Brevundimonas naejangsanensis and Sphingobacterium mizutaii strains. J. Hazard. Mater. 2021, 411, 125126. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Z.; Yu, Q.; Cheng, Y.; Liu, Z.; Zhang, T.; Zhou, S. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef]
- Wan, Y.; Jia, A.; Zhu, Z.; Hu, J. Transformation of tetracycline during chloramination: Kinetics, products and pathways. Chemosphere 2013, 90, 1427–1434. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Leng, Y.; Bao, J.; Song, D.; Li, J.; Ye, M.; Li, X. Background Nutrients Affect the Biotransformation of Tetracycline by Stenotrophomonas maltophilia as revealed by Genomics and Proteomics. Environ. Sci. Technol. 2017, 51, 10476–10484. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-R.; Huang, C.-H. Transformation kinetics and pathways of tetracycline antibiotics with manganese oxide. Environ. Pollut. 2011, 159, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Mahamallik, P.; Saha, S.; Majumdar, A. Degradation of tetracycline antibiotics by advanced oxidation processes: Application of MnO2 nanomaterials. Nat. Resour. Eng. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol 2018, 9, 1058. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and stable photocatalytic degradation of tetracycline wastewater by 3D Polyaniline/Perylene diimide organic heterojunction under visible light irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Kaczorek, E.; Jesionowski, T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catal. Today 2020, 348, 127–136. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Jakimska-Nagórska, A.; Wasik, A.; Kot-Wasik, A. Phototransformation of three selected pharmaceuticals, naproxen, 17α-Ethinylestradiol and tetracycline in water: Identification of photoproducts and transformation pathways. Microchem. J. 2019, 148, 673–683. [Google Scholar] [CrossRef]
- Bermek, H.; Yazıcı, H.; Öztürk, H.; Tamerler, C.; Jung, H.; Li, K.; Brown, K.M.; Ding, H.; Xu, F. Purification and characterization of manganese peroxidase from wood-degrading fungus Trichophyton rubrum LSK-27. Enzym. Microb. Technol. 2004, 35, 87–92. [Google Scholar] [CrossRef]
- Cheng, X.; Jia, R.; Li, P.; Tu, S.; Zhu, Q.; Tang, W.; Li, X. Purification of a new manganese peroxidase of the white-rot fungus Schizophyllum sp. F17, and decolorization of azo dyes by the enzyme. Enzym. Microb. Technol. 2007, 41, 258–264. [Google Scholar] [CrossRef]
- Paszczyński, A.; Crawford, R.L.; Huynh, V.-B. Manganese peroxidase of Phanerochaete chrysosporium: Purification. Methods Enzymol. 1988, 161, 264–270. [Google Scholar]
- Llorca, M.; Rodriguez-Mozaz, S.; Couillerot, O.; Panigoni, K.; de Gunzburg, J.; Bayer, S.; Czaja, R.; Barcelo, D. Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere 2015, 119, 90–98. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin peroxidase of Phanerochaete chrysosporium. Meth. Enzymol. 1988, 161, 238–249. [Google Scholar]


| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Glucose | 1 | 24,200 | 24,200 | 2.78 | 0.171 |
| Ammonium tartrate | 1 | 163,020 | 163,020 | 18.74 | 0.012 |
| MnSO4 | 1 | 12,800 | 12,800 | 1.47 | 0.292 |
| Error | 4 | 34,791 | 8698 | ||
| Total | 1 | 24,200 | 24,200 | 2.78 | 0.171 |
| R2 = 85.18% |
| Retention Time (min) | Compound | Ion | Predicted Mass (m/z) | Measured Mass (m/z) | Elemental Composition | Ring Double Bond Equivalent Value (RDB) | Intensity | |
|---|---|---|---|---|---|---|---|---|
| Parents compound | 4.19 | ISO or ETC | [M+H]+ | 445.1605 | 445.1602 | C22H25O8N2 | 11.5 | 2.69 × 108 |
| 4.72 | TC * | [M+H]+ | 445.1605 | 445.1602 | C22H25O8N2 | 11.5 | 3.33 × 108 | |
| Transformation products | 4.03 | TP417 | [M+H−CO]+ | 417.1656 | 417.1661 | C21H25O7N2 | 10.5 | 2.52 × 105 |
| 5.26 | TP431 | [M+H−CH2]+ | 431.1449 | 431.1456 | C21H23O8N2 | 11.5 | 1.57 × 107 | |
| 3.62 | ISO-TP461 | [M+H+O]+ | 461.1555 | 461.1585 | C22H25O9N2 | 11.5 | 1.09 × 106 | |
| 3.80 | TP461 | [M+H+O]+ | 461.1555 | 461.1566 | C22H25O9N2 | 11.5 | 1.88 × 106 | |
| 5.44 | TP459 | [M+H+O−2H]+ | 459.1398 | 459.1402 | C22H23O9N2 | 12.5 | 2.06 × 108 | |
| 3.18 | TP477 | [M+H+O+O]+ | 477.1504 | 477.1508 | C22H25O10N2 | 11.5 | 4.13 × 107 | |
| 4.40 | TP475 | [M+H+O+O−2H]+ | 475.1347 | 475.1353 | C22H23O10N2 | 12.5 | 3.40 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Leng, Y.; Wan, D.; Chang, F.; Huang, Y.; Li, Z.; Xiong, W.; Wang, J. Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium. Molecules 2021, 26, 6803. https://doi.org/10.3390/molecules26226803
Sun X, Leng Y, Wan D, Chang F, Huang Y, Li Z, Xiong W, Wang J. Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium. Molecules. 2021; 26(22):6803. https://doi.org/10.3390/molecules26226803
Chicago/Turabian StyleSun, Xuemei, Yifei Leng, Duanji Wan, Fengyi Chang, Yu Huang, Zhu Li, Wen Xiong, and Jun Wang. 2021. "Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium" Molecules 26, no. 22: 6803. https://doi.org/10.3390/molecules26226803
APA StyleSun, X., Leng, Y., Wan, D., Chang, F., Huang, Y., Li, Z., Xiong, W., & Wang, J. (2021). Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete chrysosporium. Molecules, 26(22), 6803. https://doi.org/10.3390/molecules26226803







