Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem
Abstract
:1. Introduction
2. Mechanisms of Resistance
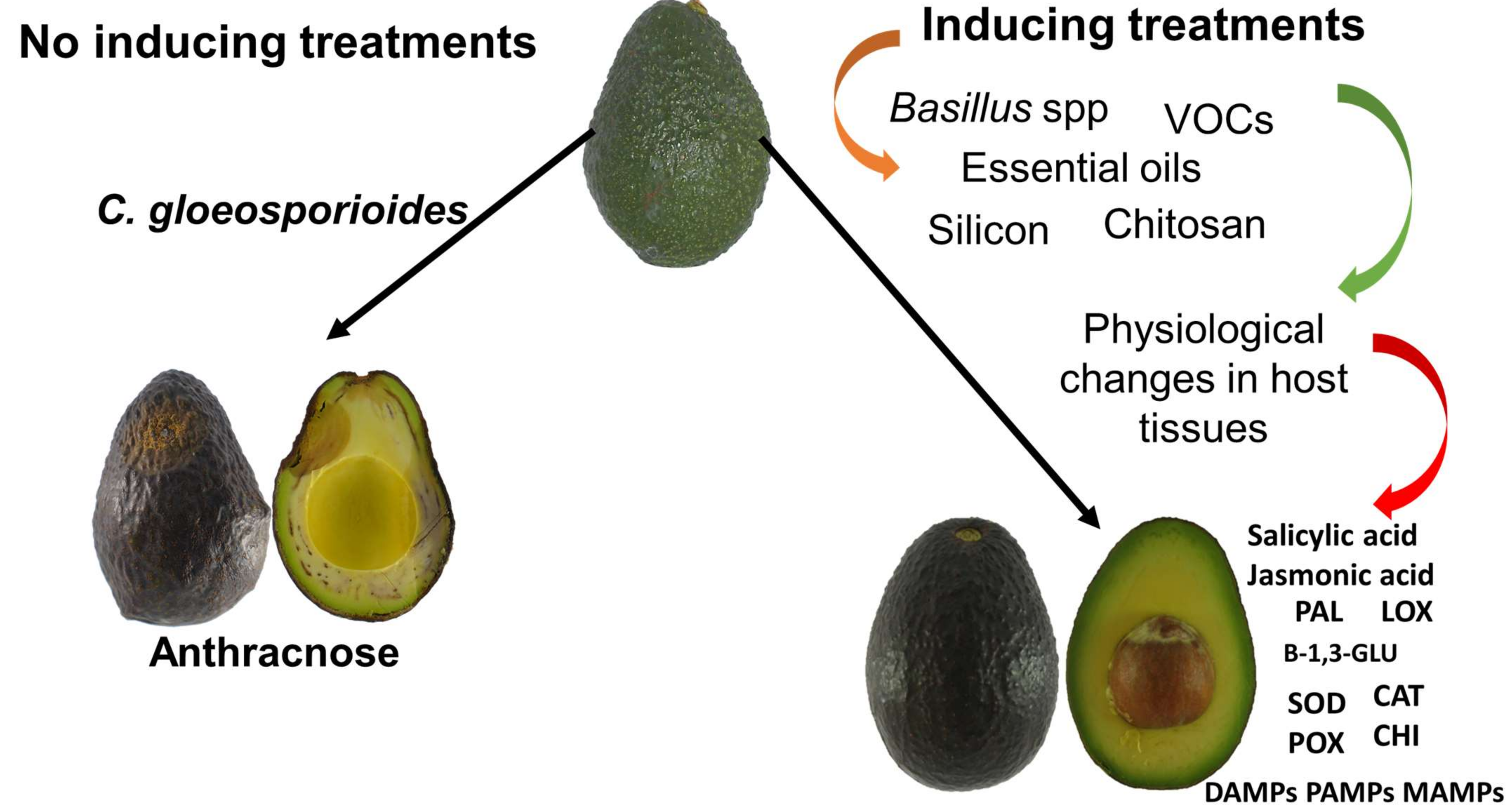
3. Alternative Control Methods That Involve the Activation of Defense Mechanisms in Avocado
3.1. Essential Oils
3.2. Bacillus spp.
3.3. Volatile Organic Compounds
3.4. Chitosan (Coating, Elicitor, and Biofungicide)
3.4.1. Deposition of Chitosan
3.4.2. Chitosan as an Elicitor
3.4.3. Chitosan–Nucleus Interaction
| In Vitro | Fruit Defense Mechanism | Reference No. | |||
|---|---|---|---|---|---|
| GRAS Compound | Mycelial Growth Inhibition (%) | Antifungal Response | Defense Enzymes | Antioxidant Enzymes | |
| Essential oils | 60–100 | Production of phenols | CHI, 1,3-β-GLU, PAL, and POX | SOD and CAT | [36] |
| Essential oils | Production of monoterpene phenol derivative | Upregulation of PAL gene expression | Enhanced biosynthesis of epicatechin | [35] | |
| Bacillus sp. | 30–55 | Production of volatile compounds | [47] | ||
| Volatile compounds | 100 | PAL, CHI, and β-1,3 GLU | Total phenolic contents | [52] | |
| Chitosan | Upregulation of PAL and downregulation of LOX genes. Upregulation of CHI genes | Higher epicatechin contents and higher SOD activity | [67] | ||
| Chitosan | Induced unigenes related to systemic acquired resistance | Induction of genes involved in response to both biotic and abiotic stress | [76] | ||
| Silicon | CHI, 1,3-β-GLU, PAL, and POX | SOD and CAT | [63] | ||
| GRAS Compound | Postharvest Quality | Disease | Microorganism Involved | Reference No. |
|---|---|---|---|---|
| Essential oils | Anthracnose | C. gloeosporioides | [35,36] | |
| Bacillus sp. | Fusarium dieback, anthracnosis, and Phytophthora root rot | Fusarium solani, Fusarium sp., C. gloeosporioides | [47] | |
| Volatile compounds | Stem-end rot | Lasiodiplodia theobromae | [52] | |
| Chitosan | Stem-end rot and anthracnose | Lasiodiplodia theobromae, C. gloeosporioides | [67] | |
| Chitosan | Decreased respiratory rate, ethylene production, and fresh mass loss. Increased pulp firmness | Anthracnose | C. gloeosporioides | [65,76] |
| Chitosan | Reduced severity and incidence of anthracnose, maintained fruit quality | Anthracnose | C. gloeosporioides | [67] |
| Silicon | Decreased respiratory rate, ethylene production, and fresh mass loss | Anthracnose | C. gloeosporioides | [63] |
3.5. Silicon
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly (lactic acid) films containing thymol and r-(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; López, J.H.; Henao-Rojas, J.C. Causes of Hass avocado fruit rejection in preharvest, harvest, and packinghouse: Economic losses and associated variables. Agronomy 2020, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Herrera-González, J.A.; Bautista-Baños, S.; Salazar-García, S.; Gutiérrez-Martínez, P. Current situation of postharvest handling and fungal diseases of avocado ‘Hass’ for export in Michoacán, Mexico. Rev. Mex. Cienc. Agrícolas 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Thompson, A.K.; Korsten, L. Avocado fruit quality management during the postharvest supply chain. Food Rev. Int. 2014, 30, 169–202. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Baños, S.; Ventura-Aguilar, R.I.; Ramos-García, M.D.L. Avocado. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 227–256. ISBN 9781315209180. [Google Scholar]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Chávez, R.A.; Martínez-Peniche, R.Á.; Hernández-Iturriaga, M.; Teixidó-Espasa, N.; Usall-Rodié, J.; Viñas-Almenar, I.; Torres-Sanchis, R. Mechanisms of resistance in postharvest fruit-pathogen interaction. Revista Chapingo Serie Horticultura 2015, 21, 185–198. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA Poly-D-Glucosamine (Chitosan) 8 128930 (EPA/OPP Chemical Code). USDA National Organic Program 2004. Available online: https://www.ams.usda.gov/sites/default/files/media/Chitosan%20TR.pdf (accessed on 5 November 2021).
- Taghavi, M.; Khosravi, A.; Mortaz, E.; Nikaein, D.; Athari, S.S. Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. Eur. J. Pharmacol. 2017, 808, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tafolla-Arellano, J.C.; González-León, A.; Tiznado-Hernández, M.E.; Garcia, L.Z.; Báez-Sañudo, R. Composition, physiology and biosynthesis of the plant cuticle. Rev. Fitotec. Mex. 2013, 36, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Vázquez, C.; Ruiz-May, E.; Guerrero-Analco, J.A.; Elizalde-Contreras, J.M.; Enciso-Ortiz, E.J.; Rosas-Saito, G.; López-Sánchez, L.; Kiel-Martínez, A.L.; Bonilla-Landa, I.; Monribot-Villanueva, J.L.; et al. Filling gaps in our knowledge on the cuticle of mangoes (Mangifera Indica) by analyzing six fruit cultivars: Architecture/structure, postharvest physiology and possible resistance to fruit fly (Tephritidae) attack. Postharvest Biol. Technol. 2019, 148, 83–96. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- el Hadrami, A.; Adam, L.R.; el Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ojito-Ramos, K.; Orelvis, P. Introducción al sistema inmune en plantas. Biotecnol. Veg. 2010, 10, 3–19. [Google Scholar]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial biocontrol as an alternative to synthetic fungicides: Boundaries between pre- and postharvest applications on vegetables and fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Serrano, M.; L’Haridon, F.; Tjamos, S.E.; Metraux, J.-P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive oxygen species: The good and the bad. In Reactive Oxygen Species (ROS) in Living Cells; InTech: London, UK, 2018; pp. 8–20. ISBN 9789537619992. [Google Scholar]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, C.C.; Martins, A.D.; Pereira, R.V.; Willett, D.S. The ecology of salicylic acid signaling: Primary, secondary and tertiary effects with applications in agriculture. Int. J. Mol. Sci. 2019, 20, 5851. [Google Scholar] [CrossRef] [Green Version]
- Dzhavakhiya, V.G.; Shcherbakova, L.A. Creation of disease-resistant plants by gene engineering. In Comprehensive and Molecular Phytopathology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 439–466. [Google Scholar]
- Valentines, M.C.; Vilaplana, R.; Torres, R.; Usall, J.; Larrigaudière, C. Specific roles of enzymatic browning and lignification in apple disease resistance. Postharvest Biol. Technol. 2005, 36, 227–234. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Regenass, M.; Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993, 4, 307–316. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-M.; Shih, S.-L.; Lin, W.-C.; Wu, J.-W.; Chen, Y.-T.; Hsieh, C.-Y.; Guan, L.-C.; Lin, L.; Cheng, C.-P. Phytoalexin biosynthesis genes are regulated and involved in plant response to Ralstonia solanacearum infection. Plant Sci. 2014, 224, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Hashmi, M.S.; Qazi, I.M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J.K. Pre-storage chitosan-thyme oil coating control anthracnose in mango fruit. Sci. Hortic. 2021, 284, 110139. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Kumar, A.; Kudachikar, V.B. Antifungal properties of essential oils against anthracnose disease: A critical appraisal. J. Plant Dis. Prot. 2017, 125, 133–144. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Bill, M.; Korsten, L.; Remize, F.; Glowacz, M.; Sivakumar, D. Effect of thyme oil vapours exposure on phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX) genes expression, and control of anthracnose in ‘Hass’ and ‘Ryan’ avocado fruit. Sci. Hortic. 2017, 224, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P. Antifungal activity and chemical composition of thyme, peppermint and citronella oils in vapor phase against avocado and peach postharvest pathogens. J. Food Saf. 2013, 33, 86–93. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv. Hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A. In vitro evaluation of eight plant essential oils for controlling Colletotrichum, Botryosphaeria, Fusarium and Phytophthora fruit rots of avocado, mango and papaya. Plant Prot. Sci. 2018, 54, 153–162. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Magdaleno, M.; Luque-Alcaraz, A.; Gutiérrez-Martínez, P.; Cortez-Rocha, M.; Burgos-Hernández, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of chitosan-pepper tree (Schinus molle) essential oil biocomposites on the growth kinetics, viability and membrane integrity of Colletotrichum gloeosporioides. Rev. Mex. Ing. Química 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velázquez-Del Valle, M.G.; Hernández-Rodríguez, A. Uso de microorganismos antagonistas en el control de enfermedades postcosecha en frutos. Rev. Mex. Fitopatol. 2007, 25, 66–74. [Google Scholar]
- Granada, D.; López-Lujan, L.; Ramírez-Restrepo, S.; Morales, J.; Peláez-Jaramillo, C.; Andrade, G.; Bedoya-Pérez, J.C. Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integr. Agric. 2020, 19, 748–758. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Valdivia, L.; Tovar-Pérez, E.; Chacón-López, A.; López-García, U.; Gutiérrez-Martínez, P.; Stoll, A.; Aguilera, S. Identification and characterization of a new Bacillus Atrophaeus strain b5 as biocontrol agent of postharvest anthracnose disease in soursop (Annona muricata) and Avocado (Persea Americana). Microbiol. Res. 2018, 210, 26–32. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bravo-Castillo, K.R.; Monribot-Villanueva, J.L.; Kiel-Martínez, A.L.; Ramírez-Vázquez, M.; Guerrero-Analco, J.A.; Reverchon, F. Diffusible and volatile organic compounds produced by avocado rhizobacteria exhibit antifungal effects against Fusarium kuroshium. Braz. J. Microbiol. 2020, 51, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Demoz, B.T.; Korsten, L. Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stem-end rot pathogens. Biol. Control 2006, 37, 68–74. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.-L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef]
- Guerrero-Barajas, C.; Constantino-Salinas, E.A.; Amora-Lazcano, E.; Tlalapango-Ángeles, D.; Mendoza-Figueroa, J.S.; Cruz-Maya, J.A.; Jan-Roblero, J. Bacillus mycoides A1 and Bacillus tequilensis A3 inhibit the growth of a member of the phytopathogen Colletotrichum gloeosporioides species complex in avocado. J. Sci. Food Agric. 2020, 100, jsfa.10450. [Google Scholar] [CrossRef]
- Anand, S.S.; Philip, B.K.; Mehendale, H.M. Volatile organic compounds. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 967–970. ISBN 9780123864543. [Google Scholar]
- Xiaokang, W.; Brunton, N.P.; Lyng, J.G.; Harrison, S.M.; Carpes, S.T.; Papoutsis, K. Volatile and non-volatile compounds of shiitake mushrooms treated with pulsed light after twenty-four hour storage at different conditions. Food Biosci. 2020, 36, 100619. [Google Scholar] [CrossRef]
- Bill, M.; Obianom, P.C.; Korsten, L.; Mavuso, Z.; van Rooyen, Z.; Sivakumar, D. Using plant volatiles in biodegradable sachets and exposure of volatiles under pallet covers to control postharvest decay in avocados. S. Afr. Avocado Grow. Assoc. Yearb. 2016, 39, 44–49. [Google Scholar]
- Obianom, C.; Sivakumar, D. Natural plant volatiles as an alternative approach to control stem-end rot in avocado cultivars. J. Phytopathol. 2018, 166, 1–9. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Erin Lim, S.H.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Pedroso, A.T.; Ramírez-Arrebato, M.A.; Rivero-González, D.; Bosquez-Molina, E.; Barrera-Necha, L.L.; Bautista-Baños, S. Propiedades químico-estructurales y actividad biológica de la quitosana en microorganismos fitopatógenos. Rev. Chapingo Ser. Hortic. 2009, 15, 307–317. [Google Scholar]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Baños, S.; Necha, L.L.B.; Hernández-López, M.; Rodríguez-González, F. Morphological and ultrastructural modifications of chitosan-treated fungal phytopathogens. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Elsevier: Morelos, México, 2016; pp. 251–275. ISBN 9780128027356. [Google Scholar]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical characteristics and functional properties of chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–31. ISBN 9780128027356. [Google Scholar]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- de Oliveira-Pedro, R.; Takaki, M.; Castilho-Gorayeb, T.C.; del Bianchi, V.L.; Thomeo, J.C.; Tiera, M.J.; de Oliveira-Tiera, V.A. Synthesis, Characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus. Microbiol. Res. 2013, 168, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, USA, 2009; pp. 57–112. ISBN 978-0-387-92823-4. [Google Scholar]
- Rodríguez-López, E.; González-Prieto, J.; Mayek-Pérez, N. La infección de Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. en aguacatero (Persea americana Mill.): Aspectos bioquímicos y genéticos. Rev. Mex. Fitopatol. 2009, 27, 53–63. [Google Scholar]
- Marques, K.M.; Galati, V.C.; Fernandes, J.D.R.; Guimarães, J.E.R.; Silva, J.P.; Mattiuz, B.H.; Mattiuz, C.F.M. Use of chitosan for the control of postharvest anthracnose and quality in avocados. Acta Hortic. 2016, 1120, 225–231. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A Review of the structure-activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Kaleda-Marino, A.; Pinsetta-Junior, J.S.; Marques-Magalhães, K.; Mattiuz, B.-H. Chitosan-propolis combination inhibits anthracnose in “Hass” avocados. Emir. J. Food Agric. 2018, 30, 681. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Sashiwa, H. Chimical Aspects of Chitin and Chitosan Derivatives; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9780429099434. [Google Scholar]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Sedlaříková, J.; Janalíková, M.; Rudolf, O.; Pavlačková, J.; Egner, P.; Peer, P.; Varaďová, V.; Krejčí, J. Chitosan/thyme oil systems as affected by stabilizing agent: Physical and antimicrobial properties. Coatings 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- González-Estrada, R.; Blancas-Benítez, F.; M. Velázquez-Estrada, R.; Montaño-Leyva, B.; Ramos-Guerrero, A.; Aguirre-Güitrón, L.; Moreno-Hernández, C.; Coronado-Partida, L.; Herrera-González, J.A.; Rodríguez-Guzmán, C.A.; et al. Alternative eco-friendly methods in the control of post-harvest decay of tropical and subtropical fruits. In Modern Fruit Industry; IntechOpen: London, UK, 2020; pp. 1–22. [Google Scholar]
- Bautista-Baños, S.; Hernández-López, M. El Control biológico en la reducción de enfermedades postcosecha en productos hortofrutícolas: Uso de microorganismos antagónicos. Biótica 2006, 3, 3–9. [Google Scholar]
- Bi, Y.; Li, Y.; Ge, Y. Induced resistance in postharvest fruits and vegetables by chemicals and its mechanism. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.-Á.; Cuellar-Torres, E.A.; González-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguín, J.; Chacón-López, A. Transcriptomic analysis of avocado Hass (Persea americana Mill) in the interaction system fruit-chitosan-Colletotrichum. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Martinez, P.; Ledezma-Morales, A.; Romero-Islas, L.d.C.; Ramos-Guerrero, A.; Romero-Islas, J.; Rodríguez-Pereida, C.; Casas-Junco, P.; Coronado-Partida, L.; González-Estrada, R. Antifungal activity of chitosan against postharvest fungi of tropical and subtropical fruits. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; InTech: London, UK, 2018; pp. 311–327. [Google Scholar]
- Guo, H.; Qiao, B.; Ji, X.; Wang, X.; Zhu, E. Antifungal activity and possible mechanisms of submicron chitosan dispersions against Alteraria alternata. Postharvest Biol. Technol. 2020, 161, 110883. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.; Ramos-Guerrero, A.; Rodríguez-Pereida, C.; Coronado-Partida, L.; Angulo-Parra, J.; González-Estrada, R. Chitosan for postharvest disinfection of fruits and vegetables. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–241. ISBN 9780128126981. [Google Scholar]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Effects of postharvest potassium silicate application on phenolics and other anti-oxidant systems aligned to avocado fruit quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Obianom, C.; Romanazzi, G.; Sivakumar, D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 2019, 147, 214–221. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant-pathogen interactions. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bosse, R.J.; Bower, J.P.; Bertling, I. Pre- and post-harvest treatments on ‘Fuerte’ avocados to control anthracnose (Colletotrichum gloeosporioides) during ripening. S. Afr. Avocado Grow. Assoc. Yearb. 2011, 34, 65–69. [Google Scholar]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kaluwa, K.; Bertling, I.; Bower, J.P.; Tesfay, S.Z. Silicon application effects on ‘Hass’ avocado fruit physiology. S. Afr. Avocado Grow. Assoc. Yearb. 2010, 33, 44–47. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Massart, S.; Perazzolli, M.; Höfte, M.; Pertot, I.; Jijakli, M.H. Impact of the omic technologies for understanding the modes of action of biological control agents against plant pathogens. BioControl 2015, 60, 725–746. [Google Scholar] [CrossRef]
- Rendón-Anaya, M.; Ibarra-Laclette, E.; Méndez Bravo, A.; Lan, T.; Zheng, C.; Carretero-Paulet, L.; Perez-Torres, C.A.; Chacón-López, A.; Hernandez-Guzmán, G.; Chang, T.-H.; et al. The avocado genome informs deep angiosperm phylogeny, highlights introgressive hybridization, and reveals pathogen-influenced gene space adaptation. bioRxiv 2019, 654285. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules 2021, 26, 6819. https://doi.org/10.3390/molecules26226819
Herrera-González JA, Bautista-Baños S, Serrano M, Romanazzi G, Gutiérrez-Martínez P. Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules. 2021; 26(22):6819. https://doi.org/10.3390/molecules26226819
Chicago/Turabian StyleHerrera-González, Juan Antonio, Silvia Bautista-Baños, Mario Serrano, Gianfranco Romanazzi, and Porfirio Gutiérrez-Martínez. 2021. "Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem" Molecules 26, no. 22: 6819. https://doi.org/10.3390/molecules26226819
APA StyleHerrera-González, J. A., Bautista-Baños, S., Serrano, M., Romanazzi, G., & Gutiérrez-Martínez, P. (2021). Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules, 26(22), 6819. https://doi.org/10.3390/molecules26226819










