Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of α-Pinene Concentration in HP-β-CD/α-Pinene Inclusion Complex Solutions
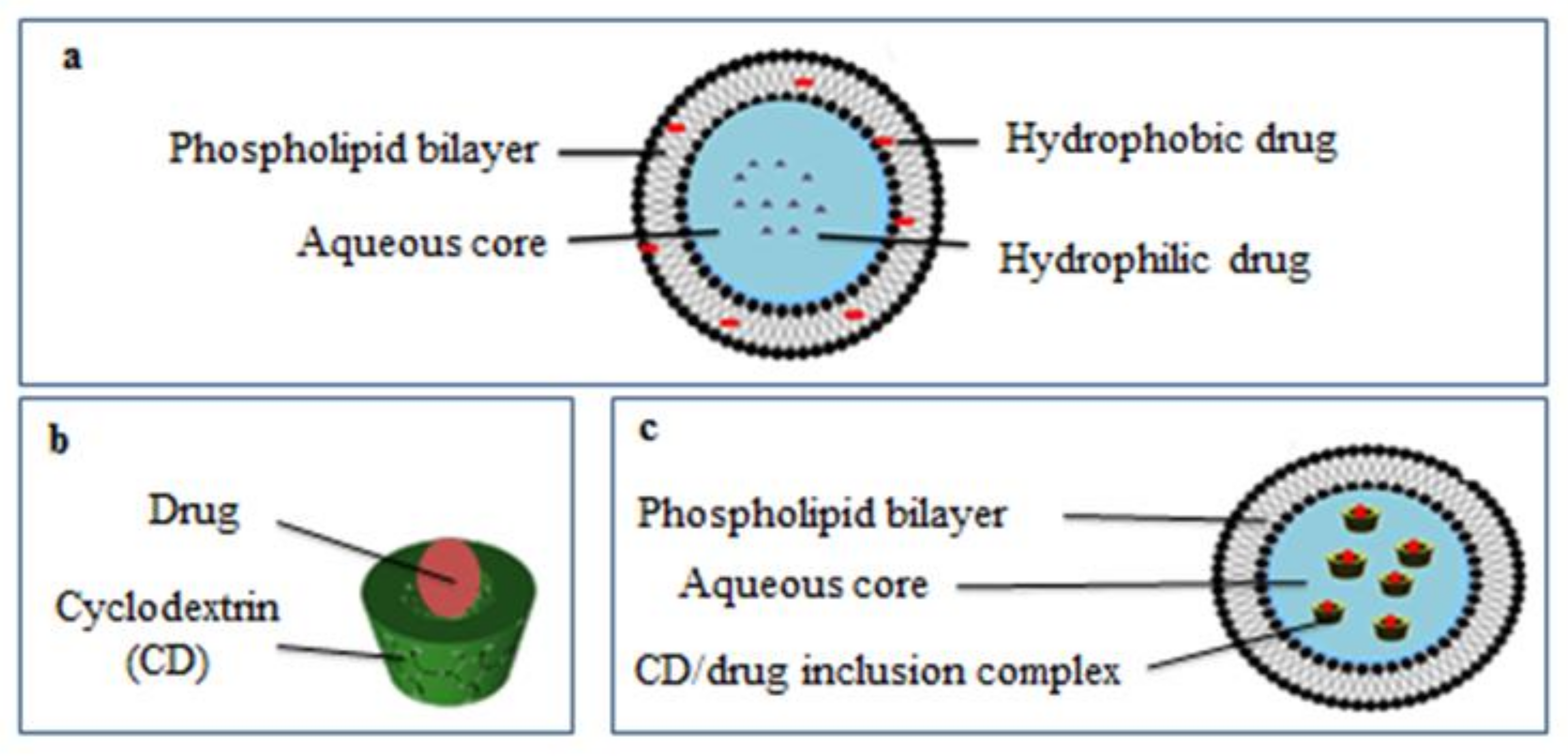
2.2. Characterization of Liposome Formulations
2.2.1. Determination of Liposome Particle Size
2.2.2. Determination of Phospholipid: Chol:α-Pinene Molar Ratio
Phospholipid Incorporation Rate
Cholesterol Incorporation Rate
α-Pinene Encapsulation Efficiency and Loading Rate
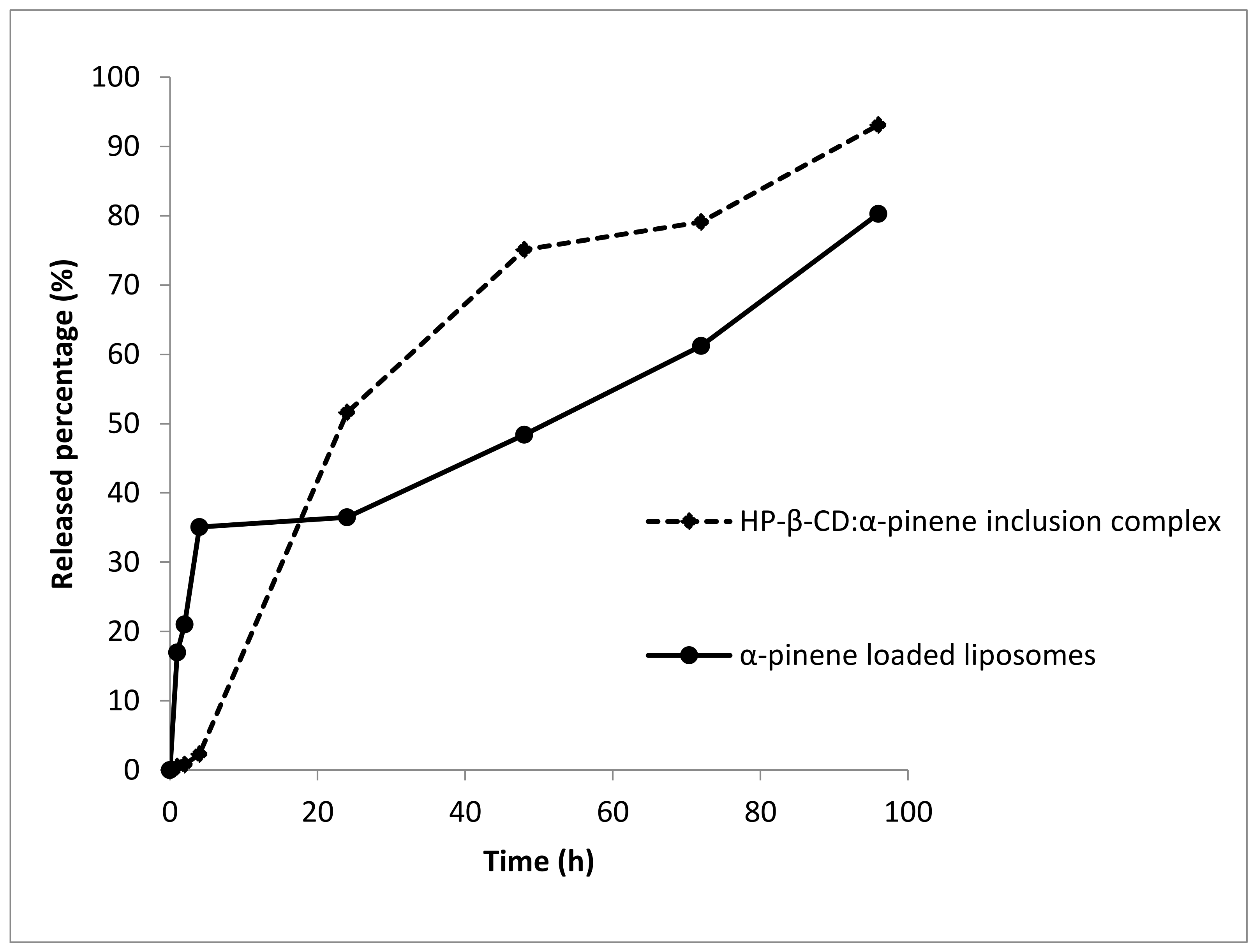
2.2.3. In Vitro Release Study
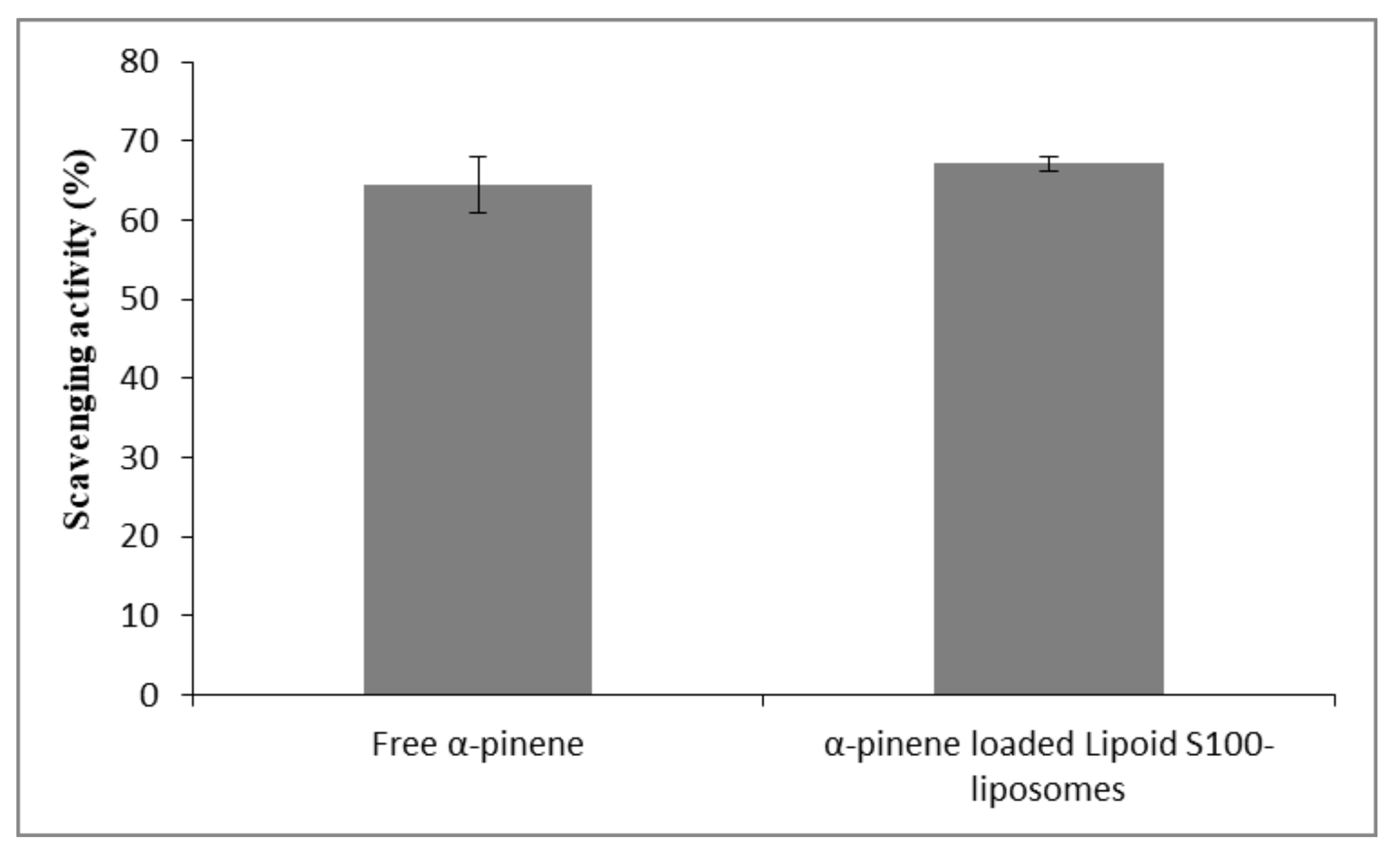
2.2.4. Determination of DPPH Radical Scavenging Activity
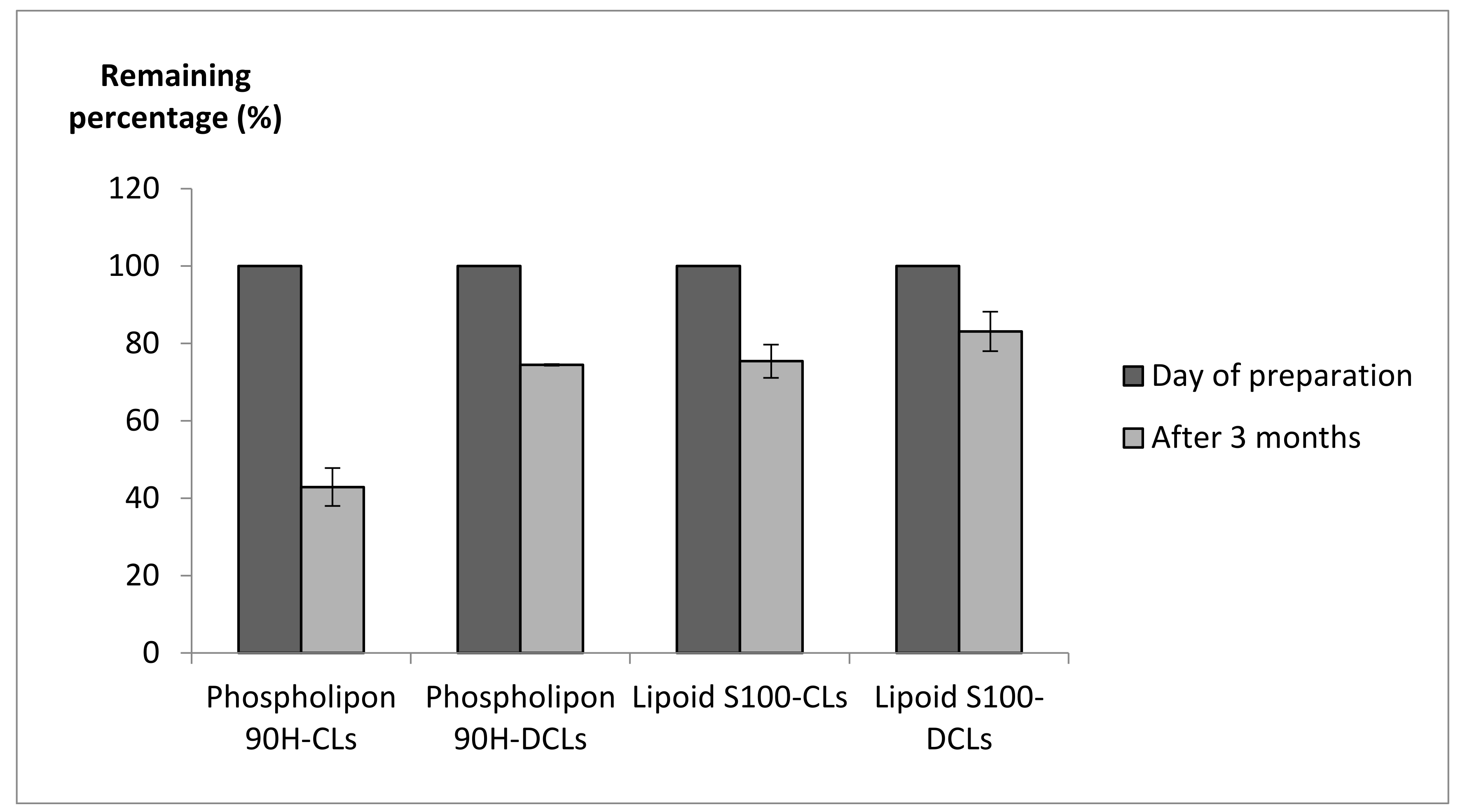
2.2.5. Storage Stability
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis of α-Pinene
3.3. Determination of the Optimal HP-β-CD Concentration for α-Pinene Solubilization
3.4. Preparation of HP-β-CD/α-Pinene Inclusion Complex for DCL Preparations
3.5. Preparation of Liposomes
3.6. Characterization of Liposomes
3.6.1. Particle Size Analysis
3.6.2. Determination of Phospholipid:Chol:α-Pinene Molar Ratio in the Formulations
Determination of Phospholipid Incorporation Rate
Determination of Cholesterol Incorporation Rate
Quantification of α-Pinene in the Formulations
3.6.3. In Vitro Release Study
3.6.4. DPPH Radical Scavenging Activity Measurements
3.6.5. Storage Stability
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Zielińska, A.; Ferreira, N.R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Mamouni, S.E.; Silva, A.M.; Nowak, I.; Santini, A.; Souto, E.B. Development and Optimization of Alpha-Pinene-Loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and Dispersion Analysis. Molecules 2019, 24, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula Angustifolia and Lavandula Latifolia Essential Oils from Spain: Aromatic Profile and Bioactivities. Planta Med. 2015, 82, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus Officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Maravelias, C.T. Synthesis and Techno-Economic Assessment of Microbial-Based Processes for Terpenes Production. Biotechnol. Biofuels 2018, 11, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche J. Entomol. 2016, 2016, 459582. [Google Scholar] [CrossRef] [Green Version]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential Protective Effects of Alpha-Pinene against Cytotoxicity Caused by Aspirin in the IEC-6 Cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Yang, L.; Qiu, J. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oil from Wedelia Prostrata. EXCLI J. 2013, 12, 479–490. [Google Scholar] [PubMed]
- Sieniawska, E.; Los, R.; Baj, T.; Malm, A.; Glowniak, K. Antimicrobial Efficacy of Mutellina Purpurea Essential Oil and α-Pinene against Staphylococcus Epidermidis Grown in Planktonic and Biofilm Cultures. Ind. Crop. Prod. 2013, 51, 152–157. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity through the Suppression of MAPKs and the NF-ΚB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-Tumor Effect of α-Pinene on Human Hepatoma Cell Lines through Inducing G2/M Cell Cycle Arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, D.C.; Gu, X.-J.; Xiao, P.G. Phytochemical and Biological Research of Cannabis Pharmaceutical Resources. In Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2015; pp. 431–464. ISBN 978-0-08-100085-4. [Google Scholar]
- Neeman, E.M.; Avilés Moreno, J.R.; Huet, T.R. The Gas Phase Structure of α-Pinene, a Main Biogenic Volatile Organic Compound. J. Chem. Phys. 2017, 147, 214305. [Google Scholar] [CrossRef]
- Pavlovic, J.; Hopke, P.K. Technical Note: Detection and Identification of Radical Species Formed from α-Pinene/Ozone Reaction Using DMPO Spin Trap. Atmos. Chem. Phys. Discuss. 2009, 9, 23695–23717. [Google Scholar] [CrossRef] [Green Version]
- Pinho, P.G.; Pio, C.A.; Carter, W.P.L.; Jenkin, M.E. Evaluation of α- and β-Pinene Degradation in the Detailed Tropospheric Chemistry Mechanism, MCM v3.1, Using Environmental Chamber Data. J. Atmos. Chem. 2007, 57, 171–202. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of Nanotechnology for the Encapsulation of Botanical Insecticides for Sustainable Agriculture: Prospects and Promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Wang, D.; Chi, D.F. Morphology and Release Profile of Microcapsules Encapsulated Alpha-Pinene by Complex Coacervation. Adv. Mater. Res. 2012, 602–604, 1285–1288. [Google Scholar] [CrossRef]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation Efficiency of Cyclodextrins for Volatile Flavor Compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Investigation of Monoterpenes Complexation with Hydroxypropyl-β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 51–60. [Google Scholar] [CrossRef]
- Kfoury, M.; Pipkin, J.D.; Antle, V.; Fourmentin, S. Captisol®: An Efficient Carrier and Solubilizing Agent for Essential Oils and Their Components. Flavour Fragr. J. 2017, 32, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes Incorporating Cyclodextrin–Drug Inclusion Complexes: Current State of Knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- McCormack, B.; Gregoriadis, G. Drugs-in-Cyclodextrins-in Liposomes: A Novel Concept in Drug Delivery. Int. J. Pharm. 1994, 112, 249–258. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove Essential Oil-in-Cyclodextrin-in-Liposomes in the Aqueous and Lyophilized States: From Laboratory to Large Scale Using a Membrane Contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-Cyclodextrin-in-Liposomes as a Carrier System for Volatile Essential Oil Components: Application to Anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef]
- Gharib, R.; Haydar, S.; Charcosset, C.; Fourmentin, S.; Greige-Gerges, H. First Study on the Release of a Natural Antimicrobial Agent, Estragole, from Freeze-Dried Delivery Systems Based on Cyclodextrins and Liposomes. J. Drug Deliv. Sci. Technol. 2019, 52, 794–802. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Drug-in-Hydroxypropyl-β-Cyclodextrin-in-Lipoid S100/Cholesterol Liposomes: Effect of the Characteristics of Essential Oil Components on Their Encapsulation and Release. Int. J. Pharm. 2020, 579, 119151. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.; Auezova, L.; Danjou, P.-E.; Fourmentin, S.; Greige-Gerges, H. First Evaluation of Drug-in-Cyclodextrin-in-Liposomes as an Encapsulating System for Nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bragagni, M.; Maestrelli, F.; Mennini, N.; Ghelardini, C.; Mura, P. Liposomal Formulations of Prilocaine: Effect of Complexation with Hydroxypropyl-ß-Cyclodextrin on Drug Anesthetic Efficacy. J. Liposome Res. 2010, 20, 315–322. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. New “Drug-in Cyclodextrin-in Deformable Liposomes” Formulations to Improve the Therapeutic Efficacy of Local Anaesthetics. Int. J. Pharm. 2010, 395, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, Z.; Khreich, N.; Auezova, L.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Cyclodextrin-Membrane Interaction in Drug Delivery and Membrane Structure Maintenance. Int. J. Pharm. 2019, 564, 59–76. [Google Scholar] [CrossRef]
- Hatzi, P.; Mourtas, S.; Klepetsanis, P.G.; Antimisiaris, S.G. Integrity of Liposomes in Presence of Cyclodextrins: Effect of Liposome Type and Lipid Composition. Int. J. Pharm. 2007, 333, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, G.; Fresta, M.; Ventura, C.A. Interaction of Natural and Modified β-Cyclodextrins with a Biological Membrane Model of Dipalmitoylphosphatidylcholine. J. Colloid Interface Sci. 1996, 180, 542–547. [Google Scholar] [CrossRef]
- Denz, M.; Haralampiev, I.; Schiller, S.; Szente, L.; Herrmann, A.; Huster, D.; Müller, P. Interaction of Fluorescent Phospholipids with Cyclodextrins. Chem. Phys. Lipids 2016, 194, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Milles, S.; Meyer, T.; Scheidt, H.A.; Schwarzer, R.; Thomas, L.; Marek, M.; Szente, L.; Bittman, R.; Herrmann, A.; Pomorski, T.G.; et al. Organization of Fluorescent Cholesterol Analogs in Lipid Bilayers—Lessons from Cyclodextrin Extraction. Biochim. Biophys. Acta 2013, 1828, 1822–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohvo-Rekilä, H.; Akerlund, B.; Slotte, J.P. Cyclodextrin-Catalyzed Extraction of Fluorescent Sterols from Monolayer Membranes and Small Unilamellar Vesicles. Chem. Phys. Lipids 2000, 105, 167–178. [Google Scholar] [CrossRef]
- Nishijo, J.; Shiota, S.; Mazima, K.; Inoue, Y.; Mizuno, H.; Yoshida, J. Interactions of Cyclodextrins with Dipalmitoyl, Distearoyl, and Dimyristoyl Phosphatidyl Choline Liposomes. A Study by Leakage of Carboxyfluorescein in Inner Aqueous Phase of Unilamellar Liposomes. Chem. Pharm. Bull. 2000, 48, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Study of the Relationship between Lipid Binding Properties of Cyclodextrins and Their Effect on the Integrity of Liposomes. Int. J. Pharm. 2007, 338, 35–42. [Google Scholar] [CrossRef]
- Li, J.; Perdue, E.M. Physicochemical Properties of Selected Monoterpenes. In Proceedings of the 209th ACS National Meeting, Anaheim, CA, USA, 2–7 April 1995; Elsevier: Amsterdam, The Netherlands, 1995; pp. 134–137. [Google Scholar]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel Findings for Quercetin Encapsulation and Preservation with Cyclodextrins, Liposomes, and Drug-in-Cyclodextrin-in-Liposomes. Food Hydrocoll. 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New Findings on the Incorporation of Essential Oil Components into Liposomes Composed of Lipoid S100 and Cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.A.; Pinto, O.A.; Hollmann, A. Interaction of Semiochemicals with Model Lipid Membranes: A Biophysical Approach. Colloids Surf. B Biointerfaces 2018, 161, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Fourmentin, S.; Charcosset, C.; Greige-Gerges, H. Effect of Hydroxypropyl-β–Cyclodextrin on Lipid Membrane Fluidity, Stability and Freeze-Drying of Liposomes. J. Drug Deliv. Sci. Technol. 2018, 44, 101–107. [Google Scholar] [CrossRef]
- Yancey, P.G.; Rodrigueza, W.V.; Kilsdonk, E.P.; Stoudt, G.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular Cholesterol Efflux Mediated by Cyclodextrins. Demonstration of Kinetic Pools and Mechanism of Efflux. J. Biol. Chem. 1996, 271, 16026–16034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steck, T.L.; Ye, J.; Lange, Y. Probing Red Cell Membrane Cholesterol Movement with Cyclodextrin. Biophys. J. 2002, 83, 2118–2125. [Google Scholar] [CrossRef] [Green Version]
- Ermilova, I.; Lyubartsev, A.P. Cholesterol in Phospholipid Bilayers: Positions and Orientations inside Membranes with Different Unsaturation Degrees. Soft Matter 2019, 15, 78–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harroun, T.A.; Katsaras, J.; Wassall, S.R. Cholesterol Hydroxyl Group Is Found to Reside in the Center of a Polyunsaturated Lipid Membrane. Biochemistry 2006, 45, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Heberle, F.A.; Greathouse, D.V.; Koeppe, R.E.; Standaert, R.F.; Van Oosten, B.J.; Harroun, T.A.; Kinnun, J.J.; Williams, J.A.; Wassall, S.R.; et al. Lipid Bilayer Thickness Determines Cholesterol’s Location in Model Membranes. Soft Matter 2016, 12, 9417–9428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.-F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-Encapsulated Vincristine, Vinblastine and Vinorelbine: A Comparative Study of Drug Loading and Retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Sebaaly, C.; Greige-Gerges, H.; Stainmesse, S.; Fessi, H.; Charcosset, C. Effect of Composition, Hydrogenation of Phospholipids and Lyophilization on the Characteristics of Eugenol-Loaded Liposomes Prepared by Ethanol Injection Method. Food Biosci. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-Cyclodextrin-in-Liposome: The Effect of Cyclodextrins on Encapsulation Efficiency and Release Kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef]
- Qiu, N.; Cai, L.; Wang, W.; Wang, G.; Cheng, X.; Xu, Q.; Wen, J.; Liu, J.; Wei, Y.; Chen, L. Barbigerone-in-Hydroxypropyl-β-Cyclodextrin-Liposomal Nanoparticle: Preparation, Characterization and Anti-Cancer Activities. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 505–514. [Google Scholar] [CrossRef]
- Wang, W.-X.; Feng, S.-S.; Zheng, C.-H. A Comparison between Conventional Liposome and Drug-Cyclodextrin Complex in Liposome System. Int. J. Pharm. 2016, 513, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative Activity of Rosmarinus Officinalis L. Essential Oil Compared to Its Main Components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.-J.; Qin, F.; Tu, J.-L.; Li, B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef] [Green Version]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and Characterization of Clove Essential Oil-Loaded Liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Ohkubo, K.; Imai, K.; Kamibayashi, M.; Yoshihashi, Y.; Matsumoto, K.; Fukuhara, K.; Terada, K.; Itoh, S.; Ozawa, T.; et al. Solubilisation of a 2,2-Diphenyl-1-Picrylhydrazyl Radical in Water by β-Cyclodextrin to Evaluate the Radical-Scavenging Activity of Antioxidants in Aqueous Media. Chem. Commun. 2015, 51, 8311–8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhle, L.; Kfoury, M.; Greige-Gerges, H.; Fourmentin, S. Effect of Dimethylsulfoxide, Ethanol, α- and β-Cyclodextrins and Their Association on the Solubility of Natural Bioactive Compounds. J. Mol. Liq. 2020, 310, 113156. [Google Scholar] [CrossRef]
- Wiącek, A.; Chibowski, E. Zeta Potential, Effective Diameter and Multimodal Size Distribution in Oil/Water Emulsion. Colloids Surf. Physicochem. Eng. Asp. 1999, 159, 253–261. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C. Hydroxypropyl-ß-Cyclodextrin as a Membrane Protectant during Freeze-Drying of Hydrogenated and Non-Hydrogenated Liposomes and Molecule-in-Cyclodextrin-in- Liposomes: Application to Trans-Anethole. Food Chem. 2018, 267, 67–74. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| HP-β-CD:α-Pinene Molar Ratio | CE (%) |
|---|---|
| 1:1 | 14.3 ± 1.3 |
| 2.5:1 | 40.6 ± 3.5 |
| 5:1 | 65.4 ± 6.2 |
| 7.5:1 | 80.6 ± 7.7 |
| 10:1 | 76.9 ± 3.8 |
| Size Distribution | Vesicle Characterization | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Population 1 | Population 2 | Population 3 | IR of PL (%) | IR of Chol (%) | EE of α-Pinene (%) | LR of α-Pinene (%) | Final PL:Chol: α-Pinene Molar Ratio | ||||
| % | Mean Size (nm) | % | Mean Size (µm) | % | Mean Size (µm) | |||||||
| Phospholipon 90H:Chol formulations | ||||||||||||
| Blank CLs | 87.7 ± 6.7 87.3 ± 6.5 | 150 ± 0.0 150 ± 0.0 | 12.3 ± 6.7 12.7 ± 6.5 | 6.2 ± 0.9 6.2 ± 0.5 | - | - | 89.5 ± 6.3 | 75.9 ± 3.5 | - | - | 122:98:0 | |
| α-pinene-CLs | 84.3 ± 7.2 82.5 ± 11.6 | 140 ± 13.4 123 ± 25.4 | 15.7 ± 7.2 17.5 ± 11.6 | 7.7 ± 0.0 7.7 ± 0.0 | - | - | 88.6 ± 6.0 | 76.3 ± 4.3 | 100.0 ± 0.0 | 0.2 ± 0.01 | 124:98:0.36 | |
| Blank DCLs | 72.3 ± 7.3 61.0 ± 5.8 | 140 ± 10.9 131 ± 0.0 | 27.7 ± 7.3 17.7 ± 1.3 | 6.1 ± 0.8 6.6 ± 0.2 | - 21.3 ± 7.1 | - 48.3 ± 4.8 | 66.5 ±3.9 | 41.1 ± 1.3 | - | - | 90:59:0 | |
| α-pinene-DCLs | 82.5 ± 1.9 74.3 ± 2.2 | 140 ± 10.9 115 ± 17.8 | 17.5 ± 1.9 10.8 ± 1.3 | 5.7 ± 0.4 5.8 ± 0.6 | - 14.9 ± 2.0 | - 83.5 ± 3.5 | 87.8 ± 3.8 | 63.2 ± 3.1 | 72.9 ± 6.8 | 0.2 ± 0.02 | 118:82:0.31 | |
| Lipoid S100:Chol formulations | ||||||||||||
| Blank CLs | 17.0 ± 1.4 14.2 ± 0.3 | 166 ± 33.3 172 ± 41.1 | 83.0 ± 1.4 85.8 ± 0.3 | 6.7 ± 0.0 8.4 ± 2.9 | - | - | 95.9 ± 1.5 | 71.2 ± 5.4 | - | - | 119:92:0 | |
| α-pinene-CLs | - | - | 33.4 ± 5.3 12.3 ± 2.5 | 9.7 ± 0.7 10.6 ± 0.8 | 66.6 ± 5.3 87.6 ± 2.5 | 83.5 ± 8.7 84.8 ± 6.4 | 48.6 ± 2.4 | 66.4 ± 4.9 | 100.0 ± 0.0 | 22.9 ± 2.2 | 61:93:42 | |
| Blank DCLs | - | - | - | - | 100 ± 0.0 100 ± 0.0 | 26.1 ± 0.0 34.2 ± 4.7 | 83.1 ± 0.7 | 74.5 ± 1.7 | - | - | 103:100:0 | |
| α-pinene-DCLs | 34.0 ± 0.0 18.5 ± 2.2 | 131 ± 8.3 296 ± 9.7 | 66.0 ± 0.0 61.0 ± 2.0 | 6.8 ± 2.9 7.7 ± 1.9 | - 20.5 ± 1.4 | - 29.9 ± 1.8 | 89.2 ± 1.4 | 71.2 ± 1.3 | 100.0 ± 0.0 | 0.6 ± 0.02 | 111:92:1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, Z.; Kayouka, M.; Trifan, A.; Sieniawska, E.; Jemâa, J.M.B.; Elaissari, A.; Greige-Gerges, H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules 2021, 26, 6840. https://doi.org/10.3390/molecules26226840
Hammoud Z, Kayouka M, Trifan A, Sieniawska E, Jemâa JMB, Elaissari A, Greige-Gerges H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules. 2021; 26(22):6840. https://doi.org/10.3390/molecules26226840
Chicago/Turabian StyleHammoud, Zahraa, Maya Kayouka, Adriana Trifan, Elwira Sieniawska, Jouda Mediouni Ben Jemâa, Abdelhamid Elaissari, and Hélène Greige-Gerges. 2021. "Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins" Molecules 26, no. 22: 6840. https://doi.org/10.3390/molecules26226840








