In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri
Abstract
:1. Introduction
2. Results and Discussion
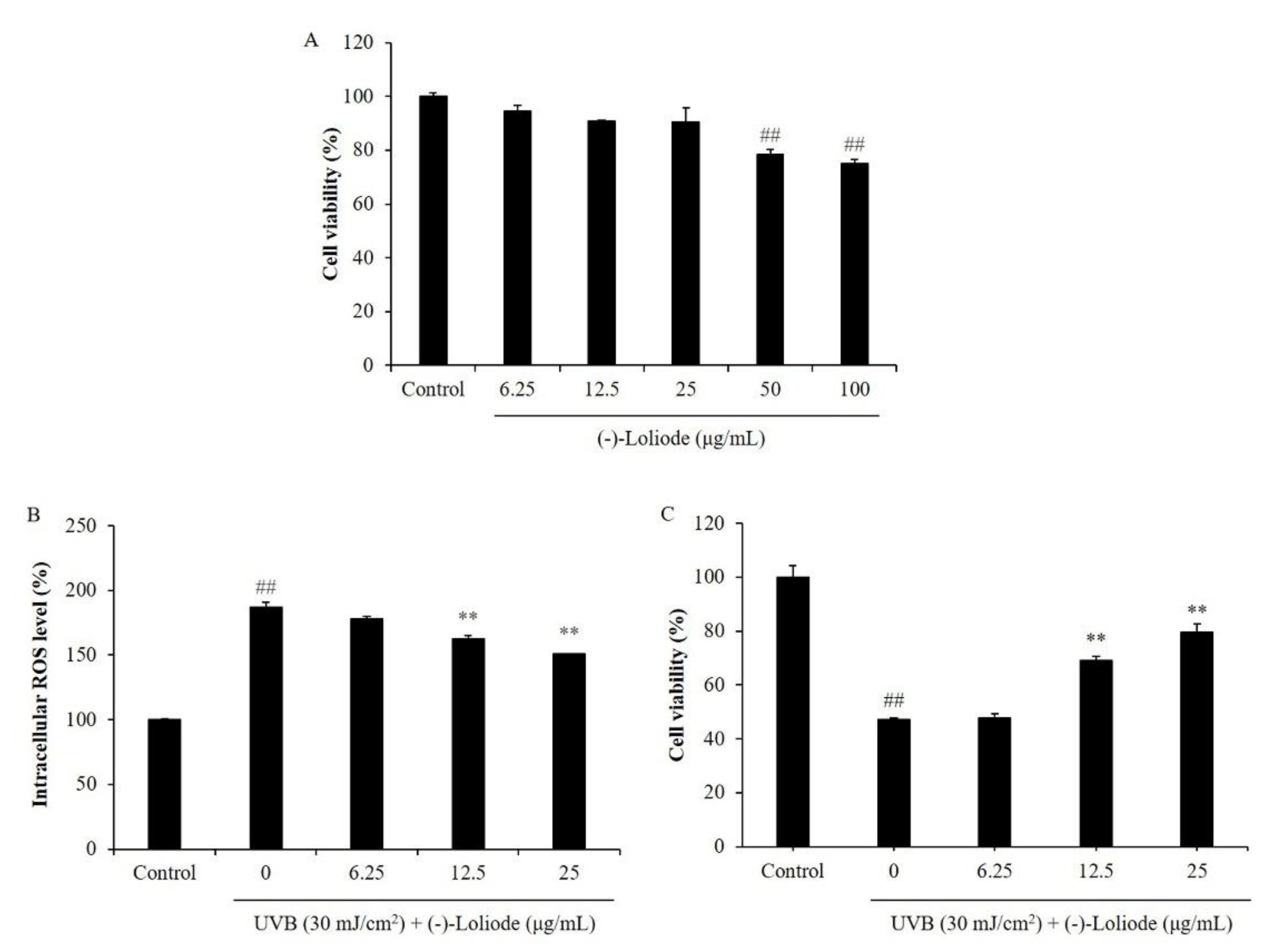
2.1. Protective Effect of (-)-Loliode against UVB-Induced HaCaT Cell Damage
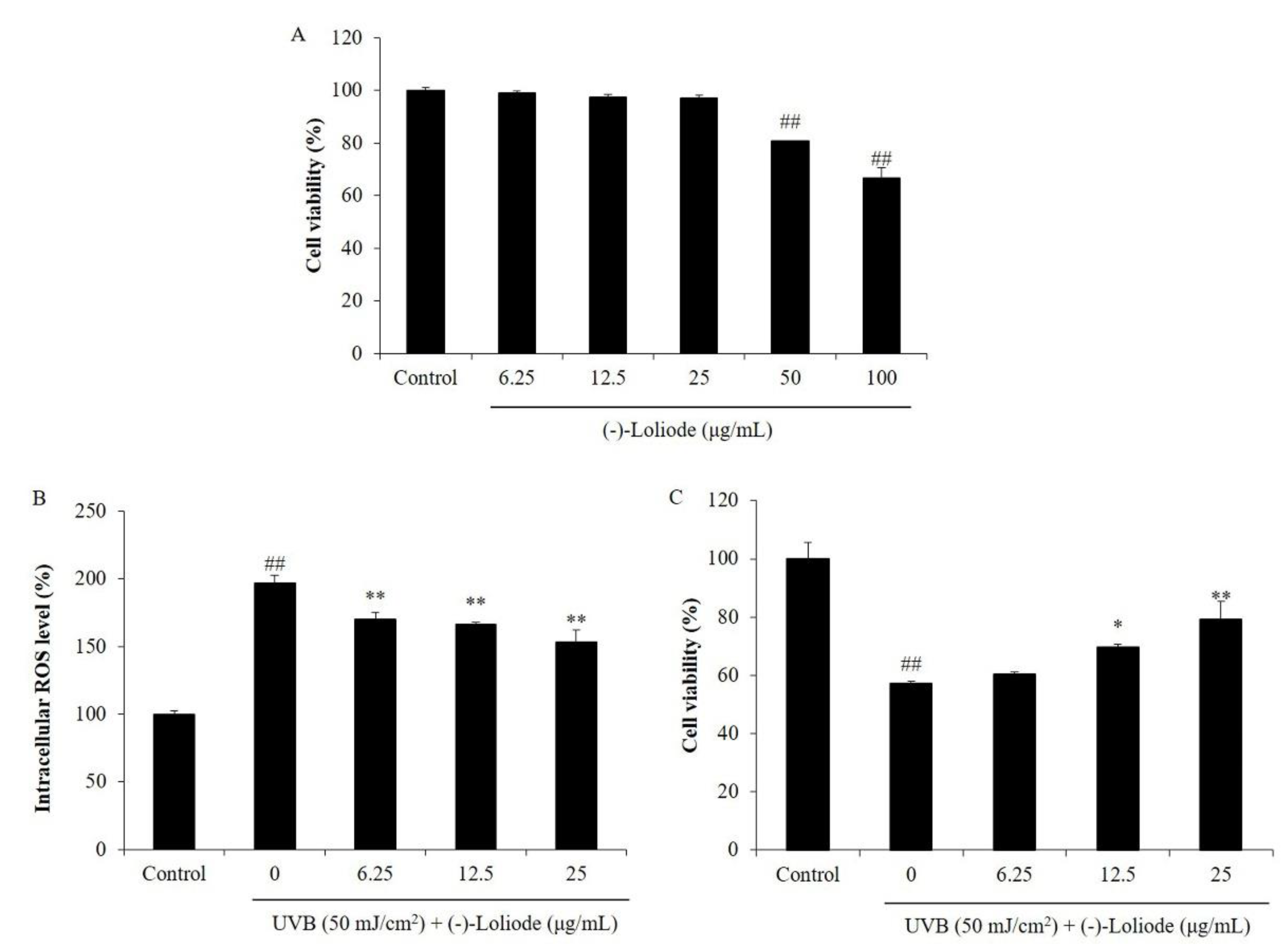
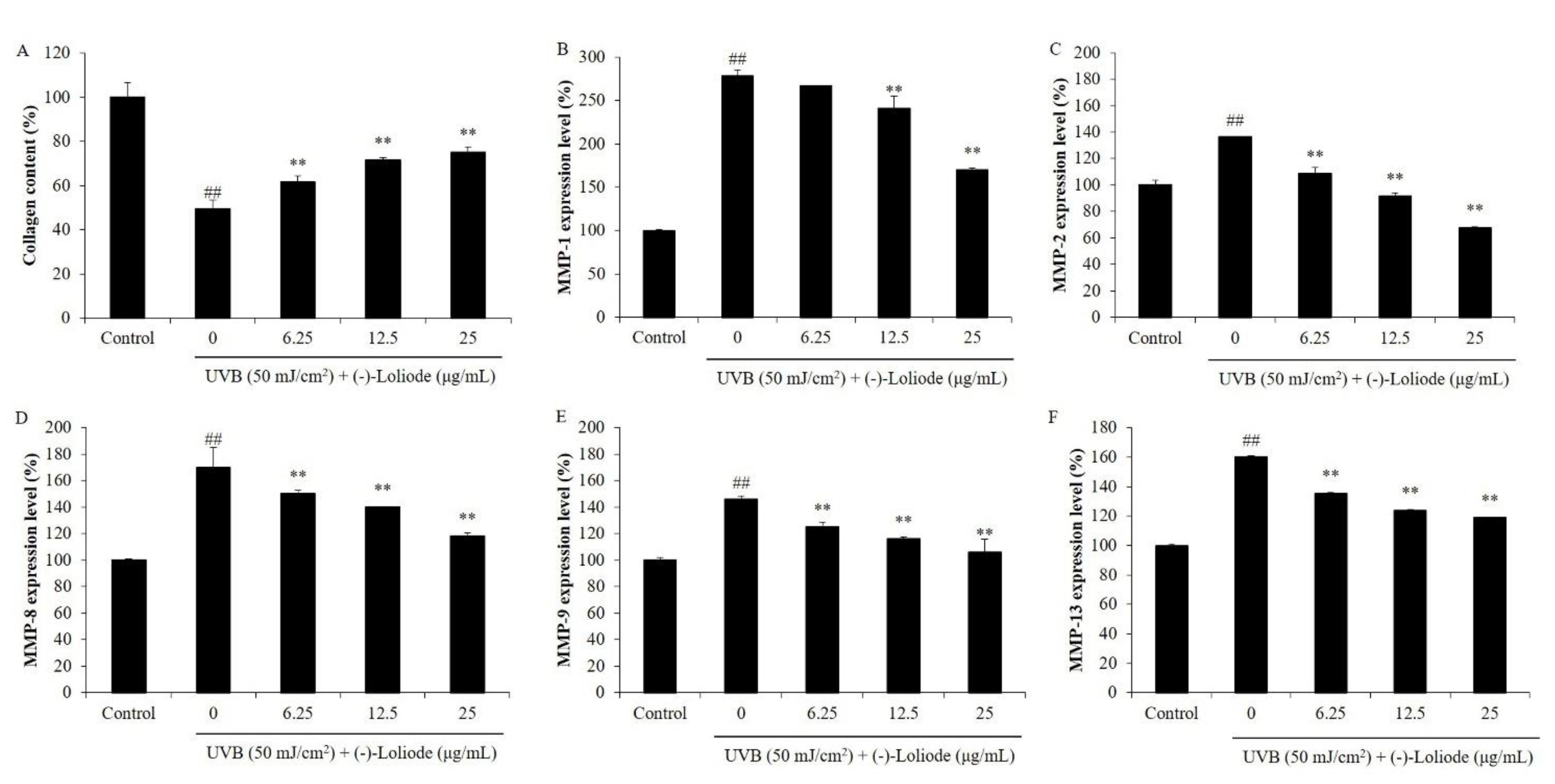
2.2. Protective Effect of (-)-Loliode against UVB-induced HDF Cell Damage
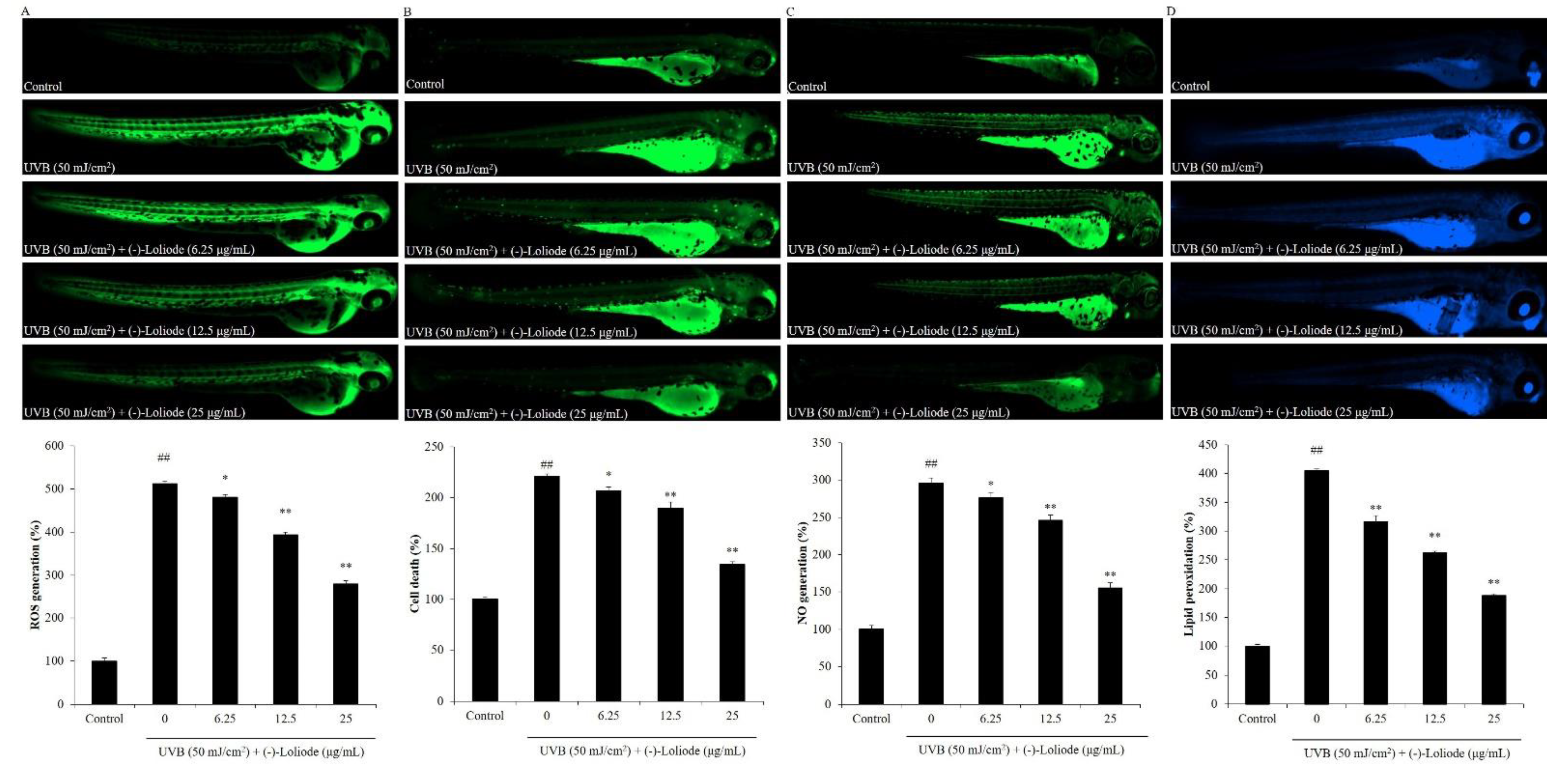
2.3. Protective Effect of (-)-Loliode against UVB-induced Zebrafish Damage
3. Materials and Methods
3.1. Chemical and Regents
3.2. Preparation of (-)-Loliode from S. horneri
3.3. In Vitro in HaCaT cells
3.4. In Vitro in HDF cells
3.5. In Vivo Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Ahn, B.-N.; Kang, K.-H.; Kim, Y.-S.; Li, Y.-X.; Kong, C.-S.; Kim, S.-K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B Biol. 2015, 153, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-K.; Kim, D.; Lee, M.; Park, S.-H.; Yamada, W.; Eun, S.; Lee, J. Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants 2021, 10, 1452. [Google Scholar] [CrossRef]
- Lee, J.-E.; Oh, J.; Song, D.; Lee, M.; Hahn, D.; Boo, Y.; Kang, N. Acetylated Resveratrol and Oxyresveratrol Suppress UVB-Induced MMP-1 Expression in Human Dermal Fibroblasts. Antioxidants 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ryu, B.; Kim, W.-S.; Kim, G.H.; Jeon, Y.-J. Protective effect of gallic acid derivatives from the freshwater green alga Spirogyra sp. against ultraviolet B-induced apoptosis through reactive oxygen species clearance in human keratinocytes and zebrafish. ALGAE 2017, 32, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Feng, M.; Wan, J.; Shi, Y.; Xie, X.; Pan, W.; Hu, B.; Wang, Y.; Wen, H.; Wang, K.; et al. Anti-damage effect of theaflavin-3′-gallate from black tea on UVB-irradiated HaCaT cells by photoprotection and maintaining cell homeostasis. J. Photochem. Photobiol. B Biol. 2021, 224, 112304. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B Biol. 2019, 191, 135–142. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Je, J.-G.; Oh, J.Y.; Kim, Y.-S.; Cha, S.-H.; Jeon, Y.-J. Protective Effect of Diphlorethohydroxycarmalol Isolated from Ishige okamurae Against Particulate Matter-Induced Skin Damage by Regulation of NF-κB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Molecules 2020, 25, 1055. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Oh, J.Y.; Hwang, J.; Jeon, Y.-J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cui, Y.R.; Yang, H.-W.; Lee, H.G.; Ko, J.-Y.; Jeon, Y.-J. A mixture of seaweed extracts and glycosaminoglycans from sea squirts inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells. Fish. Aquat. Sci. 2019, 22, 11. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-κB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.-I.; Jang, J.-H. Sargassum fulvellumProtects HaCaT Cells and BALB/c Mice from UVB-Induced Proinflammatory Responses. Evid.-Based Complement. Altern. Med. 2013, 2013, 747846. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.-J.; Jeon, Y.-J.; Lee, D.-S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.A.; Han, E.J.; Jee, Y.; Ahn, G.; Rho, J.-R.; Jeon, Y.-J. Loliolide, isolated from Sargassum horneri; abate LPS-induced inflammation via TLR mediated NF-κB, MAPK pathways in macrophages. Algal. Res. 2021, 56, 102297. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y.; et al. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, S.-Y.; Han, H.-J.; Kim, H.-S.; Kim, K.-N.; Fernando, I.P.S.; Madusanka, D.M.D.; Dias, M.K.H.M.; Cheong, S.H.; Park, S.R.; et al. UVB protective effects of Sargassum horneri through the regulation of Nrf2 mediated antioxidant mechanism. Sci. Rep. 2021, 11, 9963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and proinflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In Vitro and in vivo zebrafish model. Environ. Boil. Fishes 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Wang, L.; Jo, M.-J.; Katagiri, R.; Harata, K.; Ohta, M.; Ogawa, A.; Kamegai, M.; Ishida, Y.; Tanoue, S.; Kimura, S.; et al. Antioxidant effects of citrus pomace extracts processed by super-heated steam. LWT 2017, 90, 331–338. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.-j.; Oh, J.Y.; Fernando, I.S.; Sanjeewa, K.A.; Kang, M.-c.; Cui, Y.R.; Lee, H.G.; Ko, J.Y.; Lee, W. Protective Effects of Enzyme-assistant Extracts of Sargassum fulvellum against AAPH-induced Oxidative Stress in vitro in Vero Cells. J. Chitin Chitosan 2018, 23, 113–119. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Kim, H.-S.; Je, J.-G.; Fu, X.; Huang, C.; Ahn, G.; Oh, J.-Y.; Sanjeewa, K.K.A.; Xu, J.; Gao, X.; et al. In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri. Molecules 2021, 26, 6898. https://doi.org/10.3390/molecules26226898
Wang L, Kim H-S, Je J-G, Fu X, Huang C, Ahn G, Oh J-Y, Sanjeewa KKA, Xu J, Gao X, et al. In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri. Molecules. 2021; 26(22):6898. https://doi.org/10.3390/molecules26226898
Chicago/Turabian StyleWang, Lei, Hyun-Soo Kim, Jun-Geon Je, Xiaoting Fu, Caoxing Huang, Ginnae Ahn, Jae-Young Oh, K. K. Asanka Sanjeewa, Jiachao Xu, Xin Gao, and et al. 2021. "In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri" Molecules 26, no. 22: 6898. https://doi.org/10.3390/molecules26226898
APA StyleWang, L., Kim, H.-S., Je, J.-G., Fu, X., Huang, C., Ahn, G., Oh, J.-Y., Sanjeewa, K. K. A., Xu, J., Gao, X., Yeo, I.-K., & Jeon, Y.-J. (2021). In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri. Molecules, 26(22), 6898. https://doi.org/10.3390/molecules26226898









