The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus)
Abstract
:1. Introduction
2. Saffron as an Aromatic Spice
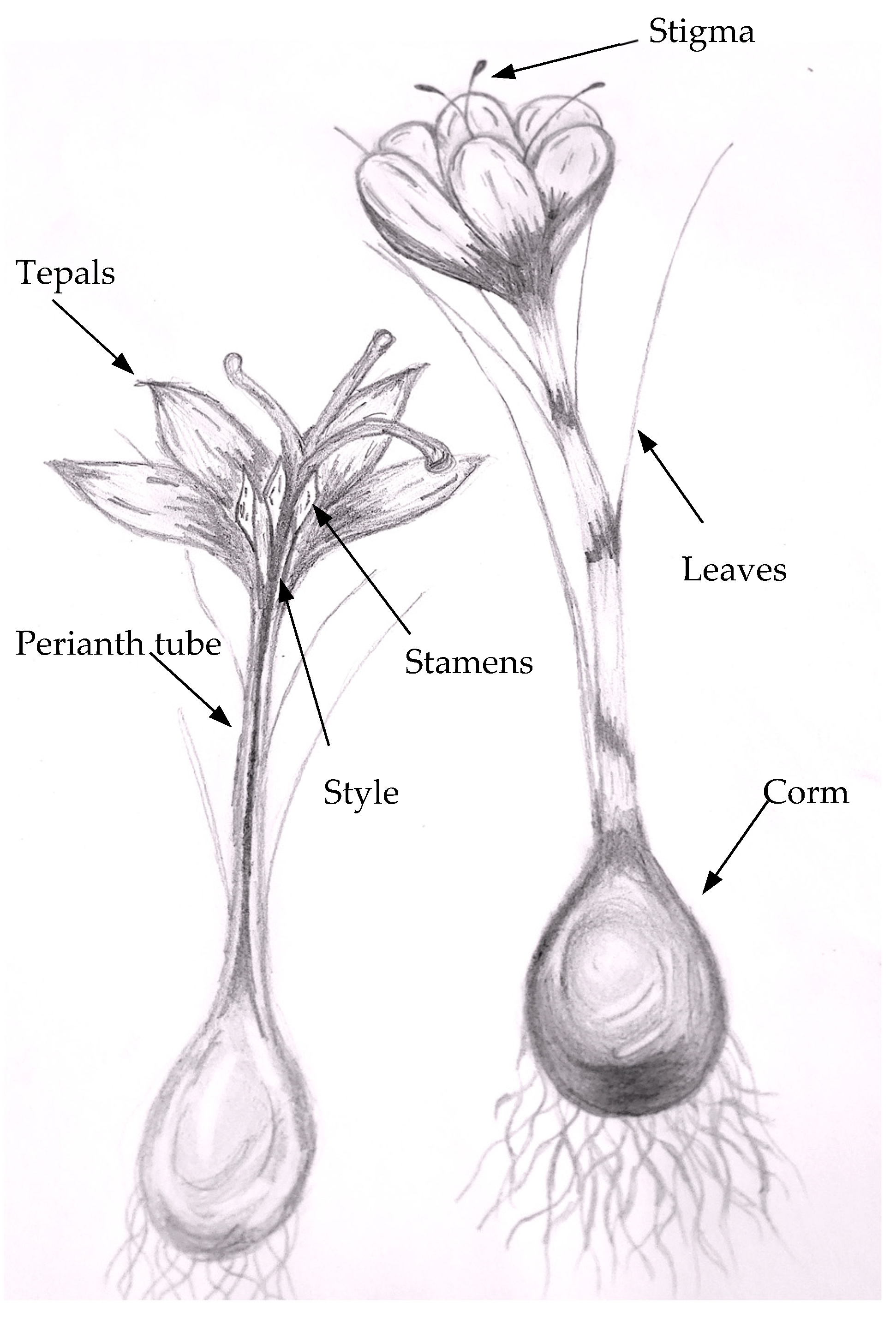
2.1. Botanical Characterisctics
2.2. Harvest
3. Post-Harvest
3.1. Drying
3.1.1. Conventional Methods
3.1.2. Non-Conventional Methods
3.2. Storage
4. Theories for the Generation of the Main Compounds in C. sativus and Degradation in Saffron
4.1. Enzymatic Theory in C. sativus
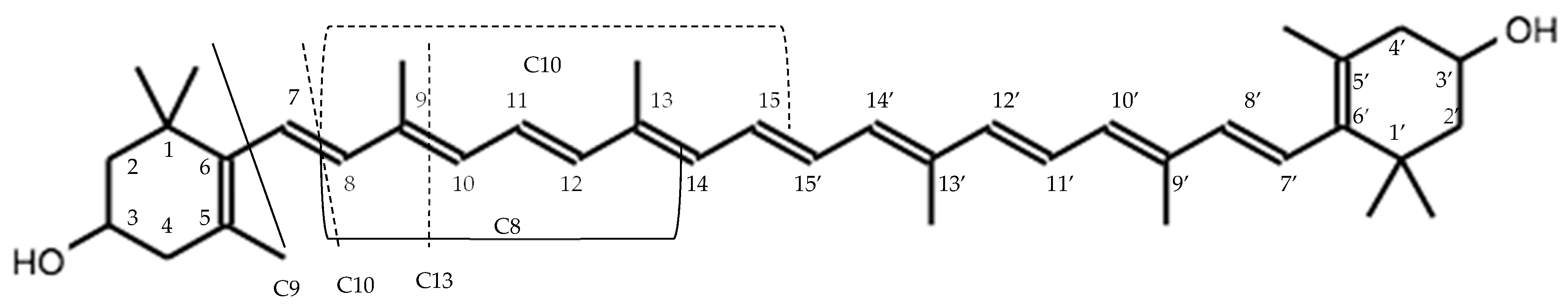
4.2. Excision of Carotenoids by CCD
4.3. Crocetin Ester Cleavage to Form Volatile Compounds
5. Determination of Volatile Chemical Compounds in Saffron
6. Saffron’s Aroma Descriptors
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bagur, M.J.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [Green Version]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Masi, E.; Taiti, C.; Heimler, D.; Vignolini, P.; Romani, A.; Mancuso, S. PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2016, 192, 75–81. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Winterhalter, P. Carotenoid cleavage products in saffron (Crocus sativus L). ACS Symp. Ser. 2013, 1134, 45–63. [Google Scholar]
- Farag, M.A.; Hegazi, N.; Dokhalahy, E.; Khattab, A.R. Chemometrics based GC-MS aroma profiling for revealing freshness, origin and roasting indices in saffron spice and its adulteration. Food Chem. 2020, 331, 127358. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Htar, T.T.; Akowuah, G.A. ATR-FTIR and spectrometric methods for the assay of crocin in commercial saffron spices (Crocus savitus L.). Int. J. Food Prop. 2015, 18, 1773–1783. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Trapero-Mozos, A.; Gómez, M.D.; Rubio-Moraga, A.; Ahrazem, O. Identification and possible role of a MYB transcription factor from saffron (Crocus sativus). J. Plant Physiol. 2012, 169, 509–515. [Google Scholar] [CrossRef]
- Winterhalter, P.; Straubinger, M. Saffron Renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Kothari, D.; Thakur, M.; Joshi, R.; Kumar, A.; Kumar, R. Agro-Climatic Suitability Evaluation for Saffron Production in Areas of Western Himalaya. Front. Plant Sci. 2021, 12, 657819. [Google Scholar] [CrossRef]
- Yue, J.; Wang, R.; Ma, X.; Liu, J.; Lu, X.; Balaso-Thakar, S.; An, N.; Liu, J.; Xia, E.; Liu, Y. Full-length transcriptome sequencing provides insights into the evolution of apocarotenoid biosynthesis in Crocus sativus. Comput. Struct. Biotechnol. J. 2020, 18, 774–783. [Google Scholar] [CrossRef]
- Fang, Q.; Li, Y.; Liu, B.; Meng, X.; Yang, Z.; Yang, S.; Bao, T.; Kimani, S.; Gao, X.; Wang, L. Cloning and functional characterization of a carotenoid cleavage dioxygenase 2 gene in safranal and crocin biosynthesis from Freesia hybrida. Plant Physiol. Biochem. 2020, 154, 439–450. [Google Scholar] [CrossRef]
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (Crocus sativus L.): Gold of the spices—a comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Maggi, L.; Carmona, M.; Alonso, G.L. Authentication of Saffron Spice (Crocus sativus L.). In Progress in Authentication of Food and Wine; Ebeler, S.E., Takeoka, G.R., Winterhalter, P., Eds.; ACS: Washington, DC, USA, 2011; pp. 309–331. [Google Scholar]
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Feizi, J. Development of an environmentally-friendly solvent-free extraction of saffron bioactives using subcritical water. LWT Food Sci. Technol. 2019, 114, 108428. [Google Scholar] [CrossRef]
- Kosar, M.; Demirci, B.; Goger, F.; Kara, I.; Baser, K.H.C. Volatile composition, antioxidant activity, and antioxidant components in saffron cultivated in Turkey. Int. J. Food Prop. 2017, 20, S746–S754. [Google Scholar] [CrossRef] [Green Version]
- Ordoudi, S.A.; Tsimidou, M. Saffron quality: Effect of agricultural practices, processing and storage. In Production Practices and Quality Assessment of Food Crops; Dris, R., Jain, S.M., Eds.; Kluwer Academic: Amsterdam, The Netherlands, 2004; Volume 1, pp. 209–260. [Google Scholar]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V.; Kontakos, S.; Kontominas, M.G. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur. Food Res. Technol. 2017, 243, 1577–1591. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, B.; Xiang, L.; Xiong, C.; Shi, Y.; Wu, L.; Meng, X.; Dong, G.; Xie, Y.; Sun, W. A novel onsite and visual molecular technique to authenticate saffron (Crocus sativus) and its adulterants based on recombinase polymerase amplification. Food Control 2019, 100, 117–121. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins pattern in saffron detected by UHPLC-MS/MS as marker of quality, process and traceability. Food Chem. 2018, 264, 241–249. [Google Scholar] [CrossRef]
- Haghighi, R.; Sayed Tabatabaei, B.E.; Maibody, S.A.M.M.; Talebi, M.; Molina, R.V.; Nebauer, S.G.; Renau-Morata, B. A flowering inhibitor of the temperature-dependent pathway in Crocus sativus L. Mol. Biol. Rep. 2020, 47, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Rezvani Moghaddam, P.; Seyyedi, S.M. Depending on mother corm size, the removal of extra lateral buds regulates sprouting mechanism and improves phosphorus acquisition efficiency in saffron (Crocus sativus L.). Ind. Crops Prod. 2019, 141, 111779. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive volatiles in Sicilian (South Italy) saffron: Safranal and its related compounds. J. Essent. Oil Res. 2017, 29, 221–227. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. The genus Crocus L.: A review of ethnobotanical uses, phytochemistry and pharmacology. Ind. Crops Prod. 2021, 171, 113923. [Google Scholar] [CrossRef]
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A Review on Medicinal Properties of Saffron toward Major Diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Sánchez, A.M.; Lorenzo, C.; López-Córcoles, H.; Alonso, G.L. Quality determination of Crocus sativus L. flower by high-performance liquid chromatography. J. Food Compos. Anal. 2020, 93, 103613. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Lu, J.; Li, J.; Yao, C.; Sun, P.; Liu, K.; Dong, Y.; Qin, L.; Qian, X. Flower cultivation regimes affect apocarotenoid accumulation and gene expression during the development of saffron stigma. Hortic. Environ. Biotechnol. 2020, 61, 473–484. [Google Scholar] [CrossRef]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gómez, M.D.; Orzaez, D.; Granell, A.; Gómez-Gómez, L. Cytosolic and Plastoglobule-targeted Carotenoid Dioxygenases from Crocus sativus Are Both Involved in b-Ionone Release. J. Biol. Chem. 2008, 238, 24816–24825. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.A.; Ashraf, N. Apocarotenoid Biosynthesis in Crocus sativus L. In Apocarotenoids of Crocus sativus L: From Biosynthesis to Pharmacology; Baba, S.A., Ashraf, N., Eds.; Springer: Singapore, 2016; pp. 1–21. [Google Scholar]
- Razavi, S.E.; Jafari, S.M. Effect of corm age on the antioxidant, bactericidal and fungicidal activities of saffron (Crocus sativus L.) stigmas. Food Control 2021, 130, 108358. [Google Scholar] [CrossRef]
- Taheri-Dehkordi, A.; Naderi, R.; Martinelli, F.; Salami, S.A. A robust workflow for indirect somatic embryogenesis and cormlet production in saffron (Crocus sativus L.) and its wild allies; C. caspius and C. speciosus. Heliyon 2020, 6, e05841. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.; Sanna, M.L.; Pintore, G.; Lage, M.; Zara, S. Isolation and characterization of microorganisms and volatiles associated with Moroccan saffron during different processing treatments. Int. J. Food Microbiol. 2018, 273, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dutt, H.C. Floral Phenology and Adequate Collection Time of Flowers of Crocus sativus L.: An Expensive Spice. Natl. Acad. Sci. Lett. 2021, 44, 275–280. [Google Scholar] [CrossRef]
- Kafi, M.; Kamili, A.N.; Husaini, A.M.; Ozturk, M.; Altay, V. An Expensive Spice Saffron (Crocus sativus L.): A Case Study from Kashmir, Iran, and Turkey. In Global Perspectives on Underutilized Crops; Springer International Publishing: Cham, Switzerland, 2018; pp. 109–149. [Google Scholar]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of art of saffron (Crocus sativus L.) agronomy: A comprehensive review. Food Rev. Int. 2009, 25, 44–85. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Ghoranneviss, M.; Abdijadid, S. Effect of cold plasma on crocin esters and volatile compounds of saffron. Food Chem. 2017, 235, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef] [PubMed]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef]
- Moras, B.; Loffredo, L.; Rey, S. Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef]
- Acar, B.; Sadikoglu, H.; Ozkaymak, M. Freeze drying of saffron (Crocus sativus L.). Dry. Technol. 2011, 29, 1622–1627. [Google Scholar] [CrossRef]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Geographical differentiation of saffron by GC-MS/FID and chemometrics. Eur. Food Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Aghaei, Z.; Jafari, S.M.; Dehnad, D. Effect of different drying methods on the physicochemical properties and bioactive components of saffron powder. Plant Foods Hum. Nutr. 2019, 74, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chaouqi, S.; Moratalla-López, N.; Lage, M.; Lorenzo, C.; Alonso, G.L.; Guedira, T. Effect of drying and storage process on Moroccan saffron quality. Food Biosci. 2018, 22, 146–153. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Cagliani, L.R.; Lalou, S.; Naziri, E.; Tsimidou, M.Z.; Consonni, R. 1H NMR-based metabolomics of saffron reveals markers for its quality deterioration. Food Res. Int. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Pardo, J.E.; López, E.; Alvarruiz, A.; Alonso, G.L. Influence of Different Drying and Aging Conditions on Saffron Constituents. J. Agric. Food Chem. 2005, 53, 3974–3979. [Google Scholar] [CrossRef]
- Gregory, M.J.; Menary, R.C.; Davies, N.W. Effect of Drying Temperature and Air Flow on the Production and Retention of Secondary Metabolites in Saffron. J. Agric. Food Chem. 2005, 53, 5969–5975. [Google Scholar] [CrossRef]
- Neri, L.; Giancaterino, M.; Rocchi, R.; Tylewicz, U.; Valbonetti, L.; Faieta, M.; Pittia, P. Pulsed electric fields (PEF) as hot air drying pre-treatment: Effect on quality and functional properties of saffron (Crocus sativus L.). Innov. Food Sci. Emerg. Technol. 2021, 67, 102592. [Google Scholar] [CrossRef]
- Yao, C.; Qian, X.-D.; Zhou, G.-F.; Zhang, S.-W.; Li, L.-Q.; Guo, Q.-S. A comprehensive analysis and comparison between vacuum and electric oven drying methods on Chinese saffron (Crocus sativus L.). Food Sci. Biotechnol. 2019, 28, 355–364. [Google Scholar] [CrossRef]
- Acar, B.; Sadikoglu, H.; Doymaz, I. Freeze-Drying Kinetics and Diffusion Modeling of Saffron (Crocus sativus L.). J. Food Process. Preserv. 2015, 39, 142–149. [Google Scholar] [CrossRef]
- Tabibian, S.A.; Labbafi, M.; Askari, G.H.; Rezaeinezhad, A.R.; Ghomi, H. Effect of gliding arc discharge plasma pretreatment on drying kinetic, energy consumption and physico-chemical properties of saffron (Crocus sativus L.). J. Food Eng. 2020, 270, 109766. [Google Scholar] [CrossRef]
- Akhondi, E.; Kazemi, A.; Maghsoodi, V. Determination of a suitable thin layer drying curve model for saffron (Crocus sativus L.) stigmas in an infrared dryer. Sci. Iran. 2011, 18, 1397–1401. [Google Scholar] [CrossRef] [Green Version]
- Torki-Harchegani, M.; Ghanbarian, D.; Maghsoodi, V.; Moheb, A. Infrared thin layer drying of saffron (Crocus sativus L.) stigmas: Mass transfer parameters and quality assessment. Chin. J. Chem. Eng. 2017, 25, 426–432. [Google Scholar] [CrossRef]
- Chen, D.; Xing, B.; Yi, H.; Li, Y.; Zheng, B.; Wang, Y.; Shao, Q. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.). LWT Food Sci. Technol. 2020, 120, 108913. [Google Scholar] [CrossRef]
- Maghsoodi, V.; Kazemi, A.; Akhondi, E. Effect of Different Drying Methods on Saffron (Crocus Sativus L.) Quality. Iran. J. Chem. Chem. Eng. 2012, 31, 85–89. [Google Scholar]
- Tong, Y.; Zhu, X.; Yan, Y.; Liu, R.; Gong, F.; Zhang, L.; Hu, J.; Fang, L.; Wang, R.; Wang, P. The Influence of Different Drying Methods on Constituents and Antioxidant Activity of Saffron from China. Int. J. Anal. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- del Campo, C.P.; Carmona, M.; Maggi, L.; Kanakis, C.D.; Anastasaki, E.G.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Effects of mild temperature conditions during dehydration procedures on saffron quality parameters. J. Sci. Food Agric. 2010, 90, 719–725. [Google Scholar] [CrossRef]
- Urbani, E.; Blasi, F.; Chiesi, C.; Maurizi, A.; Cossignani, L. Characterization of volatile fraction of saffron from central Italy (Cascia, Umbria). Int. J. Food Prop. 2015, 18, 2223–2230. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; De Los Mozos Pascual, M.; Tsimidou, M.Z. On the quality control of traded saffron by means of transmission Fourier-transform mid-infrared (FT-MIR) spectroscopy and chemometrics. Food Chem. 2014, 150, 414–421. [Google Scholar] [CrossRef]
- Ghorbani, R.; Koocheki, A. Sustainable Cultivation of Saffron in Iran. In Sustainable Agriculture Reviews 13; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; pp. 169–203. [Google Scholar]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Instrumental approaches and innovative systems for saffron quality assessment. J. Food Eng. 2018, 216, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Bustamante, E.; Sánchez, S. Microbial Production of C 13 -Norisoprenoids and Other Aroma Compounds via Carotenoid Cleavage. Crit. Rev. Microbiol. 2007, 33, 211–230. [Google Scholar] [CrossRef]
- IqbaLMzr, J.; Ahmed, N.; Mokhdomi, T.A.; Wafai, A.H.; Wani, S.H.; Bukhari, S.; Amin, A.; Qadri, R.A. Relative expression of apocarotenoid biosynthetic genes in developing stigmas of Crocus sativus L. J. Crop Sci. Biotechnol. 2013, 16, 183–188. [Google Scholar] [CrossRef]
- Torres-Montilla, S.; Rodriguez-Concepcion, M. Making extra room for carotenoids in plant cells: New opportunities for biofortification. Prog. Lipid Res. 2021, 84, 101–128. [Google Scholar] [CrossRef]
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.-A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Ataolahi, S.; Aliakbarzadeh, G.; Zarre, S.; Poursorkh, Z. Evaluation of storage time effect on saffron chemical profile using gas chromatography and spectrophotometry techniques coupled with chemometrics. J. Food Sci. Technol. 2018, 55, 1350–1359. [Google Scholar] [CrossRef]
- Cossignani, L.; Urbani, E.; Simonetti, M.S.; Maurizi, A.; Chiesi, C.; Blasi, F. Characterisation of secondary metabolites in saffron from central Italy (Cascia, Umbria). Food Chem. 2014, 143, 446–451. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. A new approach to saffron aroma. Crit. Rev. Food Sci. Nutr. 2007, 47, 145–159. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. Generation of Saffron Volatiles by Thermal Carotenoid Degradation. J. Agric. Food Chem. 2006, 54, 6825–6834. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarzadeh, G.; Parastar, H.; Sereshti, H. Classification of gas chromatographic fingerprints of saffron using partial least squares discriminant analysis together with different variable selection methods. Chemom. Intell. Lab. Syst. 2016, 158, 165–173. [Google Scholar] [CrossRef]
- Moraga, Á.R.; Rambla, J.L.; Ahrazem, O.; Granell, A.; Gómez-Gómez, L. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009, 70, 1009–1016. [Google Scholar] [CrossRef]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Jalali-Heravi, M.; Parastar, H.; Ebrahimi-Najafabadi, H. Characterization of volatile components of Iranian saffron using factorial-based response surface modeling of ultrasonic extraction combined with gas chromatography-mass spectrometry analysis. J. Chromatogr. A 2009, 1216, 6088–6097. [Google Scholar] [CrossRef]
- Jiang, M.; Kulsing, C.; Nolvachai, Y.; Marriott, P.J. Two-dimensional retention indices improve component identification in comprehensive two-dimensional gas chromatography of saffron. Anal. Chem. 2015, 87, 5753–5761. [Google Scholar] [CrossRef]
- Rocchi, R.; Mascini, M.; Faberi, A.; Sergi, M.; Compagnone, D.; Di Martino, V.; Carradori, S.; Pittia, P. Comparison of IRMS, GC-MS and E-Nose data for the discrimination of saffron samples with different origin, process and age. Food Control 2019, 106, 106736. [Google Scholar] [CrossRef]
- Amanpour, A.; Sonmezdag, A.S.; Kelebek, H.; Selli, S. GC-MS-olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.). Food Chem. 2015, 182, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Martínez, J.; Zalacain, A.; Rodríguez-Méndez, M.L.; De Saja, J.A.; Alonso, G.L. Analysis of saffron volatile fraction by TD-GC-MS and e-nose. Eur. Food Res. Technol. 2006, 223, 96–101. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G.; van Ruth, S.M.; Aghighi, S. The possibility for improvement of flowering, corm properties, bioactive compounds, and antioxidant activity in saffron (Crocus sativus L.) by different nutritional regimes. Ind. Crops Prod. 2019, 135, 301–310. [Google Scholar] [CrossRef]
- Nenadis, N.; Heenan, S.; Tsimidou, M.Z.; Van Ruth, S. Applicability of PTR-MS in the quality control of saffron. Food Chem. 2016, 196, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Heidari, R.; Samadi, S. Determination of volatile components of saffron by optimised ultrasound-assisted extraction in tandem with dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Food Chem. 2014, 143, 499–505. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G.; Erasmus, S.W.; van Ruth, S.M. Identification and characterisation of volatile fingerprints of saffron stigmas and petals using PTR-TOF-MS: Influence of nutritional treatments and corm provenance. Ind. Crops Prod. 2019, 141, 111803. [Google Scholar] [CrossRef]
- Azarabadi, N.; Özdemir, F. Determination of crocin content and volatile components in different qualities of Iranian saffron. Gida J. Food 2018, 43, 476–489. [Google Scholar] [CrossRef]
- Aliakbarzadeh, G.; Sereshti, H.; Parastar, H. Pattern recognition analysis of chromatographic fingerprints of Crocus sativus L. secondary metabolites towards source identification and quality control. Anal. Bioanal. Chem. 2016, 408, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Di Pietro, L.; Maggi, M.A.; Rossi, L. Optimization using chemometrics of HS-SPME/GC–MS profiling of saffron aroma and identification of geographical volatile markers. Eur. Food Res. Technol. 2018, 244, 1605–1613. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Galanakis, C.M. Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.). Ind. Crops Prod. 2020, 145, 111978. [Google Scholar] [CrossRef]
- Feyzi, S.; Varidi, M.; Housaindokht, M.R.; Es’haghi, Z.; Romano, R.; Piombino, P.; Genovese, A. A study on aroma release and perception of saffron ice cream using in-vitro and in-vivo approaches. Innov. Food Sci. Emerg. Technol. 2020, 65, 102455. [Google Scholar] [CrossRef]
- Du, H.; Wang, J.; Hu, Z.; Yao, X. Quantitative Structure-Retention Relationship study of the constituents of saffron aroma in SPME-GC-MS based on the Projection Pursuit Regression method. Talanta 2008, 77, 360–365. [Google Scholar] [CrossRef]
- Culleré, L.; San-Juan, F.; Cacho, J. Characterisation of aroma active compounds of Spanish saffron by gas chromatography-olfactometry: Quantitative evaluation of the most relevant aromatic compounds. Food Chem. 2011, 127, 1866–1871. [Google Scholar] [CrossRef]
- Amanpour, A.; Kelebek, H.; Selli, S. GLC/HPLC Methods for Saffron (Crocus sativus L.). In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Torelli, A.; Marieschi, M.; Bruni, R. Authentication of saffron (Crocus sativus L.) in different processed, retail products by means of SCAR markers. Food Control 2014, 36, 126–131. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Zalacain, A.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Changes in saffron volatile profile according to its storage time. Food Res. Int. 2010, 43, 1329–1334. [Google Scholar] [CrossRef]
- Guclu, G.; Kelebek, H.; Selli, S. Saffron (Crocus sativus L.): Its aroma and key odorants. In Saffron the Age-Old Panacea in a New Light; Sarwat, M., Sumaiya, S., Eds.; Academic Press: London, UK, 2020; pp. 69–82. [Google Scholar]
- Panighel, A.; Maoz, I.; De Rosso, M.; De Marchi, F.; Dalla Vedova, A.; Gardiman, M.; Bavaresco, L.; Flamini, R. Identification of saffron aroma compound β-isophorone (3,5,5-trimethyl-3-cyclohexen-1-one) in some V. vinifera grape varieties. Food Chem. 2014, 145, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Silvis, I.C.J.; Luning, P.A.; Klose, N.; Jansen, M.; van Ruth, S.M. Similarities and differences of the volatile profiles of six spices explored by Proton Transfer Reaction Mass Spectrometry. Food Chem. 2019, 271, 318–327. [Google Scholar] [CrossRef]


| Origin and Type of Extract | Compound Name | Concentration | Technique | Reference | |
|---|---|---|---|---|---|
| Iran (NaCl aqueous extract) | 2,2,6-Trimethyl-1,3-cyclohexadiene-1-carboxaldeyde (Safranal) | 7650 | μg/kg | HS-SPME/GC-MS | [19] |
| 1,5,5-Trimethyl-6-(2-butenylidene)-cyclohexene, (Megastigma-4,6(E),8(E)-triene) | 1113 | ||||
| 5,5-Dimethyl-2-methylene-3-cyclohexene-1-carboxaldehyde | 689.5 | ||||
| 3,7-Dimethyl-octa-1,6-dien-3-ol (Linalool) | 587.5 | ||||
| 2,4-Dimethylbenzenecarboxaldehyde | 130.5 | ||||
| Spain (Aqueous extract) | Safranal | 4510 | μg/kg | HS-SPME/GC-MS | [19] |
| 3,5,5-Trimethyl−3-cyclohexen-1-one (β-isophorone) | 924.5 | ||||
| 5,5-Dimethyl-2-methylene-3-cyclohexene-1-carboxaldehyde | 357.5 | ||||
| Linalool | 120.8 | ||||
| 2,6,6-Trimethyl-2-cyclohexene-1,4-dione (4-ketoisophorone) | 113.8 | ||||
| 5-Hydroxy-2,5-cyclohexadien-1-one-2,4,4-trimethyl-3 carboxaldehyde | 110.2 | ||||
| Greece (Aqueous extract) | Safranal | 6450 | μg/kg | HS-SPME–GC-MS | [19] |
| 2,4,5-Trimethyl-benzaldehyde | 1380 | ||||
| β-Isophorone | 747 | ||||
| Megastigma-4,6(E),8(E)-triene | 715.5 | ||||
| Linalool | 286 | ||||
| 5,5-Dimethyl-2-methylene-3-cyclohexene-1-carboxaldehyde | 285.5 | ||||
| 2,6,6-Trimethyl-2,4-cycloheptadien-1-one (Eucarvone) | 243 | ||||
| Morocco (Aqueous extract) | Safranal | 31,710 | μg/kg | HS-SPME–GC-MS | [19] |
| 5,5-dimethyl-2-methylene-3-cyclohexene-1-carboxaldehyde | 2890 | ||||
| 1-Methyl-3-(1-methylethyl)-benzene (m-Cymene) | 1960 | ||||
| β-isophorone | 779 | ||||
| Dodecene | 492 | ||||
| 3,5,5-Trimethyl-2-cyclohexen-1-ol (Isophorol) | 452 | ||||
| Khorasan, Iran (Methanol-ethy-acetate ultrasonic extraction) | Safranal | 26.29 | % | GC-MS | [76] |
| Bicyclo [3,2,0] hept-2-ene-4-ethoxy-endo | 5.69 | ||||
| 4-Hydroxy-2,6,6-trimethyl-1cyclohexene-1-carboxalde-hyde (β-Homocyclocitral) | 4.44 | ||||
| Linoleic acid | 4.77 | ||||
| Khorasan, Iran. (aqueous-dichloro methane) | Safranal | 2168 | μg/g | SAFE-GC-MS | [79] |
| 2-(1,1-Dimethylethyl) phenol | 1432 | ||||
| 2-Ethoxy-5-methoxybenzaldehyde | 1147 | ||||
| 3,5,5-Trimethyl-2-cyclohexen-1-one (α-Isophorone) | 845 | ||||
| 4-Ketoisophorone | 625 | ||||
| 3,5,5-Trimethyl-1,4cyclohexanedione (Dihydrooxo phorone) | 591 | ||||
| Sicily, Italy (NaCl aqueous extract) | Safranal | 84.38 | % | HS-SPME/GC-MS | [26] |
| 2,6,6-Trimethyl-1,4-cyclohexadiene-1-carboxaldehyde (Safranal isomer) | 5.72 | ||||
| α-Isophorone | 3.96 | ||||
| Furfural | 1.91 | ||||
| 2,4-Dimethylbenzenecarboxaldehyde | 1.2 | ||||
| Eskisehir, Turkey (Aqueous microdistiller) | Safranal | 64.1 | % | MD/GC-MS | [16] |
| α-Isophorone | 10.38 | ||||
| β-Isophorone | 6.4 | ||||
| Hexadecanoic acid | 3.3 | ||||
| 4-(2,2,6-Trimethyl-cyclohexan-1-yl)-3-buten-2-one (β-Ionene) | 1.25 | ||||
| Safranal isomer | 1.13 | ||||
| Safranbolou, Turkey (Aqueous microdistiller) | Safranal | 49.33 | % | MD/GC-MS | [16] |
| α-Isophorone | 16.25 | ||||
| β-Isophorone | 8.38 | ||||
| Hexadecanoic acid | 4.05 | ||||
| β-Ionene | 2.8 | ||||
| 4-Ketoisophorone | 2.5 | ||||
| 3,3,4,5-Tetramethylcyclohexan-1one | 2.28 | ||||
| Eskişehir, Turkey (Aqueous microdistiller) | Safranal | 77.9 | % | MD/GC-MS | [16] |
| α-Isophorone | 13.5 | ||||
| β-Isophorone | 2.2 | ||||
| Safranal isomer | 1.9 | ||||
| 4-Ketoisophorone | 1.2 | ||||
| Qaen, Iran (Methanol-acetonitrile ultrasound-assisted extraction) | Hexadecanoic acid | 25 | % | UAE/DLLME/GC-MS | [83] |
| Safranal | 16.8 | ||||
| Tetradecanoic acid | 14.3 | ||||
| 5,5-Dimethyl-2-methylene-3-cyclohexene-1-carboxaldehyde | 7.5 | ||||
| α-Isophorone | 4.9 | ||||
| 4,4-Dimethyl-2-cyclopenten-1-one | 4.9 | ||||
| Kerman, Iran (Ground agitated with water headspace) | 2(5H)-Furanone | 691.8 | ppb | TR/TOF/MS | [84] |
| Safranal | 610.1 | ||||
| Acetic acid | 566.3 | ||||
| Isobutanal | 451.8 | ||||
| Biogenic aldehyde | 272.6 | ||||
| 4-Ketoisophorone | 161.2 | ||||
| Acetaldehyde | 130.4 | ||||
| α-Isophorone | 106.6 | ||||
| Hangzhou, China (Microwave drying) | Safranal | 20.54 | % | GC-MS | [57] |
| Dihydrooxophorone | 18.98 | ||||
| 4-Ketoisophorone | 16.11 | ||||
| α-Isophorone | 14.57 | ||||
| 2,3-Dimethoxytoluene | 11.68 | ||||
| Hangzhou, China (Oven drying) | Safranal | 15.73 | % | GC-MS | [57] |
| 2,3-Dimethoxytoluene | 13.94 | ||||
| Dihydrooxophorone | 13.18 | ||||
| 4-Ketoisophorone | 11.73 | ||||
| Hangzhou, China (Infrared drying) | Safranal | 22.18 | % | GC-MS | [57] |
| Dihydrooxophorone | 16.88 | ||||
| 4-Ketoisophorone | 16.09 | ||||
| α-Isophorone | 15.68 | ||||
| 2,3-Dimethoxytoluene | 12.83 | ||||
| Hangzhou, China (Freeze drying) | 4-Ketoisophorone | 28.2 | % | GC-MS | [57] |
| Safranal | 10.31 | ||||
| 2,3-Dimethoxytoluene | 7.19 | ||||
| 3-Methyl-2-cyclohexen-1-one | 5.37 | ||||
| α-Isophorone | 5.17 | ||||
| Iran (Microextraction by fiber polyacrylate) | Safranal | 59.32 | % | SPME/GC-MS | [85] |
| Isophorone | 11.48 | ||||
| 4-Ketoisophorone | 10.66 | ||||
| Dihydrooxophorone | 8.35 | ||||
| Iran (Microextraction by fiber polydimethyl-siloxane) | Safranal | 49.64 | % | SPME/GC-MS | [85] |
| Acetic acid | 9.49 | ||||
| 4-Ketoisophorone | 8.72 | ||||
| Isophorone | 8.2 | ||||
| Iran (Microextraction by fiber carboxenpoly-dimethylsiloxan) | Safranal | 55.51 | % | SPME/GC-MS | [85] |
| Isophorone | 14.95 | ||||
| 4-Ketoisophorone | 10.52 | ||||
| Isophorone isomer | 10.05 | ||||
| Compound | Odor Descriptor | Reference |
|---|---|---|
| (E,E)-2,4-Decadienal | Fatty, deep-fried, fried fat | [91,95] |
| (E,E)-2,4-Nonadienal | Rancid oil | |
| (E,Z)-2,6-Nonadienal | Cucumber, sweet | |
| (E)-2-Nonenal | Melon, aldehydic | |
| (E)-Geranyl-acetone | Magnolia, green | [26] |
| (Z)-2-Nonenal | Green, metallic | [91,95] |
| 1-Octen-3-one | Mushroom, earthy | |
| 1-Tetradeacanol | Coconut | [26] |
| 2-Acetyl-1-pyrroline | Nutty, popcorn | [95] |
| 2-Ethyl-hexanol | Rose Green | [26] |
| 2-Hydroxy-3,5,5-trimethylcyclohex-2-ene-1,4-dione | Flower, woody | [26,94] |
| 2-Hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one | Caramel, Saffron, stale, dried hay | [94,95] |
| 2-Hydroxyisophorone | Woody | [26] |
| 2,2-Dimethyl-cyclohexane-1-carboxaldehyde | Flower | [94] |
| 2,3-Butanedione | Butter, cream, cream cheese | [91,95] |
| 2,4-Dimethyl-benzaldehyde | Almond, spice | [26] |
| 2,4,5-Trimethyl-benzaldehyde | Floral, violets | |
| 2,5-Dimethyl-benzaldehyde | Spice | |
| Isomer of safranal | Caramel | [94] |
| 3-[(E)-But-1-enyl]-2,4,4-trimethyl-cyclohex-2-enol | Citrus | |
| 3-Hexen-2-one | Grass, geranium | [91,95] |
| 3-Methylbutanoic acid | Cheese, rotten, sour, dried fruit | [95] |
| 3-(Methylthio) propanal | Baked potato | |
| 4-Hydroxy-2,6,6-trimethyl-3-oxocyclohex-1-en-1carboxaldehyde | Citrus, vegetable | [94] |
| HTCC | Green, Spicy, saffron, green | [26,79,94,95] |
| 4-Hydroxy-2,6,6-trimethyl-3-oxocyclohexa-1,4-diene-1carboxaldehyde | Fresh cut grass | [94] |
| 4-Hydroxy-3,5,5-trimethyl-2-cyclohex-1-one | Flower, vegetable | |
| 4-ketoisophorone | Saffron, vegetable, musty, woody | [26,79,94,95] |
| 6-Methyl-5-hepten-2-one | Clove, spicy, green, citrus like | [26,91,95] |
| Acetic acid | Vinegar, acidic | [91,95] |
| Butyric acid | Cheese | |
| Carvone | Mint | [26] |
| Dihydro-β-ionone | Earty, woody | |
| Dihydrooxophorone | Vegetable, saffron | [26,79,94,95] |
| Eucalyptol | Eucalyptus | [26] |
| Furaneol | Cotton Candy, strawberries | [91,95] |
| Furfural | Bread, almond | [26] |
| Heptanal | Fat, rancid | |
| Hexanal | Grass | [91,95] |
| Homofuraneol | Cotton candy | |
| Isophorone | Saffron, herbal, flower peppemint, woody, hay | [26,79,91,94,95] |
| Isophorone-4-methylene | Citrus | [94] |
| Isovalerianic acid | Cheese | [91] |
| Limonene | Green, citrus | [26] |
| Linalool | Floral, honeysuckle | [79,95] |
| Octanal | Lemon, fat, green | [26,91,95] |
| Oxo-β-cyclocitral | Caramel | [94] |
| Phenol | Phenol | [26] |
| Phorone | Geranium | |
| Rose oxide | Rose, flower | |
| Safranal | Saffron, tea, herbal | [26,79,91,94] |
| Teroinolene | Fresh, pine | [26] |
| α-Cyclocitral | Green | |
| α-Pinene | Pine, terpenin | |
| α-Terpinene | Lemon | |
| β-Cyclocitral | Mint | |
| β-Ionone | Floral | |
| -Phenylethanol | Roses, floral, flower | [79,91,94,95] |
| -Pinene | Pine, resin | [26] |
| -Terpinene | Turpenin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Avila-Sosa, R. The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). Molecules 2021, 26, 6954. https://doi.org/10.3390/molecules26226954
Cid-Pérez TS, Nevárez-Moorillón GV, Ochoa-Velasco CE, Navarro-Cruz AR, Hernández-Carranza P, Avila-Sosa R. The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). Molecules. 2021; 26(22):6954. https://doi.org/10.3390/molecules26226954
Chicago/Turabian StyleCid-Pérez, Teresa Soledad, Guadalupe Virginia Nevárez-Moorillón, Carlos Enrique Ochoa-Velasco, Addí Rhode Navarro-Cruz, Paola Hernández-Carranza, and Raúl Avila-Sosa. 2021. "The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus)" Molecules 26, no. 22: 6954. https://doi.org/10.3390/molecules26226954
APA StyleCid-Pérez, T. S., Nevárez-Moorillón, G. V., Ochoa-Velasco, C. E., Navarro-Cruz, A. R., Hernández-Carranza, P., & Avila-Sosa, R. (2021). The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). Molecules, 26(22), 6954. https://doi.org/10.3390/molecules26226954









