Towards Understanding the Involvement of H+-ATPase in Programmed Cell Death of Psammosilene tunicoides after Oxalic Acid Application
Abstract
:1. Introduction
2. Results
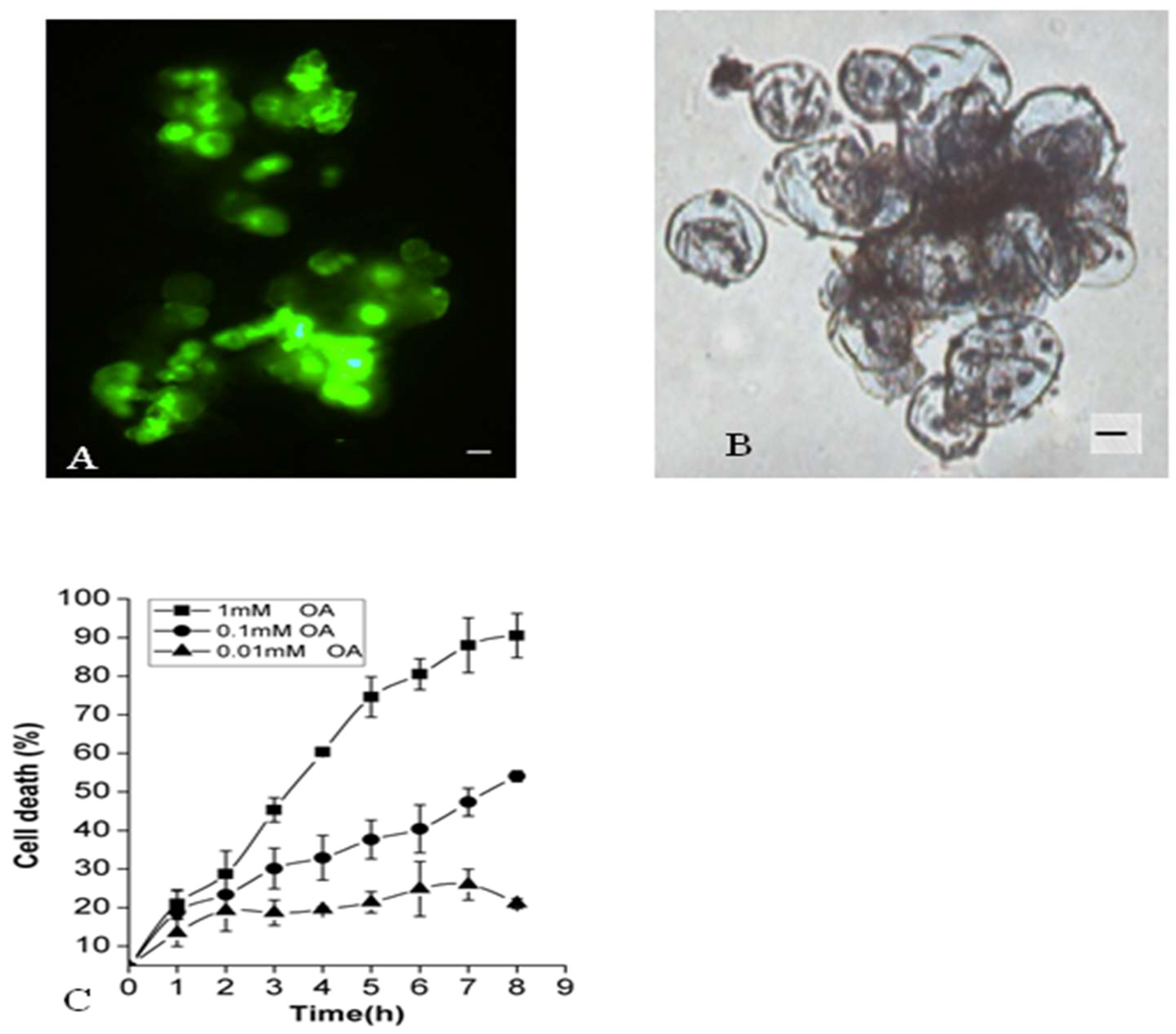
2.1. Effects of OA on Suspension Cell Viability of P. tunicoides
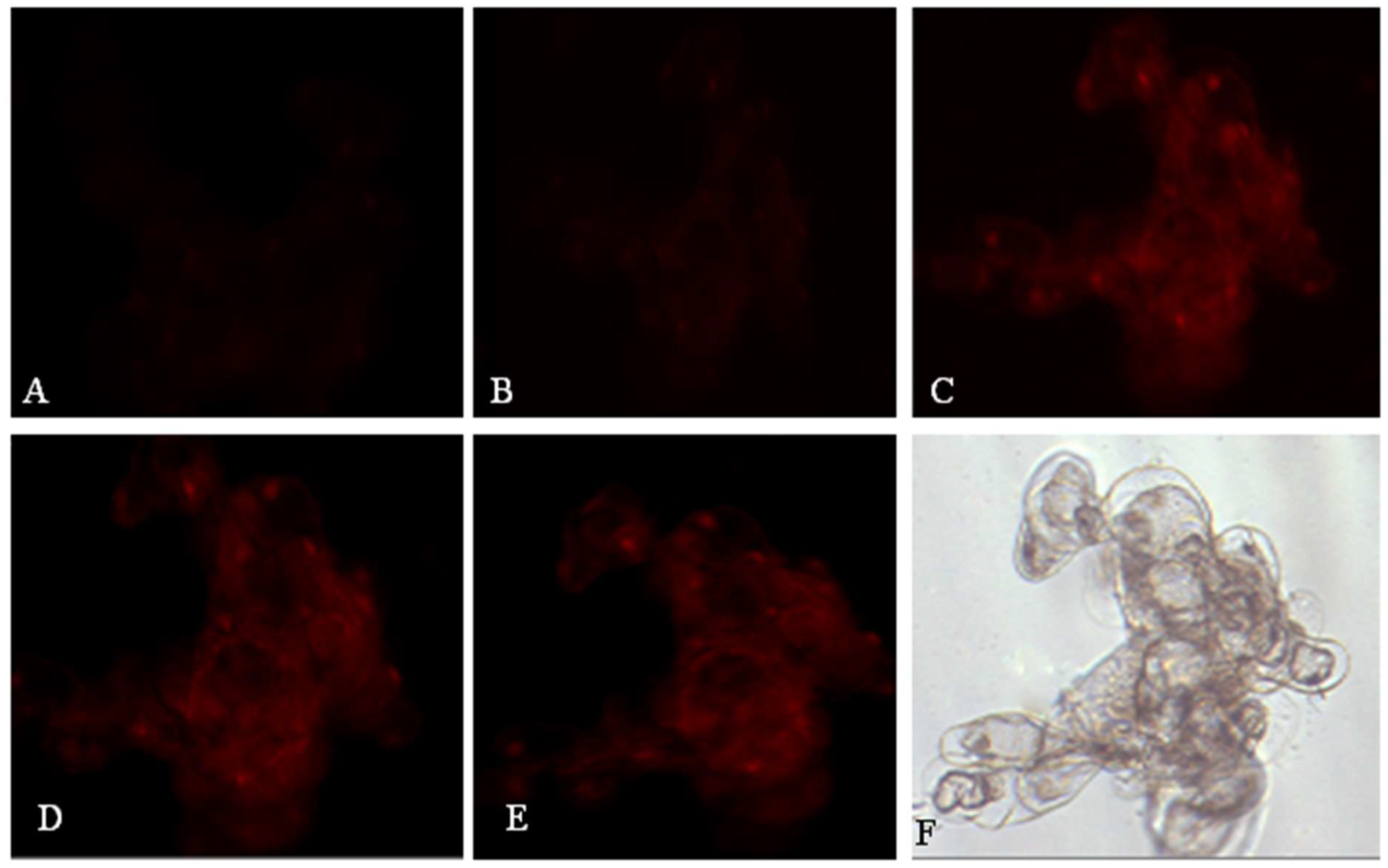
2.2. Effects of OA on Respiratory Electron Transporter Chains
2.3. Effects of OA on Nuclear Membrane Integrity
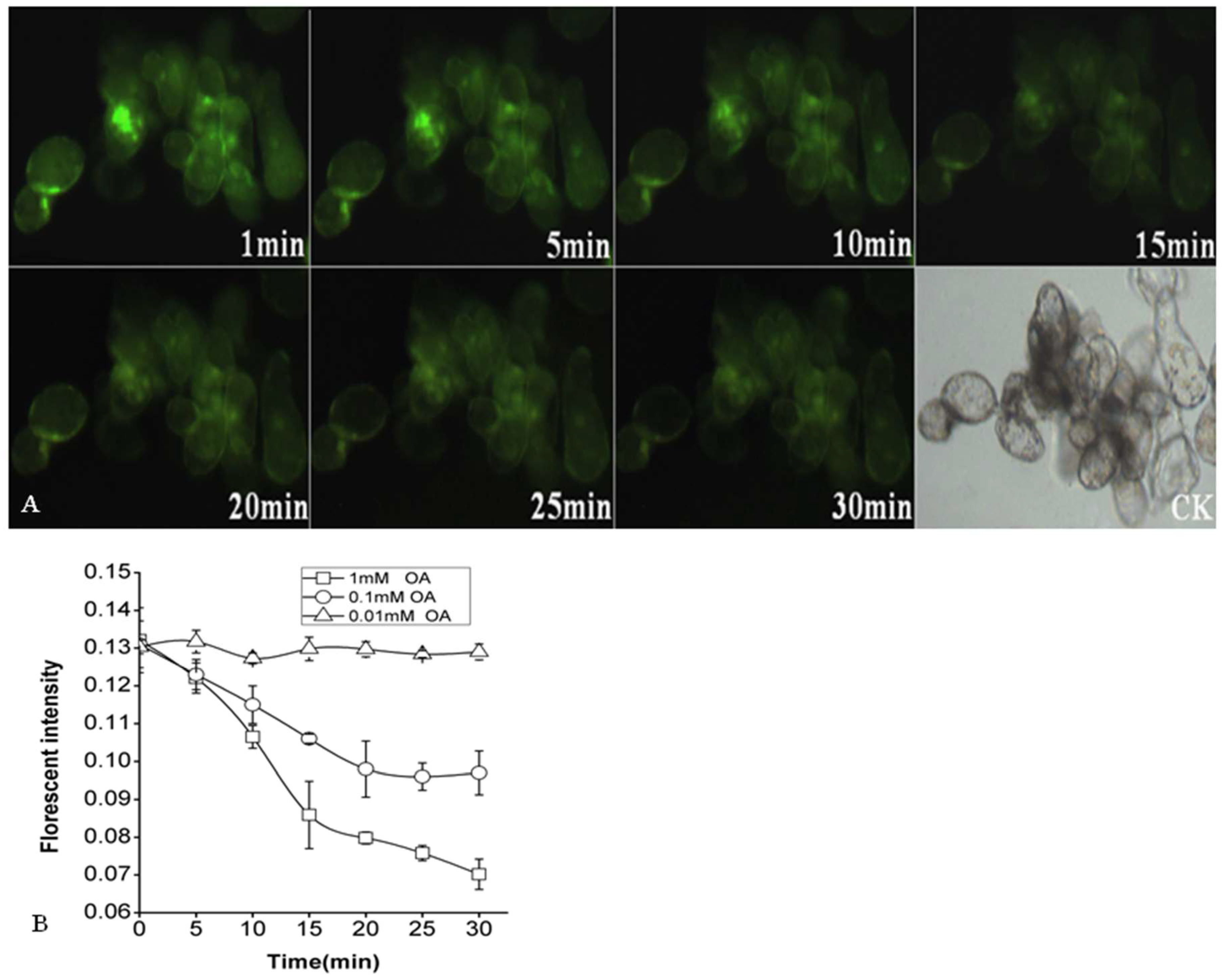
2.4. Effects of OA on Cytoplasmic pH of P. tunicoides
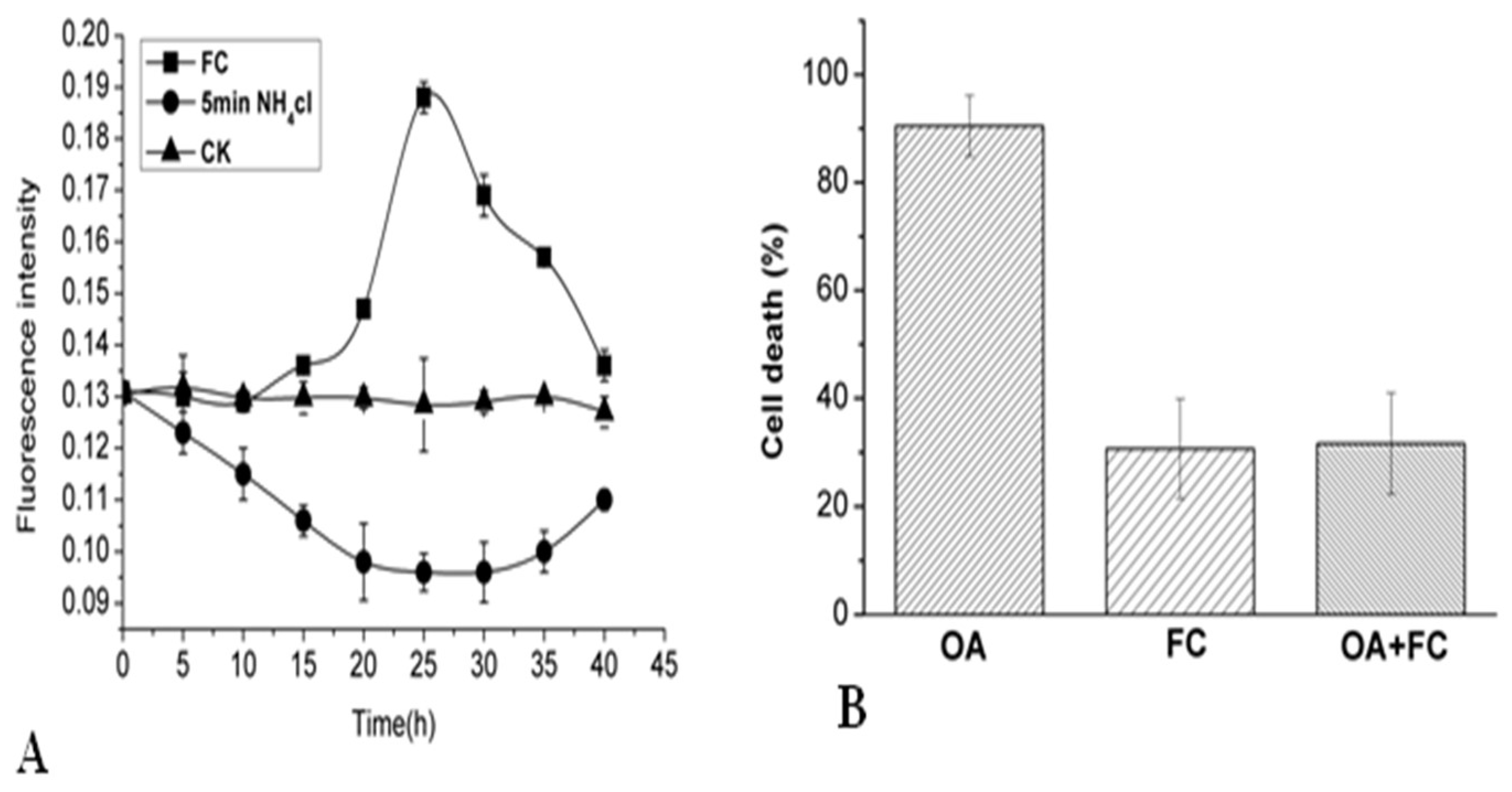
2.5. Effects of NH4Cl and Fusicoccinon on Cytoplasmic pH and Cell Viability of P. tunicoides
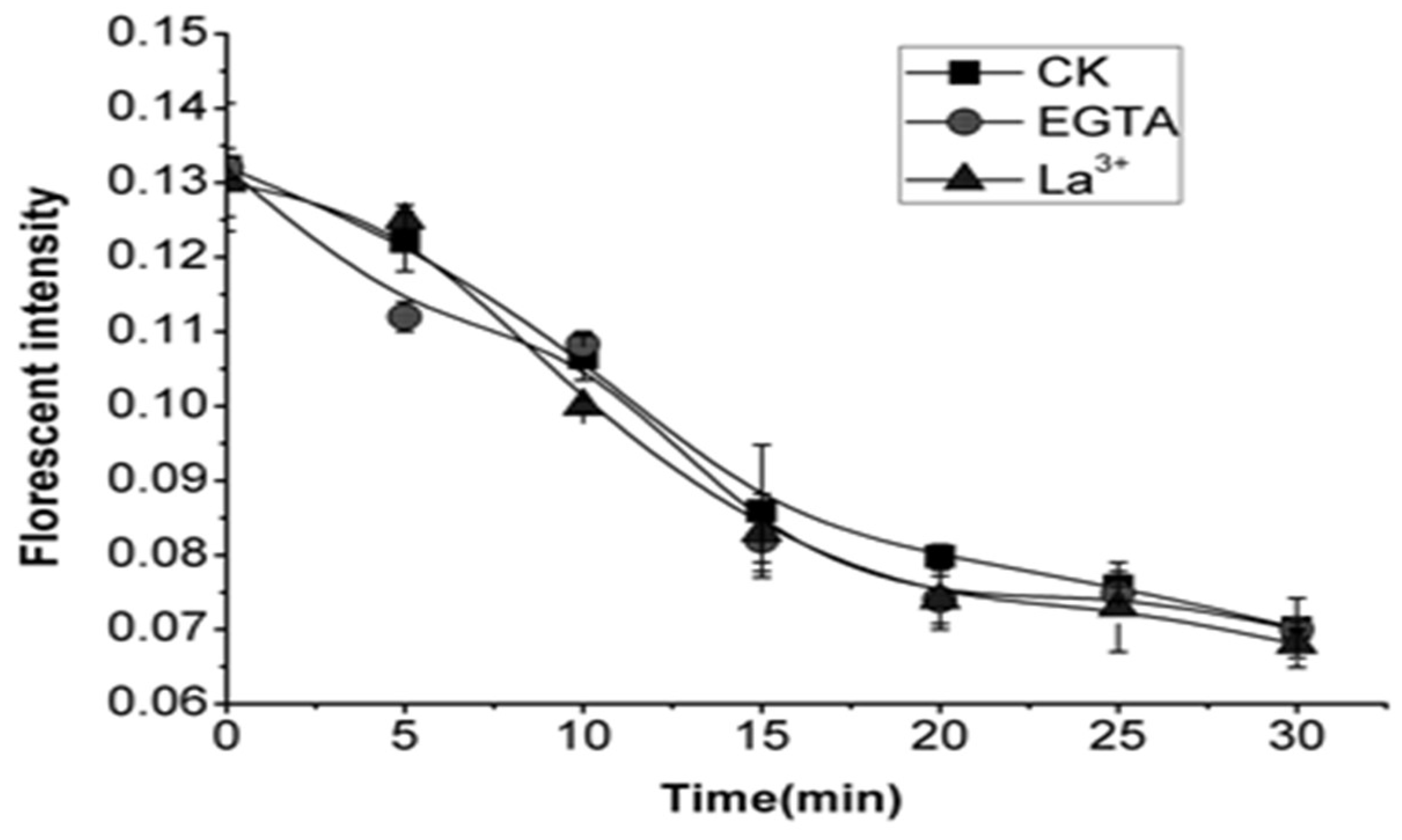
2.6. Effects of EGTA and La3+ on Cytoplasmic pH of OA-Treated Cells
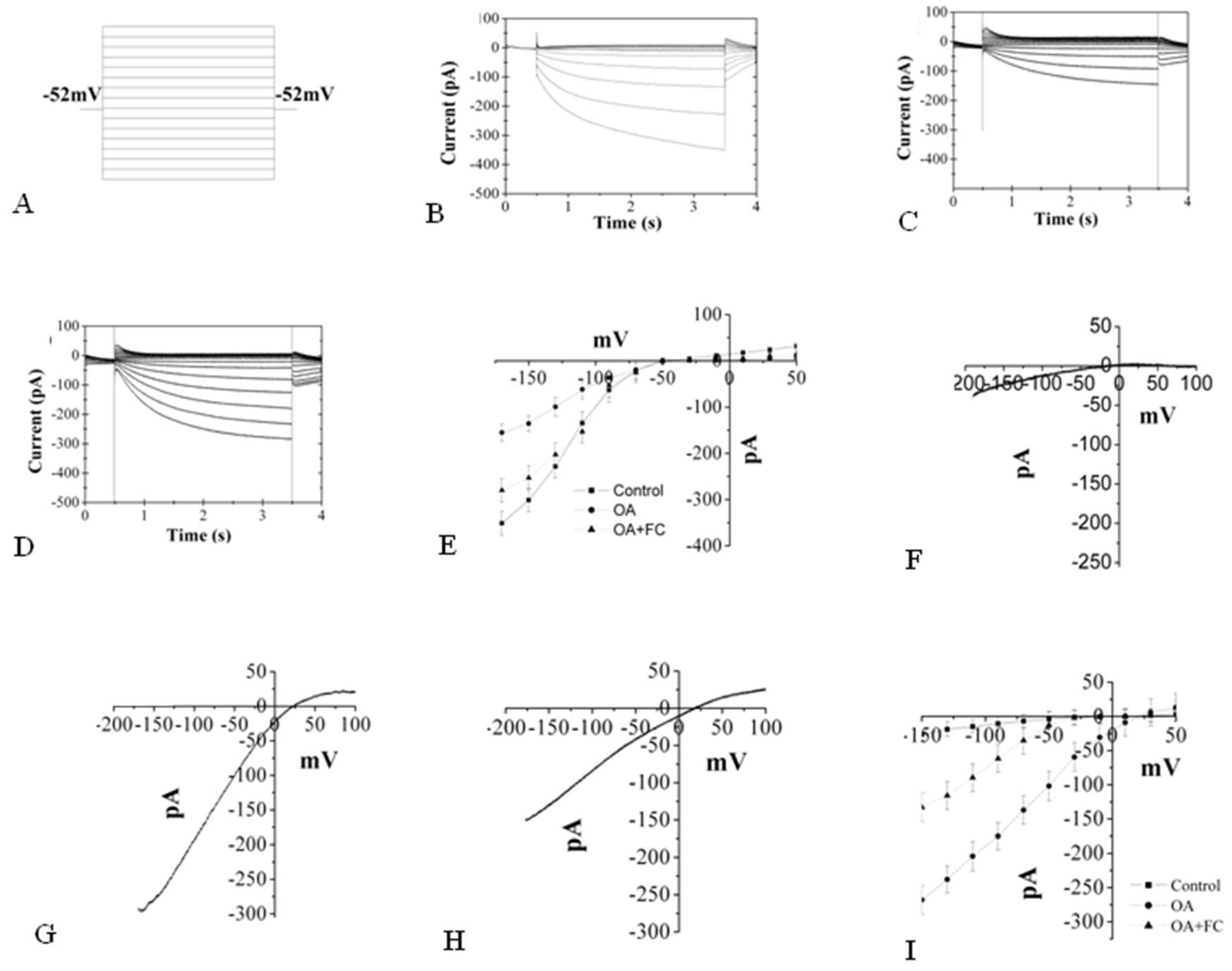
2.7. The Changes Induced by OA in K+ and Ca2+ Inward
3. Discussion
4. Materials and Methods
4.1. Chemical Materials
4.2. Plant Materials
4.3. Determination of Cell Viability and Death
4.4. Rd 123 and PI Staining Procedures
4.5. Intracellular pH (pHi) Measurement
4.6. Patch Clamp and Data Acquisition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vidhyasekaran, P. Manipulation of Calcium Ion Influx—Mediated Immune Signaling Systems for Crop Disease Management. In Plant Innate Immunity Signals and Signaling Systems; Springer: New York, NY, USA, 2020; pp. 23–49. [Google Scholar]
- Sun, G.; Feng, C.; Zhang, A.; Zhang, Y.; Chang, D.; Wang, Y.; Ma, Q. The dual role of oxalic acid on the resistance of tomato against Botrytis cinerea. World J. Microbiol. Biotechnol. 2019, 35, 36. [Google Scholar] [CrossRef]
- Monazzah, M.; Rabiei, Z.; Enferadi, S.T. The effect of oxalic acid, the pathogenicity factor of Sclerotinia Sclerotiorum on the two susceptible and moderately resistant lines of sunflower. Iran. J. Biotechnol. 2018, 16, e1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, M.A.; Cheng, Y.; Aslam, M.; Jakada, B.H.; Wai, M.H.; Ye, K.; He, X.; Luo, T.; Ye, L.; Dong, C. ROS and Oxidative Response Systems in Plants Under Biotic and Abiotic Stresses: Revisiting the Crucial Role of Phosphite Triggered Plants Defense Response. Front. Microbiol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Mohammed, H.A. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Zhang, Z.; Xi, Y.; Han, H.; Lan, F.; Zhang, B.; Wang-Pruski, G. Effects of potassium phosphite on biochemical contents and enzymatic activities of Chinese potatoes inoculated by Phytophthora infestans. Appl. Ecol. Environ. Res. 2019, 17, 4499–4514. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest Treatment with Oxalic Acid Improves Postharvest Storage of Lemon Fruit by Stimulation of the Antioxidant System and Phenolic Content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Effect of postharvest oxalic acid application on enzymatic browning and quality of lotus (Nelumbo nucifera Gaertn.) root slices. Food Chem. 2020, 312, 126051. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Han, X.; Zhang, Z.; Xi, Y.; Boorboori, M.; Wang-Pruski, G. Phosphite application alleviates Pythophthora infestans by modulation of photosynthetic and physio-biochemical metabolites in potato leaves. Pathogens 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Yu, J.; Shen, L.; Yu, Z.; Yu, M.; Du, Y.; Zhang, R.; Song, T.; Yin, X.; Zhou, Y. Enhanced resistance to rice blast and sheath blight in rice (Oryza sativa L.) by expressing the oxalate decarboxylase protein Bacisubin from Bacillus subtilis. Plant Sci. 2017, 265, 51–60. [Google Scholar] [CrossRef]
- Williams, B.; Kabbage, M.; Kim, H.-J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef] [Green Version]
- Fagundes-Nacarath, I.; Debona, D.; Rodrigues, F. Oxalic acid-mediated biochemical and physiological changes in the common bean-Sclerotinia sclerotiorum interaction. Plant Physiol. Biochem. 2018, 129, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ji, R.; Guo, X.; Foster, S.J.; Chen, H.; Dong, C.; Liu, Y.; Hu, Q.; Liu, S. Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus). Planta 2008, 228, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nacarath, I.R.F.F. Biochemical and Physiological Responses of Common Bean Infected by Sclerotinia sclerotiorum Mediated by Oxalic Acid and Phosphites and Diagrammatic Scale for Assessment of White Mold Severity. Fed. Univ. Viçosa 2018, 153, 27–45. [Google Scholar]
- Ravi, K.; Pareek, S.; Kaushik, R.; Ameta, K. Effect of Exogenous Application of Oxalic Acid on Physiological and Biochemical Characteristics in ‘Gola’Ber (Ziziphus mauritiana Lamk.) Fruit during Storage. Res. J. Agric. Sci. 2017, 8, 1249–1252. [Google Scholar]
- Errakhi, R.; Meimoun, P.; Lehner, A.; Vidal, G.; Briand, J.; Corbineau, F.; Rona, J.-P.; Bouteau, F. Anion channel activity is necessary to induce ethylene synthesis and programmed cell death in response to oxalic acid. J. Exp. Bot. 2008, 59, 3121–3129. [Google Scholar] [CrossRef]
- Borrelli, N.; Benvenuto, M.L.; Osterrieth, M. Calcium oxalate crystal production and density at different phenological stages of soybean plants (Glycine max L.) from the southeast of the Pampean Plain, Argentina. Plant Biol. 2016, 18, 1016–1024. [Google Scholar] [CrossRef]
- Sadak, M.S.; Orabi, S.A. Improving thermo tolerance of wheat plant by foliar application of citric acid or oxalic acid. Int. J. ChemTech Res. 2015, 8, 333–345. [Google Scholar]
- Li, P.; Yin, F.; Song, L.; Zheng, X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016, 202, 125–132. [Google Scholar] [CrossRef]
- Gębura, J.; Winiarczyk, K. A study on calcium oxalate crystals in Tinantia anomala (Commelinaceae) with special reference to ultrastructural changes during anther development. J. Plant Res. 2016, 129, 685–695. [Google Scholar] [CrossRef]
- Mazen, A.M.; Zhang, D.; Franceschi, V.R. Calcium oxalate formation in Lemna minor: Physiological and ultrastructural aspects of high capacity calcium sequestration. New Phytol. 2004, 161, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Chairiyah, N.; Harijati, N.; Mastuti, R. The Dynamic of Calcium Oxalate (CaOx) in Porang Corms (Amorphophallus muelleri Blume) at Different Harvest Time. J. Trop. Life Sci. 2021, 11, 33–44. [Google Scholar] [CrossRef]
- Sze, H.; Li, X.; Palmgren, M.G. Energization of plant cell membranes by H+-pumping ATPases: Regulation and biosynthesis. Plant Cell 1999, 11, 677–689. [Google Scholar] [PubMed] [Green Version]
- Gonugunta, V.K.; Srivastava, N.; Raghavendra, A.S. Cytosolic alkalinization is a common and early messenger preceding the production of ROS and NO during stomatal closure by variable signals, including abscisic acid, methyl jasmonate and chitosan. Plant Signal. Behav. 2009, 4, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staal, M.; De Cnodder, T.; Simon, D.; Vandenbussche, F.; Van Der Straeten, D.; Verbelen, J.-P.; Elzenga, T.; Vissenberg, K. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Plant Physiol. 2011, 155, 2049–2055. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Kihara, Y.; Hamano, F.; Yanagida, K.; Morishita, Y.; Kunita, A.; Yamori, T.; Fukayama, M.; Aburatani, H.; Shimizu, T. The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc. Natl. Acad. Sci. USA 2010, 107, 17309–17314. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, J.; Kreitzer, M.A.; Alford, S.; Qian, H.; Tchernookova, B.K.; Naylor, E.R.; Malchow, R.P. Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods. J. Neurophysiol. 2012, 107, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Blatt, M.R.; Chen, Z.-H. Ion transport at the plant plasma membrane. eLS 2018, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Ramirez, J.; Reboutier, D.; Brault, M.; Trouverie, J.; Pennarun, A.-M.; Amiar, Z.; Biligui, B.; Galagovsky, L.; Rona, J.-P. Brassinosteroids regulate plasma membrane anion channels in addition to proton pumps during expansion of Arabidopsis thaliana cells. Plant Cell Physiol. 2005, 46, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Edris, S.; Abo-Aba, S.; Algandaby, M.M.; Makki, R.M.; Qari, S.H.; Al-Quwaie, D.A.; Jansen, R.K.; Bahieldin, A. Differential expression of genes contributing to PCD triggered by exogenous oxalic acid in tomato (Solanum lycopersicum). Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2021, 155, 871–877. [Google Scholar] [CrossRef]
- González, A.M.; Díaz, S.; Gallego, A.; Gutiérrez, J.C. Programmed nuclear death and other apoptotic-like phenomena in ciliated protozoa. In Programmed Cell Death in Protozoa; Springer: New York, NY, USA, 2008; pp. 143–159. [Google Scholar]
- Wright, H.; van Doorn, W.G.; Gunawardena, A.H. In vivo study of developmental programmed cell death using the lace plant (Aponogeton madagascariensis; Aponogetonaceae) leaf model system. Am. J. Bot. 2009, 96, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.-K. Ultrastructural study of programmed cell death of tapetum in Panax ginseng. J. Life Sci. 2009, 19, 1016–1022. [Google Scholar]
- Gunawardena, A.H.; Pearce, D.M.; Jackson, M.B.; Hawes, C.R.; Evans, D.E. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 2001, 212, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.A.; de Pinto, M.C.; Valenti, D.; Passarella, S.; Marra, E.; De Gara, L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 2004, 134, 1100–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.D.; Bazan, I.; Zhang, Y.; Fares, W.H.; Lee, P.J. Mitochondrial dysfunction and pulmonary hypertension: Cause, effect, or both. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 314, L782–L796. [Google Scholar] [CrossRef] [Green Version]
- Grabski, S.; Xie, X.G.; Holland, J.F.; Schindler, M. Lipids trigger changes in the elasticity of the cytoskeleton in plant cells: A cell optical displacement assay for live cell measurements. J. Cell Biol. 1994, 126, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Kumar, R.; Prakash, O.; Kumar, R.; Pant, A. Chemical Signal Dissemination Through Infochemicals. In Microbial Metatranscriptomics Belowground; Springer: New York, NY, USA, 2021; pp. 91–108. [Google Scholar]
- Boller, T. Chemoperception of microbial signals in plant cells. Annu. Rev. Plant Biol. 1995, 46, 189–214. [Google Scholar] [CrossRef]
- Alkan, N.; Fluhr, R.; Sherman, A.; Prusky, D. Role of ammonia secretion and pH modulation on pathogenicity of Colletotrichum coccodes on tomato fruit. Mol. Plant-Microbe Interact. 2008, 21, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, S.; Philosoph-Hadas, S.; Riov, J.; Belausov, E.; Kochanek, B.; Tucker, M.L.; Meir, S. Abscission of flowers and floral organs is closely associated with alkalization of the cytosol in abscission zone cells. J. Exp. Bot. 2015, 66, 1355–1368. [Google Scholar] [CrossRef] [Green Version]
- Volk, G.M.; Goss, L.J.; Franceschi, V.R. Calcium channels are involved in calcium oxalate crystal formation in specialized cells of Pistia stratiotes L. Ann. Bot. 2004, 93, 741–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çetinbaş-Genç, A.; Cai, G.; Del Duca, S. Treatment with spermidine alleviates the effects of concomitantly applied cold stress by modulating Ca2+, pH and ROS homeostasis, actin filament organization and cell wall deposition in pollen tubes of Camellia sinensis. Plant Physiol. Biochem. 2020, 156, 578–590. [Google Scholar] [CrossRef]
- Fricker, M.D.; White, N.; Obermeyer, G. pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. J. Cell Sci. 1997, 110, 1729–1740. [Google Scholar] [CrossRef]
- Locato, V.; De Gara, L. Programmed cell death in plants: An overview. Plant Program. Cell Death 2018, 1743, 1–8. [Google Scholar]
- Sychta, K.; Słomka, A.; Kuta, E. Insights into plant programmed cell death induced by heavy metals—Discovering a terra incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Zancani, M.; Peresson, C.; Petrussa, E.; Casolo, V.; Krajňáková, J.; Patui, S.; Braidot, E.; Macrì, F. Plant mitochondrial pathway leading to programmed cell death. Physiol. Plant. 2007, 129, 242–252. [Google Scholar] [CrossRef]
- Valandro, F.; Menguer, P.K.; Cabreira-Cagliari, C.; Margis-Pinheiro, M.; Cagliari, A. Programmed cell death (PCD) control in plants: New insights from the Arabidopsis thaliana deathosome. Plant Sci. 2020, 299, 110603. [Google Scholar] [CrossRef]
- Madungwe, N.B.; Feng, Y.; Lie, M.; Tombo, N.; Liu, L.; Kaya, F.; Bopassa, J.C. Mitochondrial inner membrane protein (mitofilin) knockdown induces cell death by apoptosis via an AIF-PARP-dependent mechanism and cell cycle arrest. Am. J. Physiol.-Cell Physiol. 2018, 315, C28–C43. [Google Scholar] [CrossRef]
- Westphal, L.; Strehmel, N.; Eschen-Lippold, L.; Bauer, N.; Westermann, B.; Rosahl, S.; Scheel, D.; Lee, J. pH effects on plant calcium fluxes: Lessons from acidification-mediated calcium elevation induced by the γ-glutamyl-leucine dipeptide identified from Phytophthora infestans. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Afzal, M.R.; Zhang, M.; Jin, H.; Wang, G.; Zhang, M.; Ding, M.; Raza, S.; Hu, J.; Zeng, H.; Gao, X. Post-translational regulation of plasma membrane H+-ATPase is involved in the release of biological nitrification inhibitors from sorghum roots. Plant Soil 2020, 450, 357–372. [Google Scholar] [CrossRef]
- Moeder, W.; Phan, V.; Yoshioka, K. Ca2+ to the rescue–Ca2+ channels and signaling in plant immunity. Plant Sci. 2019, 279, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.M.; Baker, C.J. Role of the plasmalemma H+-ATPase in Pseudomonas syringae-induced K+/H+ exchange in suspension-cultured tobacco cells. Plant Physiol. 1989, 91, 298–303. [Google Scholar] [CrossRef]
- Würtele, M.; Jelich-Ottmann, C.; Wittinghofer, A.; Oecking, C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J. 2003, 22, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Nishimura, M.; Shimazaki, K. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 1995, 7, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahjib-Ul-Arif, M.; Munemasa, S.; Nakamura, T.; Nakamura, Y.; Murata, Y. Modulation of frequency and height of cytosolic calcium spikes by plasma membrane anion channels in guard cells. Biosci. Biotechnol. Biochem. 2021, 85, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Naumova, N.; Šachl, R. Regulation of cell death by mitochondrial transport systems of calcium and Bcl-2 proteins. Membranes 2020, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pandey, S.; Wang, X.Q.; Coursol, S.A.; Assmann, S.M. Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytol. 2002, 153, 517–526. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Fan, L.-M.; Zhang, W.-Z.; Zhang, W.; Wu, W.-H. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004, 136, 3892–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Mohammadi, M.A.; Qin, Y.; Zhang, Z. Towards Understanding the Involvement of H+-ATPase in Programmed Cell Death of Psammosilene tunicoides after Oxalic Acid Application. Molecules 2021, 26, 6957. https://doi.org/10.3390/molecules26226957
Jiang X, Mohammadi MA, Qin Y, Zhang Z. Towards Understanding the Involvement of H+-ATPase in Programmed Cell Death of Psammosilene tunicoides after Oxalic Acid Application. Molecules. 2021; 26(22):6957. https://doi.org/10.3390/molecules26226957
Chicago/Turabian StyleJiang, Xinyu, Mohammad Aqa Mohammadi, Yuan Qin, and Zongshen Zhang. 2021. "Towards Understanding the Involvement of H+-ATPase in Programmed Cell Death of Psammosilene tunicoides after Oxalic Acid Application" Molecules 26, no. 22: 6957. https://doi.org/10.3390/molecules26226957







