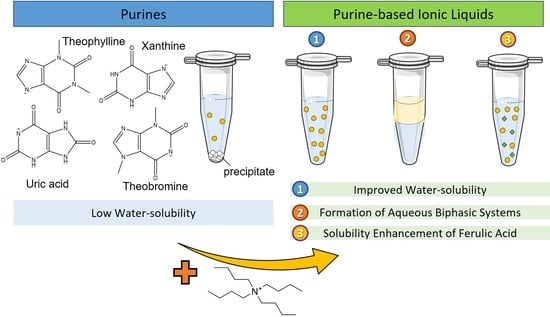
Synthesis of Purine-Based Ionic Liquids and Their Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Characterization of Purine-Based ILs
2.3. Melting and Degradation Temperatures
2.4. Solubility Assays
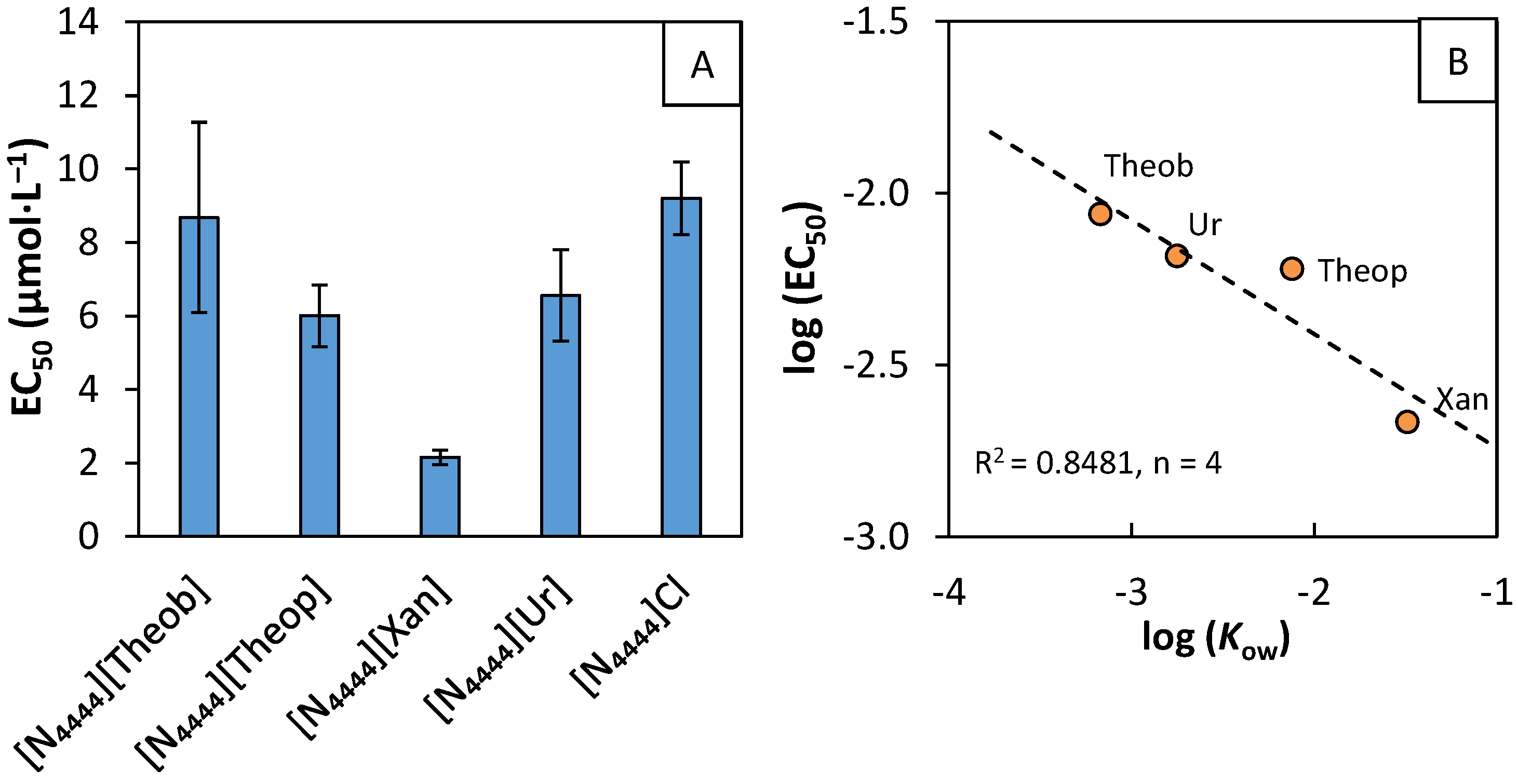
2.5. Microalgae Ecotoxicity Assays
2.6. Aqueous Biphasic Systems
2.7. COSMO-RS
3. Results and Discussion
3.1. Synthesis and Characterization of Purine-Based ILs
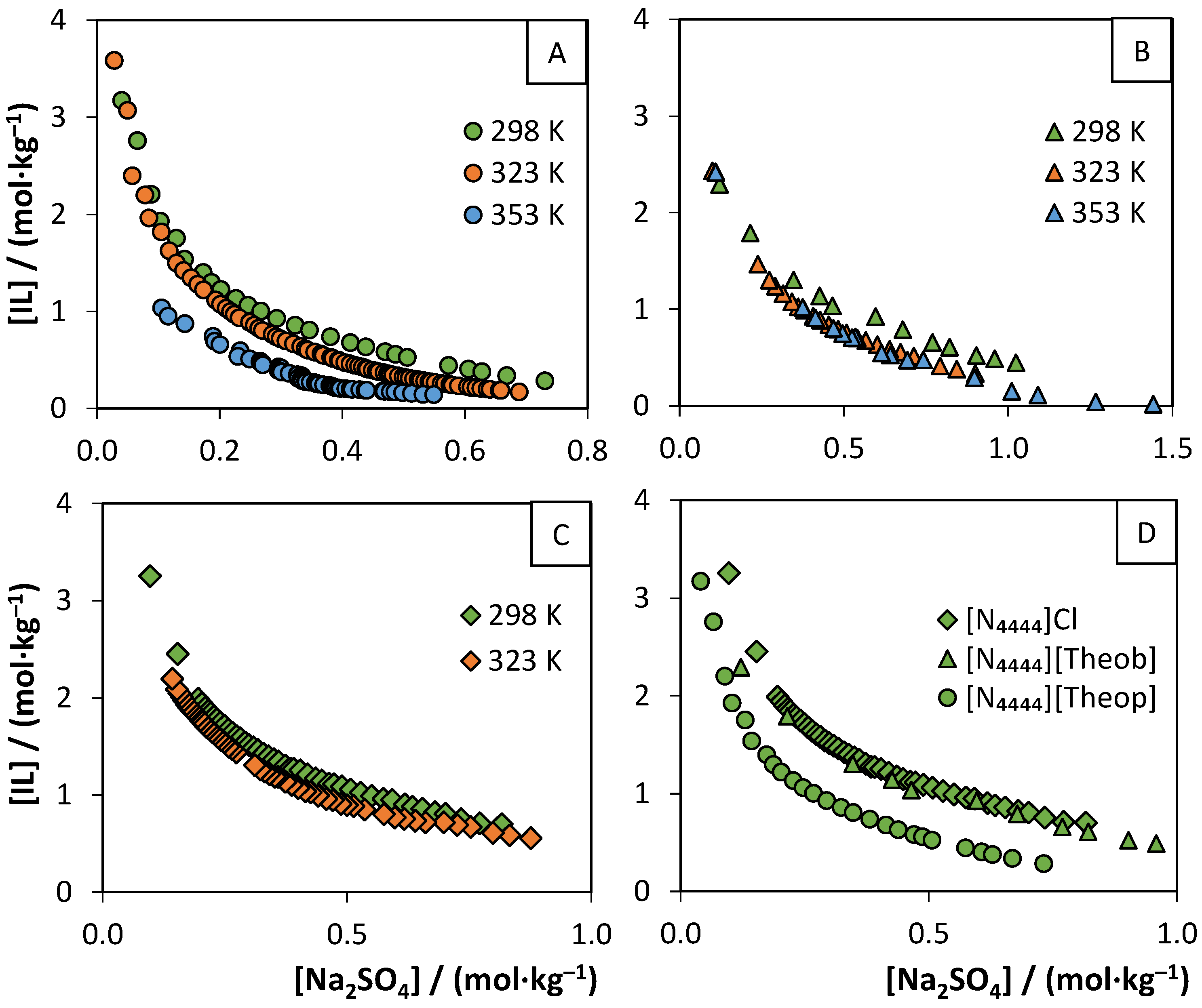
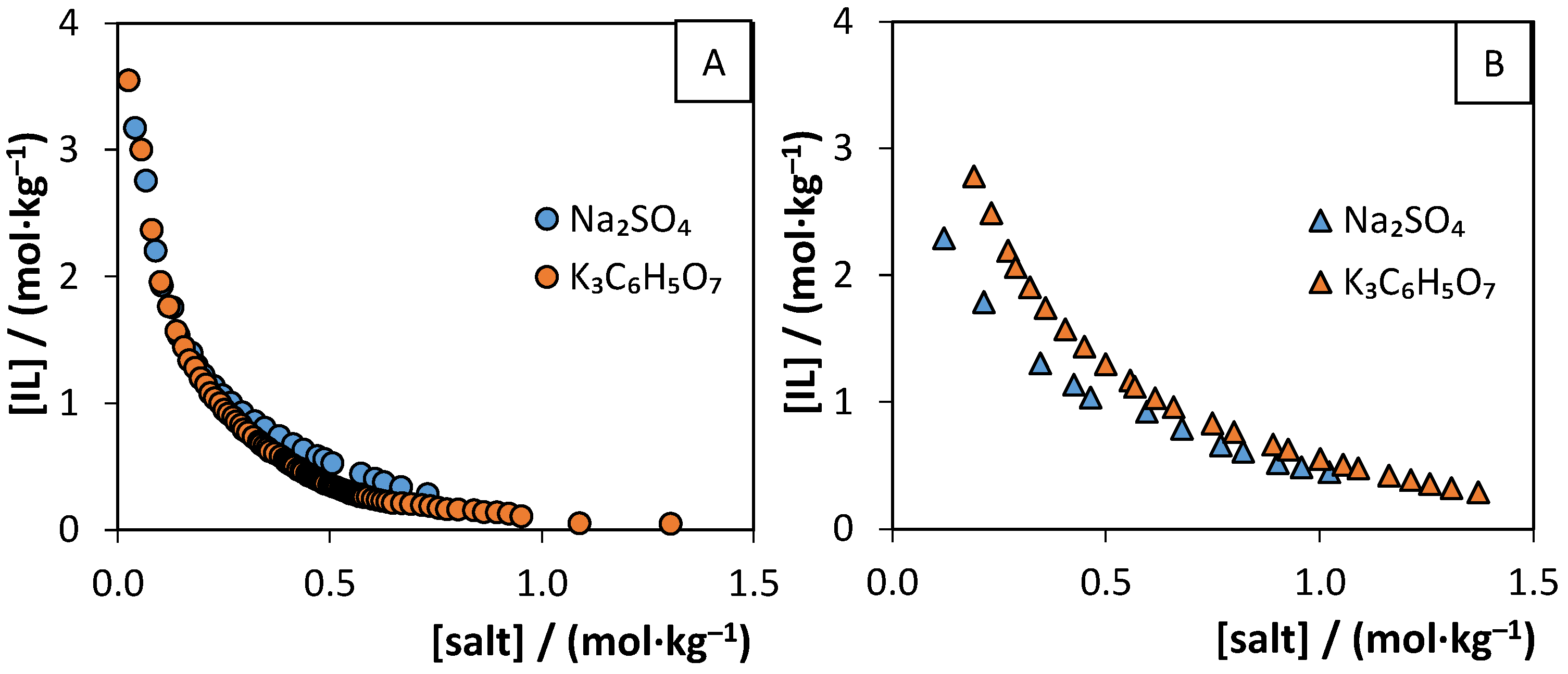
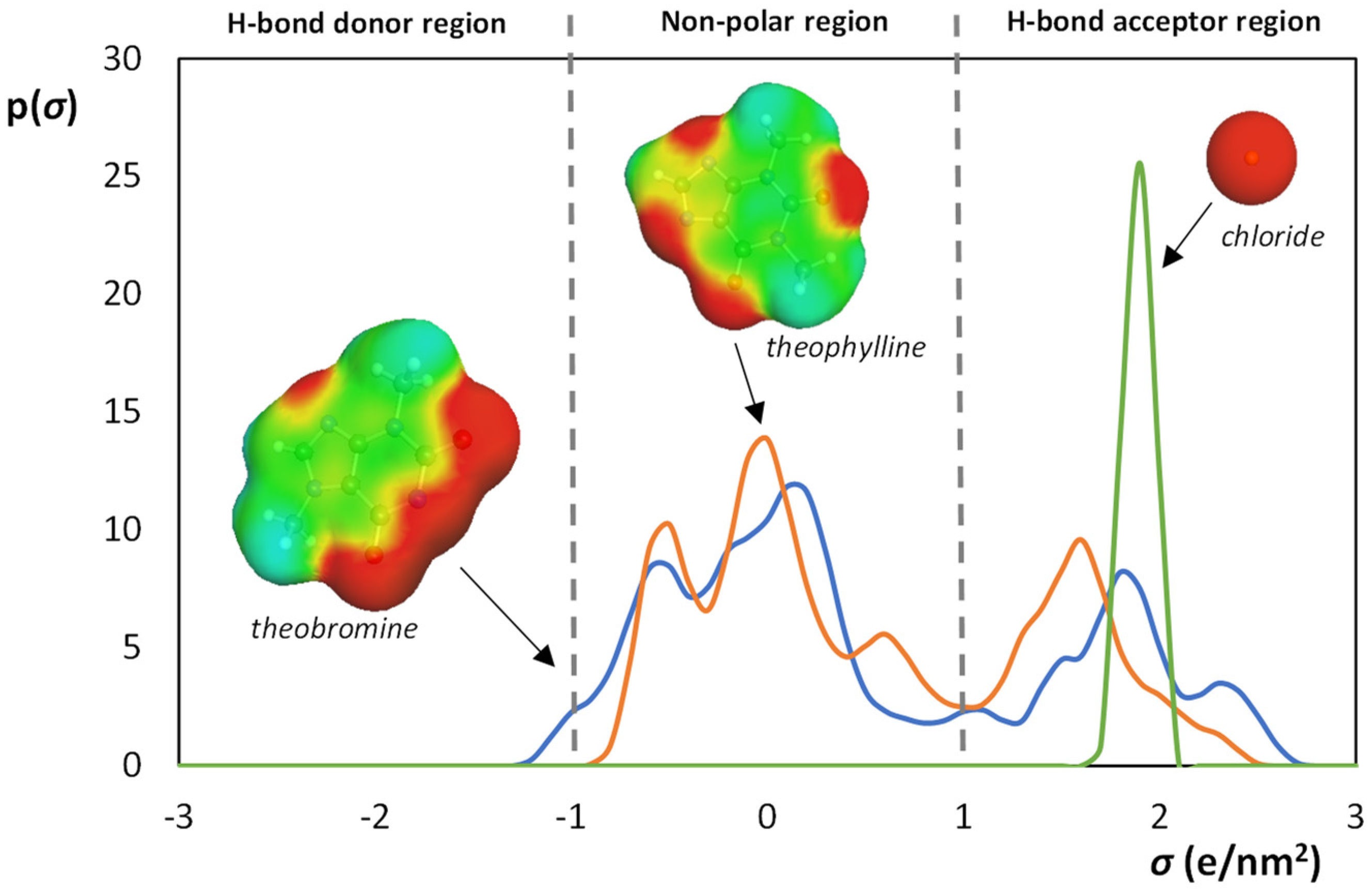
3.2. Aqueous Biphasic Systems Formation
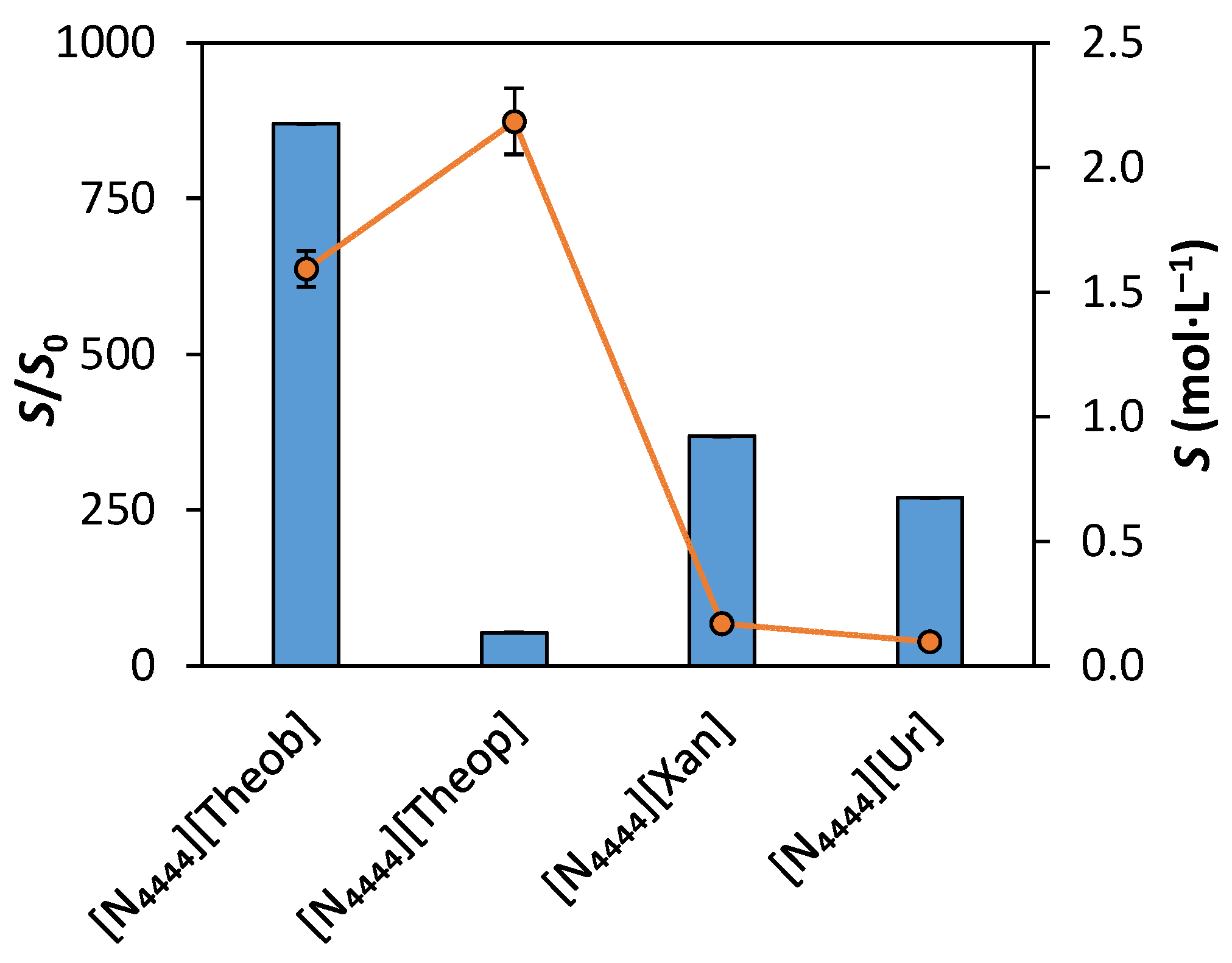
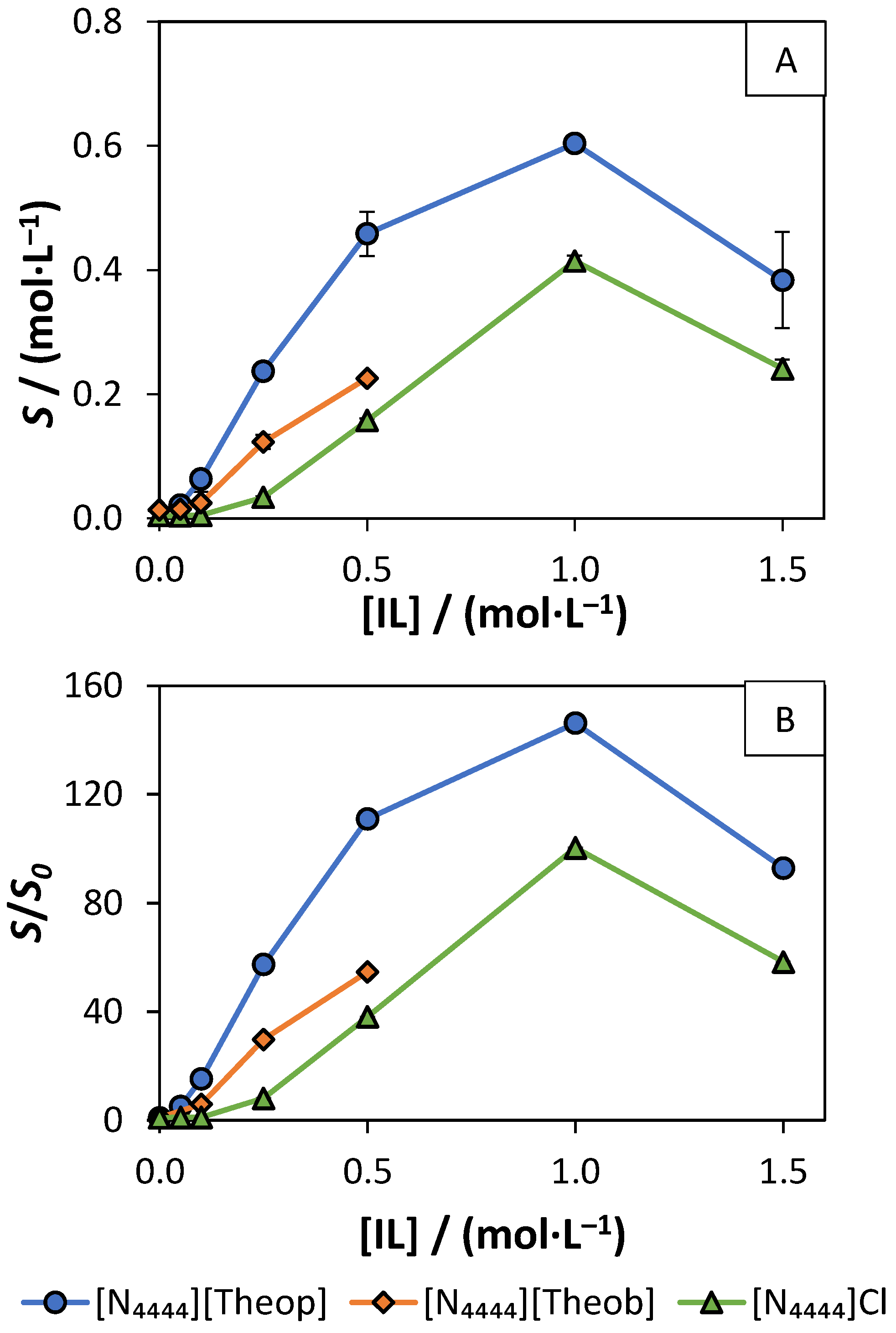
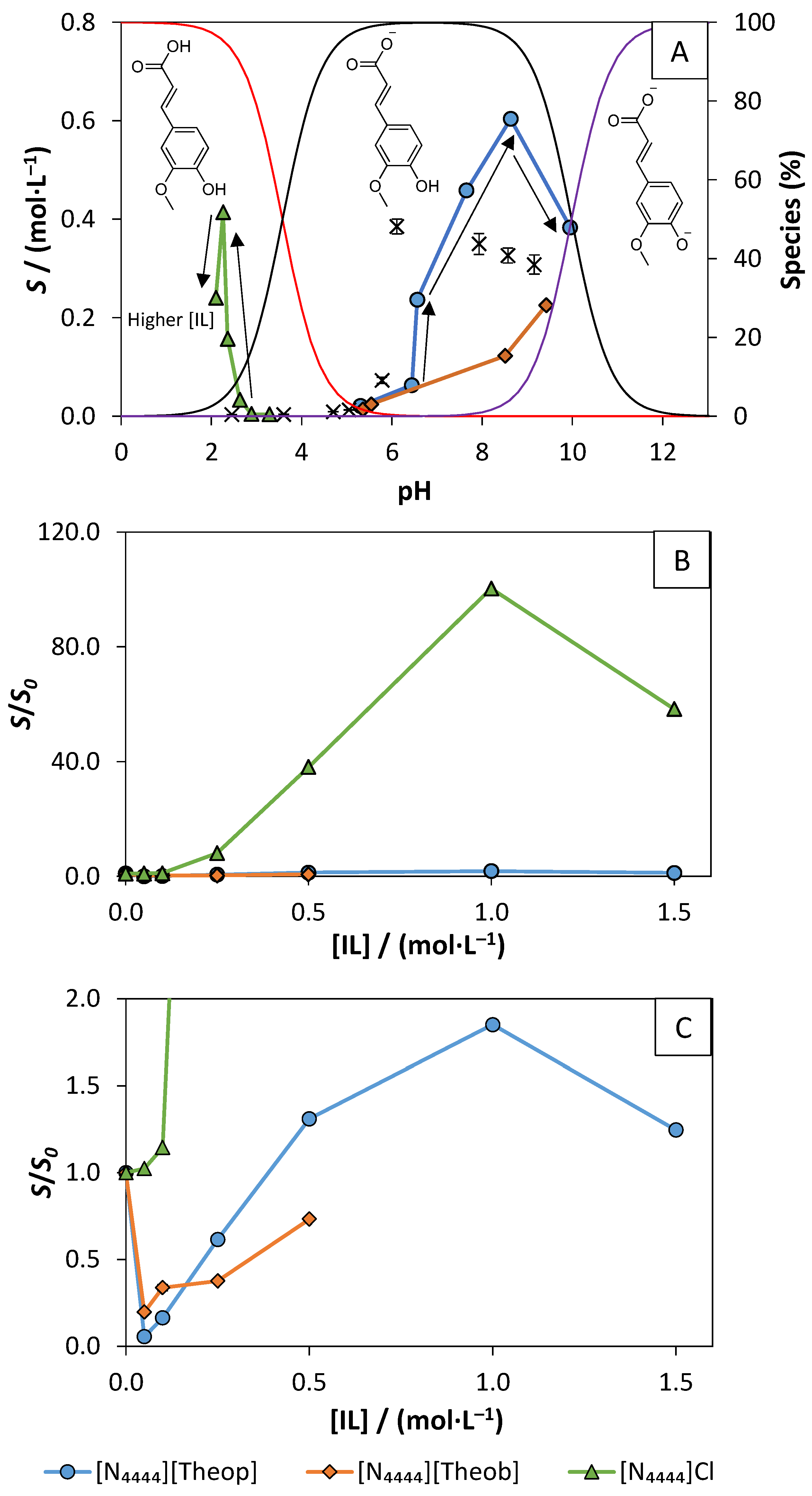
3.3. Solubility Enhancement in Aqueous Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- Mariani, A.; Bonomo, M.; Gao, X.; Centrella, B.; Nucara, A.; Buscaino, R.; Barge, A.; Barbero, N.; Gontrani, L.; Passerini, S. The unseen evidence of Reduced Ionicity: The elephant in (the) room temperature ionic liquids. J. Mol. Liq. 2021, 324, 115069. [Google Scholar] [CrossRef]
- Sintra, T.E.; Shimizu, K.; Ventura, S.P.M.; Shimizu, S.; Canongia Lopes, J.N.; Coutinho, J.A.P. Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes. Phys. Chem. Chem. Phys. 2018, 20, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Handy, S.T. Applications of Ionic Liquids in Science and Technology; InTech: Rijeka, Croatia, 2011; ISBN 9789533076058. [Google Scholar]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, D.; Guo, Y. Revisiting Characteristics of Ionic Liquids: A Review for Further Application Development. J. Environ. Prot. 2010, 1, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, N.; Courty, M.; Gatard, S.; Spulak, M.; Quilty, B.; Beadham, I.; Ghavre, M.; Haiß, A.; Kümmerer, K.; Gathergood, N.; et al. Biomass derived ionic liquids: Synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69, 6150–6161. [Google Scholar] [CrossRef]
- Chiappe, C.; Marra, A.; Mele, A. Synthesis and applications of ionic liquids derived from natural sugars. Top. Curr. Chem. 2010, 295, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Fukumoto, K. Amino acid ionic liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Feder-Kubis, J.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. Ionic Liquids with Natural Origin Component: A Path to New Plant Protection Products. ACS Sustain. Chem. Eng. 2020, 8, 842–852. [Google Scholar] [CrossRef]
- Gavhane, R.J.; Madkar, K.R.; Kurhe, D.N.; Dagade, D.H. Room Temperature Ionic Liquids from Purine and Pyrimidine Nucleobases. ChemistrySelect 2019, 4, 5823–5827. [Google Scholar] [CrossRef]
- Rosemeyer, H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, F.; Brombo, G.; Zuliani, G. Nootropics, Functional Foods, and Dietary Patterns for Prevention of Cognitive Decline. In Nutrition and Functional Foods for Healthy Aging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 211–232. ISBN 9780128092996. [Google Scholar]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Tang, N.; Asadzadeh, B.; Yan, W. Measurement and Correlation of Solubility of Theobromine, Theophylline, and Caffeine in Water and Organic Solvents at Various Temperatures. J. Chem. Eng. Data 2017, 62, 2570–2577. [Google Scholar] [CrossRef]
- Singh, N.; Shreshtha, A.K.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, N. Induced Meiotic Reductions in Root-tips I. Effect of purine derivatives. Cytologia 1986, 51, 439–448. [Google Scholar] [CrossRef] [Green Version]
- McCrudden, F.H. Uric Acid: The Chemistry, Physiology and Pathology of Uric Acid and the Physiologically Important Purin Bodies, with a Discussion of the Metabolism in Gout; Hoeber, P.B., Ed.; Facsimile: New York, NY, USA, 1906. [Google Scholar]
- Sanphui, P.; Nangia, A. Salts and Co-crystals of Theobromine and their phase transformations in water. J. Chem. Sci. 2014, 126, 1249–1264. [Google Scholar] [CrossRef]
- Albertsson, P.Å. Partition of proteins in liquid polymer-polymer two-phase systems. Nature 1958, 182, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Grilo, A.L.; Aires-Barros, M.R.; Azevedo, A.M. Partitioning in Aqueous Two-Phase Systems: Fundamentals, Applications and Trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]
- e Silva, F.A.; Kholany, M.; Sintra, T.E.; Caban, M.; Stepnowski, P.; Ventura, S.P.M.; Coutinho, J.A.P. Aqueous Biphasic Systems Using Chiral Ionic Liquids for the Enantioseparation of Mandelic Acid Enantiomers. Solvent Extr. Ion Exch. 2018, 36, 617–631. [Google Scholar] [CrossRef]
- Schaeffer, N.; Gras, M.; Passos, H.; Mogilireddy, V.; Mendonça, C.M.N.; Pereira, E.; Chainet, E.; Billard, I.; Coutinho, J.A.P.; Papaiconomou, N. Synergistic Aqueous Biphasic Systems: A New Paradigm for the “one-Pot” Hydrometallurgical Recovery of Critical Metals. ACS Sustain. Chem. Eng. 2019, 7, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- El-Hady, D.A.; Albishri, H.M.; Wätzig, H. Ionic liquids in enhancing the sensitivity of capillary electrophoresis: Off-line and on-line sample preconcentration techniques. Electrophoresis 2016, 37, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Belchior, D.C.V.; Quental, M.V.; Pereira, M.M.; Mendonça, C.M.N.; Duarte, I.F.; Freire, M.G. Performance of tetraalkylammonium-based ionic liquids as constituents of aqueous biphasic systems in the extraction of ovalbumin and lysozyme. Sep. Purif. Technol. 2020, 233. [Google Scholar] [CrossRef]
- Magri, A.; Pimenta, M.V.; Santos, J.H.P.M.; Coutinho, J.A.P.; Ventura, S.P.M.; Monteiro, G.; Rangel-Yagui, C.O.; Pereira, J.F.B. Controlling the l-asparaginase extraction and purification by the appropriate selection of polymer/salt-based aqueous biphasic systems. J. Chem. Technol. Biotechnol. 2020, 95, 1016–1027. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Freire, M.G.; Coutinho, J.A.P. Aqueous two-phase systems: Towards novel and more disruptive applications. Fluid Phase Equilib. 2020, 505, 112341. [Google Scholar] [CrossRef]
- McQueen, L.; Lai, D. Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef] [PubMed]
- De Faria, E.L.P.; Ferreira, A.M.; Cláudio, A.F.M.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Recovery of Syringic Acid from Industrial Food Waste with Aqueous Solutions of Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14143–14152. [Google Scholar] [CrossRef]
- Sintra, T.E.; Abranches, D.O.; Benfica, J.; Soares, B.P.; Ventura, S.P.; Coutinho, J.A. Cholinium-based Ionic Liquids as Bioinspired Hydrotropes to Tackle Solubility Challenges in Drug Formulation. Eur. J. Pharm. Biopharm. 2021, 164, 86–92. [Google Scholar] [CrossRef]
- Allen, C.R.; Richard, P.L.; Ward, A.J.; van de Water, L.G.A.; Masters, A.F.; Maschmeyer, T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006, 47, 7367–7370. [Google Scholar] [CrossRef]
- Soares, B.P.; Abranches, D.O.; Sintra, T.E.; Leal-Duaso, A.; García, J.I.; Pires, E.; Shimizu, S.; Pinho, S.P.; Coutinho, J.A.P. Glycerol Ethers as Hydrotropes and Their Use to Enhance the Solubility of Phenolic Acids in Water. ACS Sustain. Chem. Eng. 2020, 8, 5742–5749. [Google Scholar] [CrossRef]
- Santos, J.I.; Gonçalves, A.M.M.; Pereira, J.L.; Figueiredo, B.F.H.T.; Silva, F.A.E.; Coutinho, J.A.P.; Ventura, S.P.M.; Gonçalves, F. Environmental safety of cholinium-based ionic liquids: Assessing structure-ecotoxicity relationships. Green Chem. 2015, 17, 4657–4668. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2011; Section 2. [Google Scholar]
- Geis, S.W.; Fleming, K.L.; Korthals, E.T.; Searle, G.; Reynolds, L.; Karner, D.A. Modifications to the algal growth inhibition test for use as a regulatory assay. Environ. Toxicol. Chem. 2000, 19, 36–41. [Google Scholar] [CrossRef]
- Womersley, H.B.S. Handbook of Phycological Methods. Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; Volume 10. [Google Scholar]
- Neves, C.M.S.S.; Ventura, S.P.M.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A.P. Evaluation of cation influence on the formation and extraction capability of ionic-liquid-based aqueous biphasic systems. J. Phys. Chem. B 2009, 113, 5194–5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.M.; Sousa, S.G.; Serafim, L.S.; Lima, Á.S.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid based aqueous biphasic systems with controlled pH: The ionic liquid cation effect. J. Chem. Eng. Data 2011, 56, 4253–4260. [Google Scholar] [CrossRef]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation studies on phase inversion. J. Chromatogr. B Biomed. Appl. 1998, 711, 285–293. [Google Scholar] [CrossRef]
- Passos, H.; Dinis, T.B.V.; Capela, E.V.; Quental, M.V.; Gomes, J.; Resende, J.; Madeira, P.P.; Freire, M.G.; Coutinho, J.A.P. Mechanisms ruling the partition of solutes in ionic-liquid-based aqueous biphasic systems-the multiple effects of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8411–8422. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Lima, F.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Hydrogen-bond acidity of ionic liquids: An extended scale. Phys. Chem. Chem. Phys. 2015, 17, 18980–18990. [Google Scholar] [CrossRef] [Green Version]
- Cláudio, A.F.M.; Swift, L.; Hallett, J.P.; Welton, T.; Coutinho, J.A.P.; Freire, M.G. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 6593–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Univ. Karlsruhe Forschungszentrum Karlsruhe GmbH. TURBOMOLE V6.1 2009, 1989–2007, 25 GmbH, Since 2007. Available online: http//www.turbomole.com (accessed on 9 July 2021).
- Eckert, F.; Klamt, A. COSMOtherm, version C2.1 Release 01.08; COSMOlogic GmbH Co. KG: Leverkusen, Germany, 2006. [Google Scholar]
- Wesolowski, M.; Szynkaruk, P. Thermal decomposition of methylxanthines: Interpretation of the results by PCA. J. Therm. Anal. Calorim. 2008, 93, 739–746. [Google Scholar] [CrossRef]
- Roth, H.J.; Eger, K.; Trochütz, R. Purines and purine isomers. In Pharmaceutical Chemistry; Ellis Horwood: New York, NY, USA, 1991; pp. 598–601. [Google Scholar]
- Abranches, D.O.; Schaeffer, N.; Silva, L.P.; Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. The role of charge transfer in the formation of type i deep eutectic solvent-analogous ionic liquid mixtures. Molecules 2019, 24, 3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzafera, P. Catabolism of caffeine in plants and microorganisms. Front. Biosci. 2004, 9, 1348–1359. [Google Scholar] [CrossRef] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 March 2021).
- ChemSpider—The Free Chemical Database. 2015. Available online: http://www.chemspider.com/ (accessed on 19 January 2021).
- Bouzková, K.; Babinský, M.; Novosadová, L.; Marek, R. Intermolecular interactions in crystalline theobromine as reflected in electron deformation density and 13C NMR chemical shift tensors. J. Chem. Theory Comput. 2013, 9, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Bruns, R.F.; Fergus, J.H. Solubilities of Adenosine Antagonists Determined by Radioreceptor Assay. J. Pharm. Pharmacol. 1989, 41, 590–594. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Gardas, R.L.; Marrucho, I.M.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Mutual solubilities of water and the [Cnmim][Tf2N] hydrophobic ionic liquids. J. Phys. Chem. B 2008, 112, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, W.H.; Schmidt-Nielsen, B. Excretion in fresh-water turtle (Pseudemys scripta) and desert tortoise (Gopherus agassi). Am. J. Physiol. Content 1966, 210, 198–210. [Google Scholar] [CrossRef]
- MarvinSketch 21.14. 2021, ChemAxon. Available online: http://www.chemaxon.com (accessed on 9 April 2021).
- EU. Environmental Hazards. 10 March 2011. Available online: https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev01/English/04e_part4.pdf (accessed on 4 March 2021).
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Lee, S.Y.; Vicente, F.A.; e Silva, F.A.; Sintra, T.E.; Taha, M.; Khoiroh, I.; Coutinho, J.A.P.; Show, P.L.; Ventura, S.P.M. Evaluating Self-buffering Ionic Liquids for Biotechnological Applications. ACS Sustain. Chem. Eng. 2015, 3, 3420–3428. [Google Scholar] [CrossRef]
- Ranke, J.; Mölter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Gonçalves, A.M.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Zeng, L.; Wang, C.; Yang, Y.; Tan, Z. Assessment of the toxicity and biodegradation of amino acid-based ionic liquids. RSC Adv. 2019, 9, 10100–10108. [Google Scholar] [CrossRef] [Green Version]
- Cvjetko Bubalo, M.; Hanousek, K.; Radošević, K.; Gaurina Srček, V.; Jakovljević, T.; Redovnikovic, R.I. Imidiazolium based ionic liquids: Effects of different anions and alkyl chains lengths on the barley seedlings. Ecotoxicol. Environ. Saf. 2014, 101, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Passos, H.; Dinis, T.B.V.; Cláudio, A.F.M.; Freire, M.G.; Coutinho, J.A.P. Hydrogen bond basicity of ionic liquids and molar entropy of hydration of salts as major descriptors in the formation of aqueous biphasic systems. Phys. Chem. Chem. Phys. 2018, 20, 14234–14241. [Google Scholar] [CrossRef]
- Passos, H.; Ferreira, A.R.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Characterization of aqueous biphasic systems composed of ionic liquids and a citrate-based biodegradable salt. Biochem. Eng. J. 2012, 67, 68–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; He, D.; Cao, X.; Wan, J. Partition of spiramycin in a recyclable aqueous two-phase system based on pH-responsive and thermosensitive polymers. Process Biochem. 2020, 99, 254–264. [Google Scholar] [CrossRef]
- Passos, H.; Luís, A.; Coutinho, J.A.P.; Freire, M.G. Thermoreversible (Ionic-Liquid-Based) Aqueous Biphasic Systems. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaeffer, N.; Pérez-Sánchez, G.; Passos, H.; Gomes, J.R.B.; Papaiconomou, N.; Coutinho, J.A.P. Mechanisms of phase separation in temperature-responsive acidic aqueous biphasic systems. Phys. Chem. Chem. Phys. 2019, 21, 7462–7473. [Google Scholar] [CrossRef]
- Mota, F.L.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Aqueous solubility of some natural phenolic compounds. Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar] [CrossRef] [Green Version]
- Setschenow, J. Über die Konstitution der Salzlösungen auf Grund ihres Verhaltens zu Kohlensäure. Z. Phys. Chem. 1889, 4U, 117–125. [Google Scholar] [CrossRef]
| Name/Abbreviation | Structure | Yield (%) |
|---|---|---|
| Tetrabutylammonium theobrominate [N4444][Theob] | 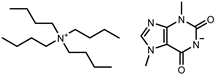 | 100 |
| Tetrabutylammonium theophyllinate [N4444][Theop] |  | 97 |
| Tetrabutylammonium xanthinate [N4444][Xan] | 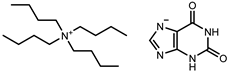 | 94 |
| Tetrabutylammonium urate [N4444][Ur] | 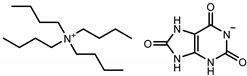 | 97 |
| Salt | Tm/(K) | ΔHm/(J·mol−1) | Td/(K) |
|---|---|---|---|
| [N4444][Theob] | 377.8 ± 0.2 | 50920 ± 51 | 457 ± 1 |
| [N4444][Theop] | 370.6 ± 0.1 | 36853 ± 1811 | 486 ± 1 |
| [N4444][Xan] | 487 ± 1 | - a | 495 ± 1 |
| [N4444][Ur] | - a | - a | 505 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira, A.R.F.; Veloso, T.; Schaeffer, N.; Pereira, J.L.; Ventura, S.P.M.; Rizzi, C.; Sirieix Plénet, J.; Passos, H.; Coutinho, J.A.P. Synthesis of Purine-Based Ionic Liquids and Their Applications. Molecules 2021, 26, 6958. https://doi.org/10.3390/molecules26226958
Carreira ARF, Veloso T, Schaeffer N, Pereira JL, Ventura SPM, Rizzi C, Sirieix Plénet J, Passos H, Coutinho JAP. Synthesis of Purine-Based Ionic Liquids and Their Applications. Molecules. 2021; 26(22):6958. https://doi.org/10.3390/molecules26226958
Chicago/Turabian StyleCarreira, Ana R. F., Telma Veloso, Nicolas Schaeffer, Joana L. Pereira, Sónia P. M. Ventura, Cécile Rizzi, Juliette Sirieix Plénet, Helena Passos, and João A. P. Coutinho. 2021. "Synthesis of Purine-Based Ionic Liquids and Their Applications" Molecules 26, no. 22: 6958. https://doi.org/10.3390/molecules26226958
APA StyleCarreira, A. R. F., Veloso, T., Schaeffer, N., Pereira, J. L., Ventura, S. P. M., Rizzi, C., Sirieix Plénet, J., Passos, H., & Coutinho, J. A. P. (2021). Synthesis of Purine-Based Ionic Liquids and Their Applications. Molecules, 26(22), 6958. https://doi.org/10.3390/molecules26226958