Identification and Quantification of Hydrocarbon Functional Groups in Gasoline Using 1H-NMR Spectroscopy for Property Prediction
Abstract
:1. Introduction
2. Experimental Method
3. Functional Group Analysis
4. Property Prediction
4.1. Octane Number (ON)
4.2. Derived Cetane Number (DCN)
4.3. Sooting Propensity
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Marshall, A.G.; Rodgers, R.P. Petroleomics: Chemistry of the underworld. Proc. Natl. Acad. Sci. USA 2008, 105, 18090–18095. [Google Scholar] [CrossRef] [Green Version]
- Abdul Jameel, A.G.; Alquaity, A.B.S.; Campuzano, F.; Emwas, A.; Saxena, S.; Sarathy, S.M.; Roberts, W.L. Surrogate formulation and molecular characterization of sulfur species in vacuum residues using APPI and ESI FT-ICR mass spectrometry. Fuel 2021, 293, 120471. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; Alkhateeb, A.; Telalović, S.; Elbaz, A.M.; Roberts, W.L.; Sarathy, S.M. Environmental Challenges and Opportunities in Marine Engine Heavy Fuel Oil Combustion. In Proceedings of the Fourth International Conference in Ocean Engineering; Springer: Singapore, 2019; pp. 1047–1055. [Google Scholar] [CrossRef]
- Pei, X.; Abdul Jameel, A.G.; Chen, C.; AlGhamdi, I.A.; AlAhmadi, K.; AlBarakati, E.; Saxena, S.; Roberts, W.L. Swirling Flame Combustion of Heavy Fuel Oil: Effect of Fuel Sulfur Content. J. Energy Resour. Technol. 2021, 143, 082103. [Google Scholar] [CrossRef]
- Guida, P.; Jameel, A.G.A.; Saxena, S.; Roberts, W.L. Fundamental Aspects and Applications of Ultrasonically Induced Cavitation in Heavy Fuel Oil with a Focus on Deasphalting, Emulsions, and Oxidative Desulfurization. In Catalytic and Noncatalytic Upgrading of Oils; American Chemical Society: Washington, DC, USA, 2021; pp. 233–293. [Google Scholar] [CrossRef]
- Ahmed, A.; Goteng, G.; Shankar, V.S.B.; Al-Qurashi, K.; Roberts, W.L.; Sarathy, S.M. A computational methodology for formulating gasoline surrogate fuels with accurate physical and chemical kinetic properties. Fuel 2015, 143, 290–300. [Google Scholar] [CrossRef]
- Yu, J.; Ju, Y.; Gou, X. Surrogate fuel formulation for oxygenated and hydrocarbon fuels by using the molecular structures and functional groups. Fuel 2016, 166, 211–218. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; Naser, N.; Issayev, G.; Touitou, J.; Ghosh, M.K.; Emwas, A.; Farooq, A.; Dooley, S.; Sarathy, S.M. A minimalist functional group (MFG) approach for surrogate fuel formulation. Combust. Flame 2018, 192, 250–271. [Google Scholar] [CrossRef] [Green Version]
- Allenn, D.; Petrakis, L.; Grandy, D.; Gavalas, G.; Gates, B. Determination of functional groups of coal-derived liquids by nmr. and elemental analysis. Fuel 1984, 63, 803–809. [Google Scholar] [CrossRef]
- Kapur, G.S.; Ecker, A.; Meusinger, R. Establishing Quantitative Structure-Property Relationships (QSPR) of Diesel Samples by Proton-NMR & Multiple Linear Regression (MLR) Analysis. Energy Fuels 2001, 15, 943–948. [Google Scholar] [CrossRef]
- Benson, S.W.; Cruickshank, F.R.; Golden, D.M.; Haugen, G.R.; O’Neal, H.E.; Rodgers, A.S.; Shaw, R.; Walsh, R. Additivity rules for the estimation of thermochemical properties. Chem. Rev. 1969, 69, 279–324. [Google Scholar] [CrossRef]
- Thuy, T.; Allen, D. Property estimation using structural characterizationsHeat capacities of coal liquids. Fuel 1985, 64, 1754–1759. [Google Scholar] [CrossRef]
- Daly, S.R.; Niemeyer, K.E.; Cannella, W.J.; Hagen, C.L. Predicting fuel research octane number using Fourier-transform infrared absorption spectra of neat hydrocarbons. Fuel 2016, 183, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Abdul Jameel, A.G.; Naser, N.; Emwas, A.-H.; Dooley, S.; Sarathy, S.M. Predicting Fuel Ignition Quality Using 1 H NMR Spectroscopy and Multiple Linear Regression. Energy Fuels 2016, 30, 9819–9835. [Google Scholar] [CrossRef]
- Saldana, D.A.; Starck, L.; Mougin, P.; Rousseau, B.; Pidol, L.; Jeuland, N.; Creton, B. Flash Point and Cetane Number Predictions for Fuel Compounds Using Quantitative Structure Property Relationship (QSPR) Methods. Energy Fuels 2011, 25, 3900–3908. [Google Scholar] [CrossRef]
- vom Lehn, F.; Cai, L.; Copa Cáceres, B.; Pitsch, H. Exploring the fuel structure dependence of laminar burning velocity: A machine learning based group contribution approach. Combust. Flame 2021, 232, 111525. [Google Scholar] [CrossRef]
- Dragoş Ilieş, B.; Khandavilli, M.; Li, Y.; Kukkadapu, G.; Wagnon, S.W.; Gani Abdul Jameel, A.; Mani Sarathy, S. Probing the Chemical Kinetics of Minimalist Functional Group Gasoline Surrogates. Energy Fuels 2021, 35, 3315–3332. [Google Scholar] [CrossRef]
- Gani, A.; Jameel, A.; Naser, N.; Emwas, A.; Sarathy, S.M. Surrogate formulation for diesel and jet fuels using the minimalist functional group ( MFG ) approach. Proc. Combust. Inst. 2019, 37, 4663–4671. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; Van Oudenhoven, V.; Emwas, A.-H.; Sarathy, S.M. Predicting Octane Number Using Nuclear Magnetic Resonance Spectroscopy and Artificial Neural Networks. Energy Fuels 2018, 32, 6309–6329. [Google Scholar] [CrossRef] [Green Version]
- Dooley, S.; Won, S.H.; Heyne, J.; Farouk, T.I.; Ju, Y.; Dryer, F.L.; Kumar, K.; Hui, X.; Sung, C.J.; Wang, H.; et al. The experimental evaluation of a methodology for surrogate fuel formulation to emulate gas phase combustion kinetic phenomena. Combust. Flame 2012, 159, 1444–1466. [Google Scholar] [CrossRef]
- Sumathi, R.; Carstensen, H.-H.; Green, W.H. Reaction Rate Prediction via Group Additivity Part 1: H Abstraction from Alkanes by H and CH3. J. Phys. Chem. A 2001, 105, 6910–6925. [Google Scholar] [CrossRef]
- Sumathi, R.; Carstensen, H.-H.; Green, W.H. Reaction Rate Prediction via Group Additivity, Part 2: H-Abstraction from Alkenes, Alkynes, Alcohols, Aldehydes, and Acids by H Atoms. J. Phys. Chem. A 2001, 105, 8969–8984. [Google Scholar] [CrossRef]
- Dussan, K.; Dooley, S.; Dryer, F.L.; Won, S.H. A Nuclear Magnetic Resonance Orientated Combustion Property Regression. In Proceedings of the 9th US National Combustion Meeting, Cincinnati, OH, USA, 17 May 2015; pp. 17–20. [Google Scholar]
- Naser, N.; Abdul Jameel, A.G.; Emwas, A.-H.; Singh, E.; Chung, S.H.; Sarathy, S.M. The influence of chemical composition on ignition delay times of gasoline fractions. Combust. Flame 2019, 209, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Das, D.D.; St John, P.C.; McEnally, C.S.; Kim, S.; Pfefferle, L.D. Measuring and predicting sooting tendencies of oxygenates, alkanes, alkenes, cycloalkanes, and aromatics on a unified scale. Combust. Flame 2018, 190, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Abdul Jameel, A.G. A Functional Group Approach for Predicting Fuel Properties. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2019. [Google Scholar]
- Silva, S.L.; Silva, A.M.S.; Ribeiro, J.C.; Martins, F.G.; Da Silva, F.A.; Silva, C.M. Chromatographic and spectroscopic analysis of heavy crude oil mixtures with emphasis in nuclear magnetic resonance spectroscopy: A review. Anal. Chim. Acta 2011, 707, 18–37. [Google Scholar] [CrossRef]
- Rakhmatullin, I.Z.; Efimov, S.V.; Klochkov, A.V.; Gnezdilov, O.I.; Varfolomeev, M.A.; Klochkov, V. V NMR chemical shifts of carbon atoms and characteristic shift ranges in the oil sample. Pet. Res. 2021. [Google Scholar] [CrossRef]
- ASTM Int. Standard Test Method for Research Octane Number of Spark-Ignition Engine Fuel 1. 2012. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D2699-12.htm (accessed on 18 November 2021).
- ASTM Int. Standard Test Method for Motor Octane Number of Spark-Ignition Engine Fuel 1. 2011. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D2699-11.htm (accessed on 18 November 2021).
- Morgan, N.; Smallbone, A.; Bhave, A.; Kraft, M.; Cracknell, R.; Kalghatgi, G. Mapping surrogate gasoline compositions into RON/MON space. Combust. Flame 2010, 157, 1122–1131. [Google Scholar] [CrossRef]
- Elfghi, F.M. A hybrid statistical approach for modeling and optimization of RON: A comparative study and combined application of response surface methodology (RSM) and artificial neural network (ANN) based on design of experiment (DOE). Chem. Eng. Res. Des. 2016, 113, 264–272. [Google Scholar] [CrossRef]
- Li, R.; Herreros, J.M.; Tsolakis, A.; Yang, W. Machine learning regression based group contribution method for cetane and octane numbers prediction of pure fuel compounds and mixtures. Fuel 2020, 280, 118589. [Google Scholar] [CrossRef]
- Naser, N.; Sarathy, S.M.; Chung, S.H. Ignition delay time sensitivity in ignition quality tester (IQT) and its relation to octane sensitivity. Fuel 2018, 233, 412–419. [Google Scholar] [CrossRef] [Green Version]
- ASTM D613 Standard Test Method for Cetane Number of Diesel Fuel Oil. 2015. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D613-15.htm (accessed on 18 November 2021).
- ASTM D7170 Standard Test Method for Determination of Derived Cetane Number ( DCN ) of Diesel Fuel Oils—Fixed Range Injection Period, Constant Volume Combustion Chamber Method 1. 2007. Available online: https://webstore.ansi.org/standards/astm/astmd717011 (accessed on 18 November 2021).
- ASTM D6890 Standard Test Method for Determination of Ignition Delay and Derived Cetane Number (DCN) of Diesel Fuel Oils by Combustion in a Constant Volume Chamber. 2013. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D6890-13.htm (accessed on 18 November 2021).
- Li, R.; Wang, Z.; Ni, P.; Zhao, Y.; Li, M.; Li, L. Effects of cetane number improvers on the performance of diesel engine fuelled with methanol/biodiesel blend. Fuel 2014, 128, 180–187. [Google Scholar] [CrossRef]
- Ghosh, P.; Jaffe, S.B. Detailed Composition-Based Model for Predicting the Cetane Number of Diesel Fuels. Ind. Eng. Chem. Res. 2006, 45, 346–351. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; van Oudenhoven, V.C.O.; Naser, N.; Emwas, A.-H.; Gao, X.; Sarathy, S.M. Predicting Ignition Quality of Oxygenated Fuels Using Artificial Neural Networks. SAE Int. J. Fuels Lubr. 2021, 14, 57–85. [Google Scholar] [CrossRef]
- Badra, J.; Viollet, Y.; Elwardany, A.; Im, H.G.; Chang, J. Physical and chemical effects of low octane gasoline fuels on compression ignition combustion. Appl. Energy 2016, 183, 1197–1208. [Google Scholar] [CrossRef]
- Platt, S.M.; El Haddad, I.; Pieber, S.M.; Zardini, A.A.; Suarez-Bertoa, R.; Clairotte, M.; Daellenbach, K.R.; Huang, R.J.; Slowik, J.G.; Hellebust, S.; et al. Gasoline cars produce more carbonaceous particulate matter than modern filter-equipped diesel cars. Sci. Rep. 2017, 7, 4926. [Google Scholar] [CrossRef]
- Calcote, H.F.; Manos, D.M. Effect of molecular structure on incipient soot formation. Combust. Flame 1983, 49, 289–304. [Google Scholar] [CrossRef]
- Mcenally, C.; Pfefferle, L. Improved sooting tendency measurements for aromatic hydrocarbons and their implications for naphthalene formation pathways. Combust. Flame 2007, 148, 210–222. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.A. Predicting Sooting Propensity of Oxygenated Fuels Using Artificial Neural Networks. Processes 2021, 9, 1070. [Google Scholar] [CrossRef]
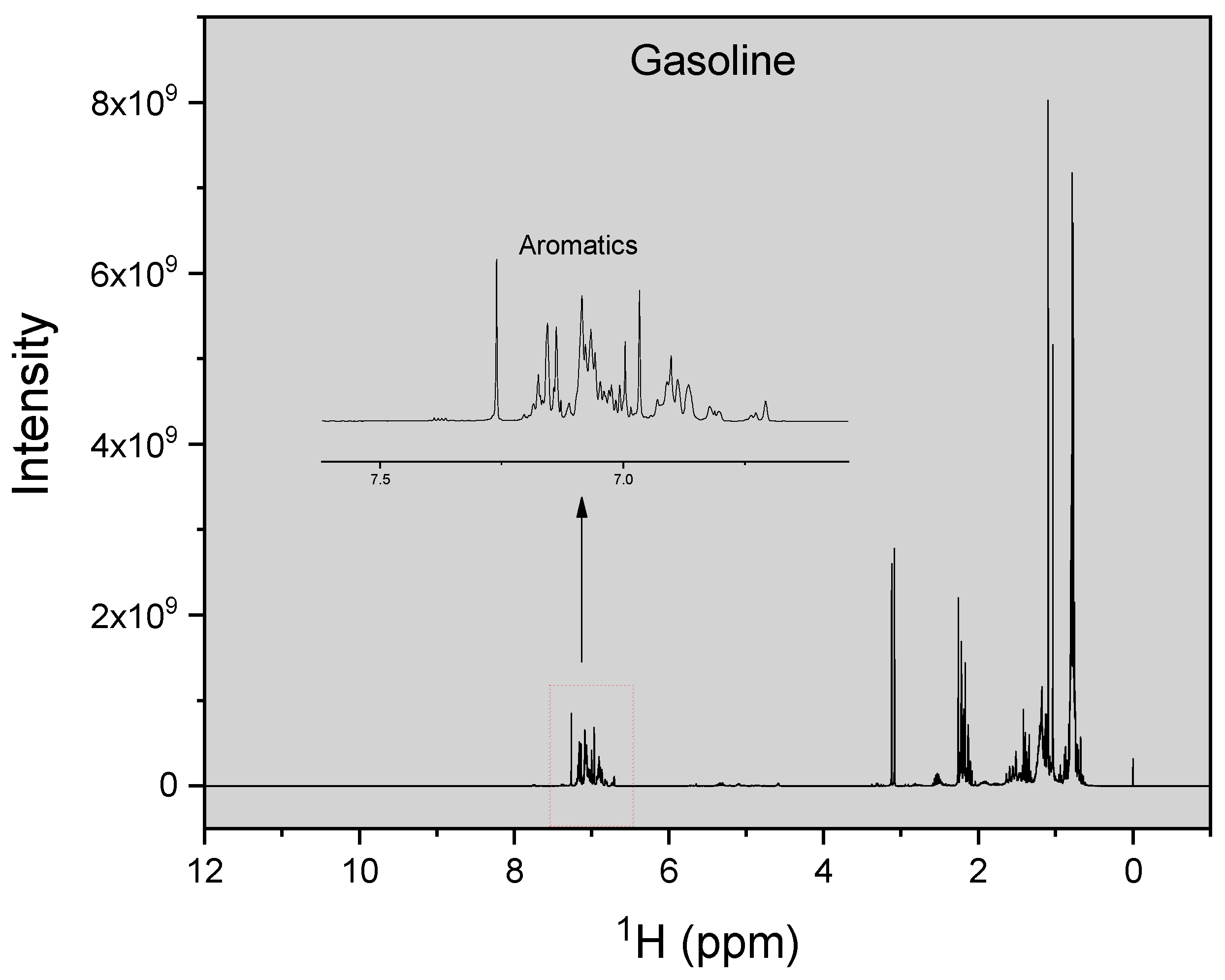
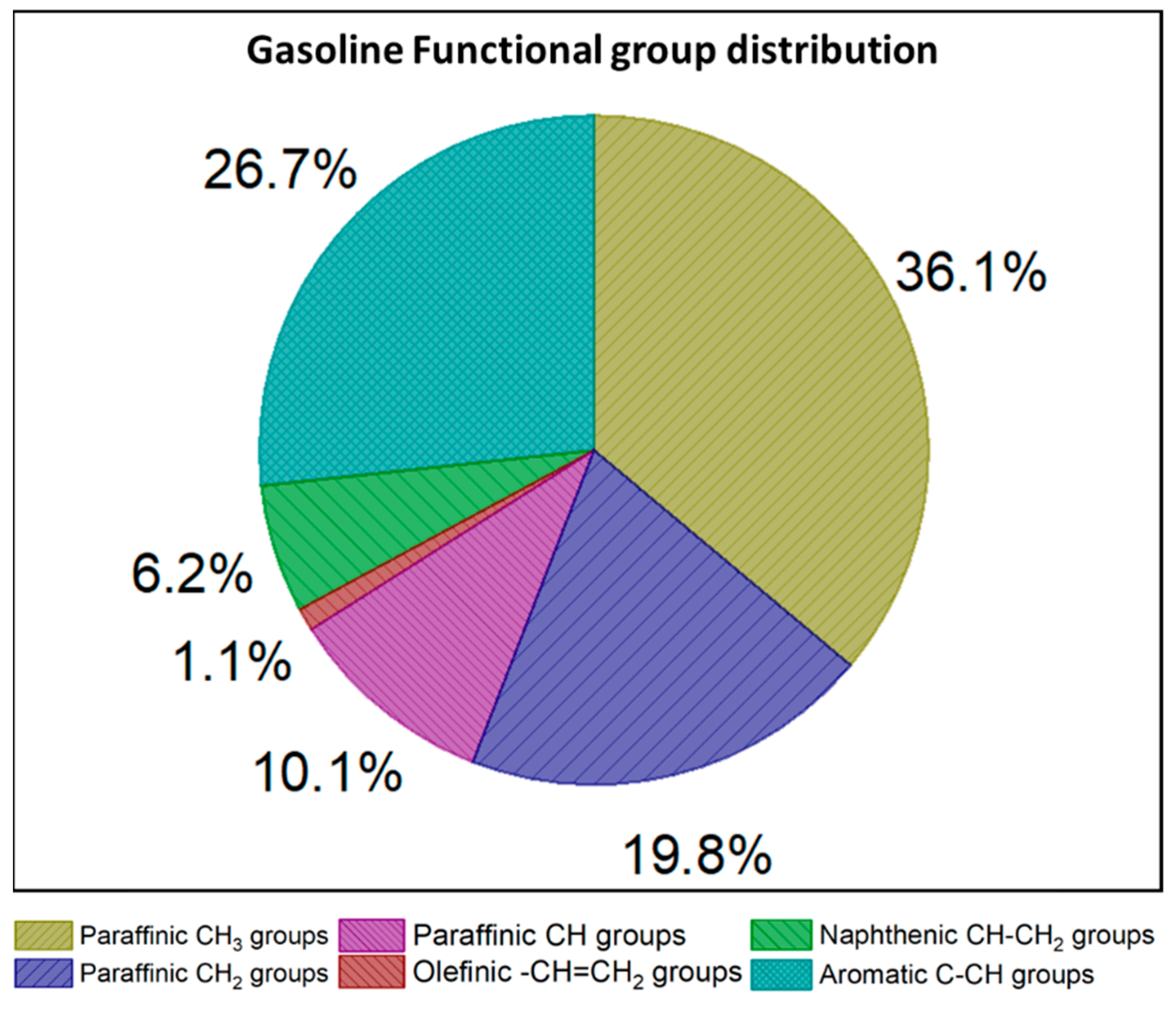
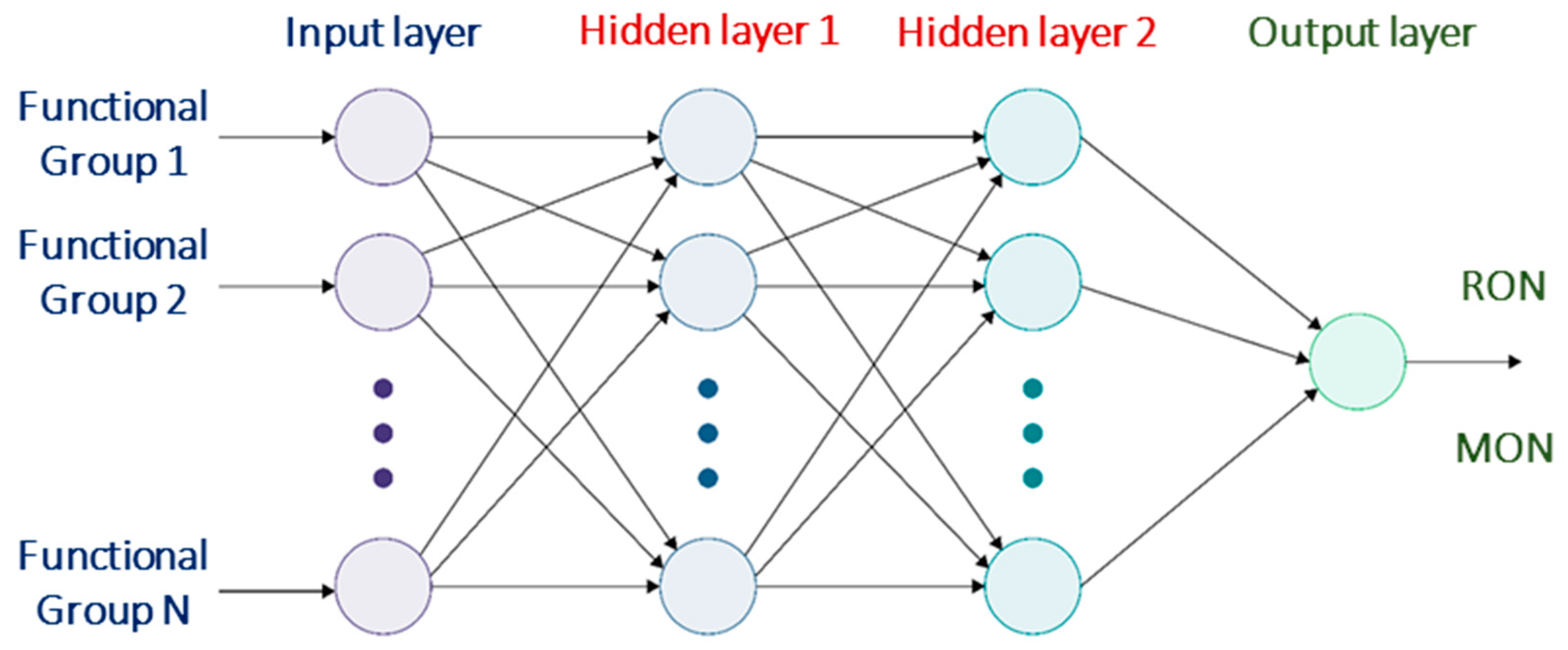
| H Atom | Chemical Shift (ppm) | Integral Intensity |
|---|---|---|
| aromatic C-CH | 6.42–8.99 | a |
| olefinic CH=CH2 | 4.50–6.42 | b |
| paraffinic CH group in alpha position to aromatic ring | 2.88–3.40 | c |
| paraffinic CH2 group in alpha position to aromatic ring | 2.64–2.88 | d |
| paraffinic CH3 group in alpha position to aromatic ring | 2.04–2.64 | e |
| naphthenic CH-CH2 group | 1.57–1.96 | f |
| paraffinic CH groups | 1.39–1.57 | g |
| paraffinic CH2 group | 0.94–1.39 | h |
| paraffinic CH3 group | 0.25–0.94 | i |
| Sum (a + b + c + d + e + f + g + h + i) | S |
| Functional Group | H Type (mol %) | Mass (Arbitrary Unit) | Mass (%) |
|---|---|---|---|
| Paraffinic CH3 | |||
| Paraffinic CH2 | |||
| Paraffinic CH | |||
| Olefinic CH=CH2 | |||
| Naphthenic CH-CH2 | |||
| α-CH | |||
| α-CH2 | |||
| α-CH3 | |||
| Aromatic C-CH |
| Property | Value (No Unit) |
|---|---|
| RON | 95.8 |
| MON | 82.5 |
| DCN | 17.5 |
| TSI | 21.7 |
| YSI | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Jameel, A.G. Identification and Quantification of Hydrocarbon Functional Groups in Gasoline Using 1H-NMR Spectroscopy for Property Prediction. Molecules 2021, 26, 6989. https://doi.org/10.3390/molecules26226989
Abdul Jameel AG. Identification and Quantification of Hydrocarbon Functional Groups in Gasoline Using 1H-NMR Spectroscopy for Property Prediction. Molecules. 2021; 26(22):6989. https://doi.org/10.3390/molecules26226989
Chicago/Turabian StyleAbdul Jameel, Abdul Gani. 2021. "Identification and Quantification of Hydrocarbon Functional Groups in Gasoline Using 1H-NMR Spectroscopy for Property Prediction" Molecules 26, no. 22: 6989. https://doi.org/10.3390/molecules26226989
APA StyleAbdul Jameel, A. G. (2021). Identification and Quantification of Hydrocarbon Functional Groups in Gasoline Using 1H-NMR Spectroscopy for Property Prediction. Molecules, 26(22), 6989. https://doi.org/10.3390/molecules26226989






