Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil
Abstract
:1. Introduction
2. Results
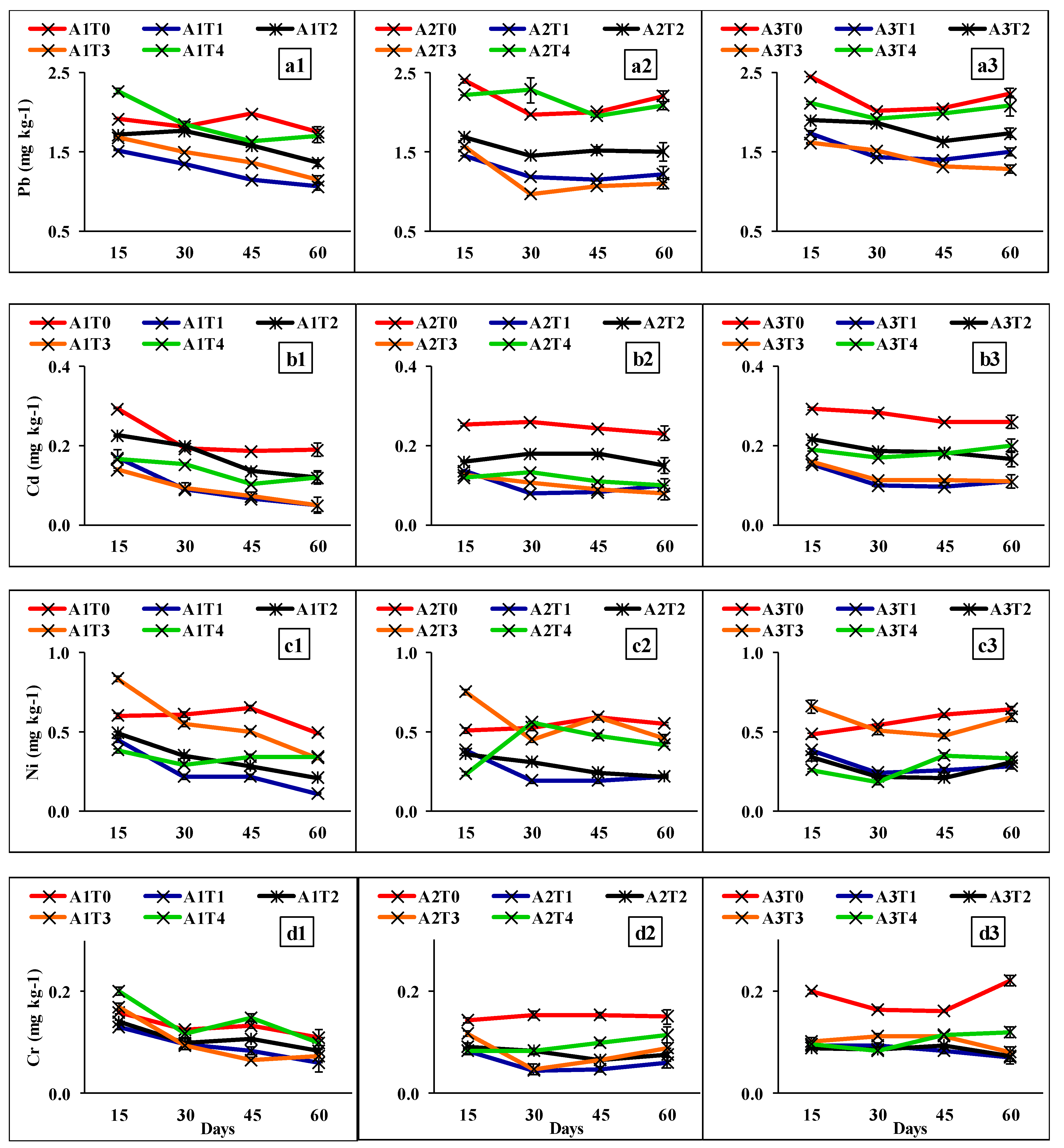
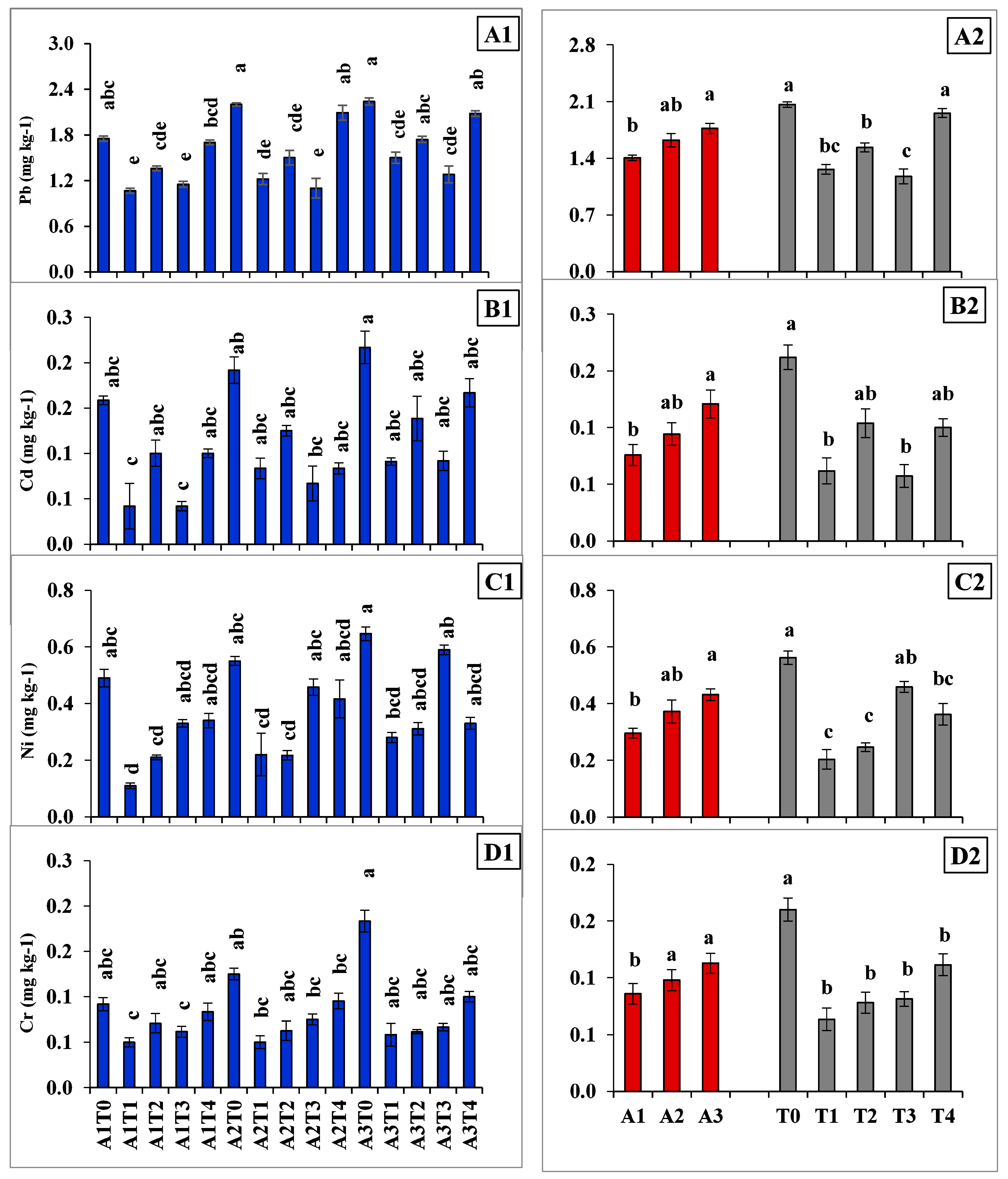
2.1. Bio-Availability of Heavy Metals
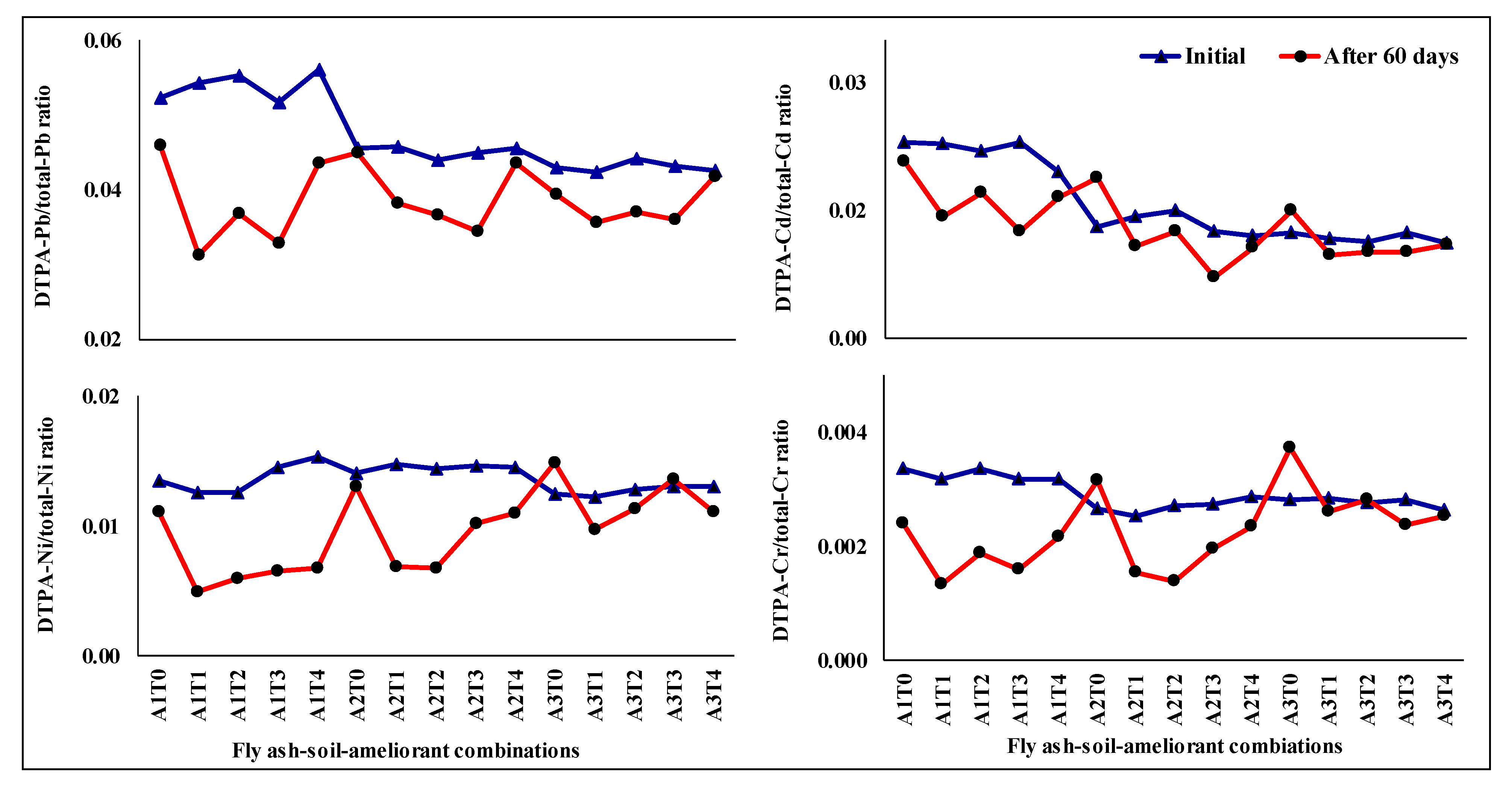
2.2. Changes in DTPA-Extractable Heavy Metal to Total Heavy Metal Ratio under Different Fly Ash-Soil-Ameliorant Combinations
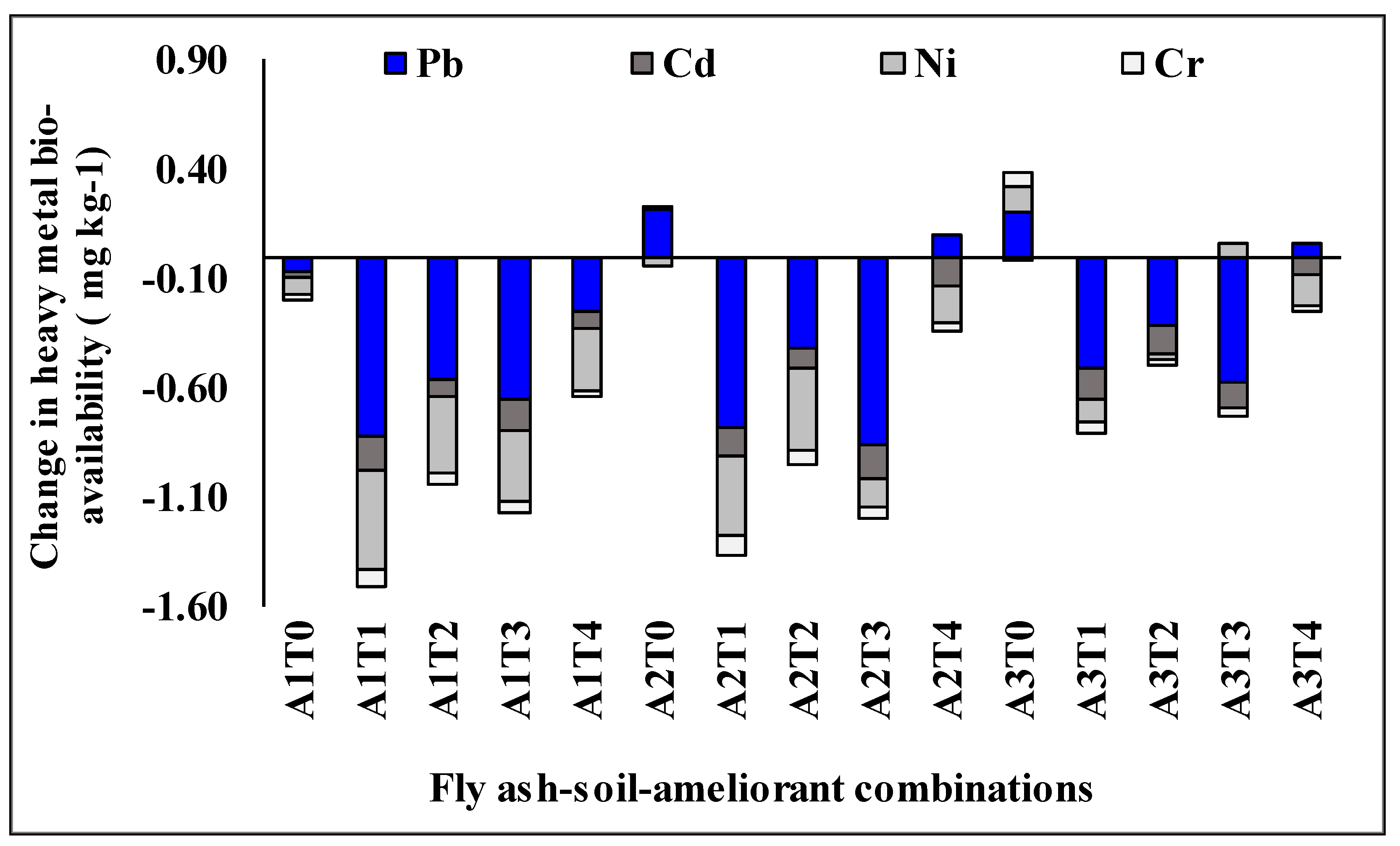
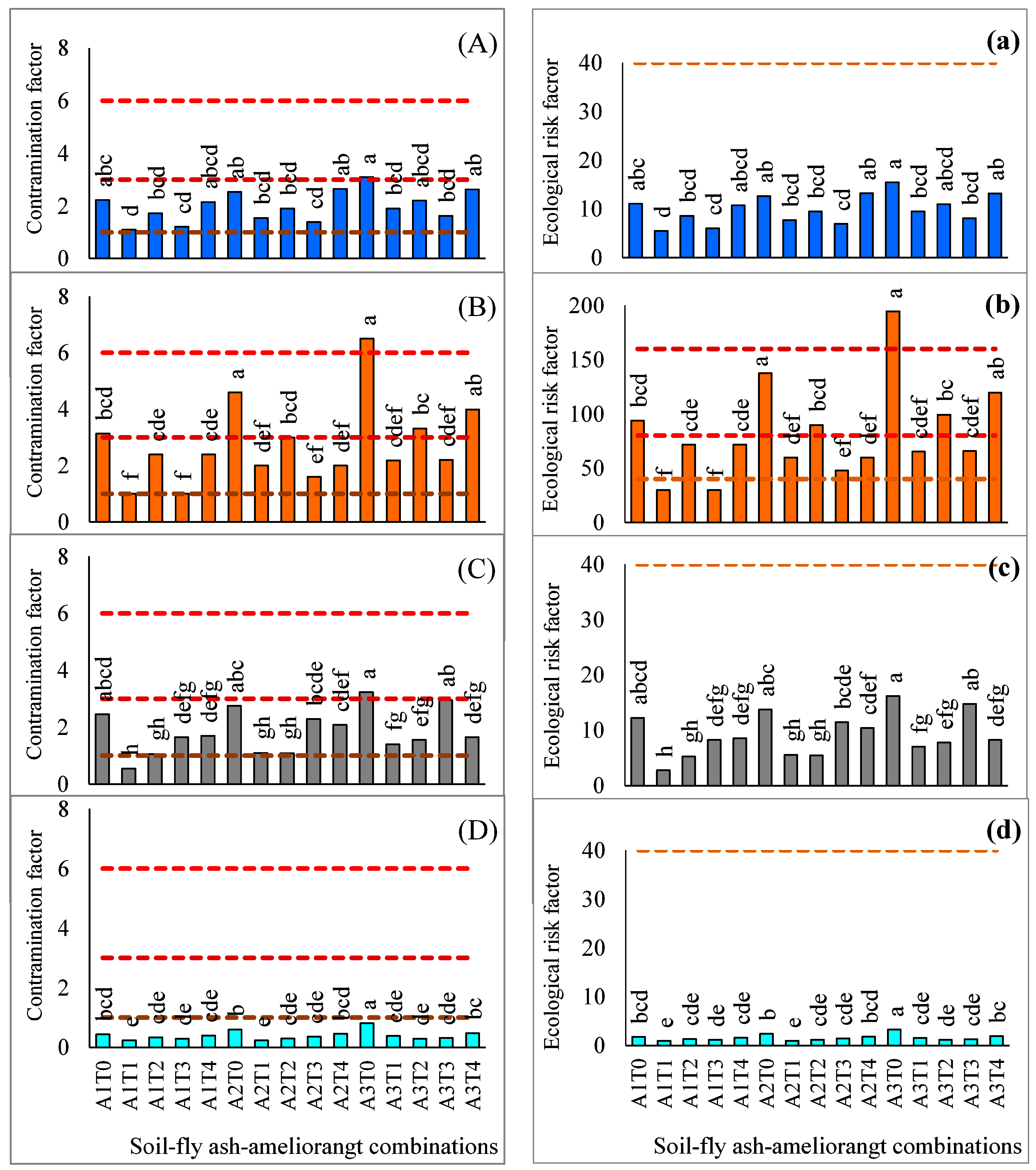
2.3. Efficacy of Different Fly Ash-Soil-Ameliorant Combinations in Minimizing Bio-Availability of Heavy Metals
2.4. Evaluation of Environmental Risk
2.5. Assessment of Microbial Endpoints under the Influence of Fly Ash-Soil-Ameliorant Combinations
3. Materials and Methods
3.1. Raw Material
3.2. Experimental Soil
3.3. Experimental Details and Analysis
3.4. Soil and Environment Risk Assessment
3.4.1. Contamination Factor (CF)
3.4.2. Ecological Risk Factor (ERF)
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Belyaeva, O.N.; Haynes, R.J. Comparison of the effects of conventional organic amendments and biochar on the chemical, physical and microbial properties of coal fly ash as a plant growth medium. Environ. Earth Sci. 2012, 66, 1987–1997. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Jha, S.K.; Ram, L.C. Impact of heavy metals in Indian fly ashes on its application as soil ameliorant. Energy Sources Part A 2016, 38, 2568–2574. [Google Scholar] [CrossRef]
- Basta, N.T.; McGowen, S.L. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 2004, 127, 73–82. [Google Scholar] [CrossRef]
- Roy, M.; Roychowdhury, R.; Mukherjee, P. Remediation of fly ash dumpsites through bioenergy crop plantation and generation: A review. Pedosphere 2018, 28, 561–580. [Google Scholar] [CrossRef]
- Bryan, A.L., Jr.; Hopkins, W.A.; Parikh, J.H.; Jackson, B.P.; Unrine, J.M. Coal fly ash basins as an attractive nuisance to birds: Parental provisioning exposes nestlings to harmful trace elements. Environ. Pollut. 2012, 161, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Chakma, S. Probabilistic human health risk assessment of groundwater contamination due to metal leaching: A case study of Indian dumping sites. Hum. Ecol. Risk Assess. 2021, 27, 101–133. [Google Scholar] [CrossRef]
- Sett, R. Flyash: Characteristics, problems and possible utilization. Adv. Appl. Sci. Res. 2017, 8, 32–50. [Google Scholar]
- Jambhulkar, H.P.; Shaikh, S.M.S.; Kumar, M.S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review. Chemosphere 2018, 213, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Sahu, G.; Bag, A.G.; Chatterjee, N.; Mukherjee, A. Potential use of flyash in agriculture: A way to improve soil health. J. Pharmacogn. Phytochem. 2017, 6, 873–880. [Google Scholar]
- Pandey, V.C.; Singh, K.; Singh, R.P.; Singh, B. Naturally growing Saccharum munja L. on the fly ash lagoons: A potential ecological engineer for the revegetation and stabilization. Ecol. Eng. 2012, 40, 95–99. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Fast green capping on coal fly ash basins through ecological engineering. Ecol. Eng. 2014, 73, 671–675. [Google Scholar] [CrossRef]
- Rai, U.N.; Pandey, K.; Sinha, S.; Singh, A.; Saxena, R.; Gupta, D.K. Revegetating fly ash landfills with Prosopis juliflora L.: Impact of different amendments and Rhizobium inoculation. Environ. Int. 2004, 30, 293–300. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dwivedi, S.; Sinha, S.; Tripathi, R.D.; Rai, U.N.; Singh, S.N. Metal accumulation and growth performance of Phaseolus vulgaris grown in fly ash amended soil. Bioresour. Technol. 2007, 98, 3404–3407. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Lahori, A.H.; Awasthi, M.K.; Wang, Z.; Zhang, Z. (Im) mobilization of soil heavy metals using CaO, FA, sulfur, and Na2S: A 1-year incubation study. Int. J. Environ. Sci. Technol. 2018, 15, 607–620. [Google Scholar] [CrossRef]
- Zhou, C.; Yuan, H.; Ning, C.; Li, S.; Xia, Z.; Zhu, M.; Ma, Q.; Yu, W. Evaluation of different types and amounts of amendments on soil Cd immobilization and its uptake to wheat. Environ. Manag. 2020, 65, 818–828. [Google Scholar] [CrossRef]
- Mukherjee, S.; Saha, N.; Sarkar, B.; Sengupta, S.; Ghosh, S.; Dey, P. Assessing Methods for Estimating Potentially Mineralisable Nitrogen Under Organic Production System in New Alluvial Soils of Lower Gangetic Plain. Soil Sci. Plant Nutr. 2021, 21, 1030–1040. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 9th ed.; USDA-NRCS: Washington, DC, USA, 2003; pp. 332–345. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of soil analysis, part 2. Chem. Microbiol. Prop. 1982, 2, 643–698. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbaiah, V.V.; Asija, G.K. A rapid procedure for utilization of available nitrogen in soil. Curr. Sci. 1956, 26, 258–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; No. 939; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Hanway, J.J.; Heidal, H. Soil analysis methods as used in Iowa State College Soil Testing Laboratory. Iowa State Coll. Agric. Bull. 1952, 57, 1–31. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Klein, D.A.; Loh, T.C.; Goulding, R.L. A rapid procedure to evaluate the dehydrogenase activity of soils low in organic matter. Soil Biol. Biochem. 1971, 3, 385–387. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publication: Oxford, UK, 1986; pp. 285–344. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Enderlein, G.; Hawkins, D.M. Identification of Outliers. Chapman and Hall, London–New York 1980. Biom. J. 2010, 29, 198. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bhattacharya, T. Mobility, ecological risk and change in surface morphology during sequential chemical extraction of heavy metals in fly ash: A case study. Environ. Technol. Innov. 2019, 13, 373–382. [Google Scholar] [CrossRef]
- Yang, Z.; Jing, F.; Chen, X.; Liu, W.; Guo, B.; Lin, G.; Huang, R.; Liu, W. Spatial distribution and sources of seven available heavy metals in the paddy soil of red region in Hunan Province of China. Environ. Monit. Assess. 2018, 190, 1–10. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- González-Alcaraz, M.N.; Conesa, H.M.; Álvarez-Rogel, J. Phytomanagement of strongly acidic, saline eutrophic wetlands polluted by mine wastes: The influence of liming and Sarcocornia fruticosa on metals mobility. Chemosphere 2013, 90, 2512–2519. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, X.; Zhang, H.; Ren, W.; Tang, M. Effects of sodium sulfide application on the growth of Robinia pseudoacacia, heavy metal immobilization, and soil microbial activity in Pb–Zn polluted soil. Ecotoxicol. Environ. Saf. 2020, 197, 110563. [Google Scholar] [CrossRef]
- Vítková, M.; Ettler, V.; Šebek, O.; Mihaljevič, M.; Grygar, T.; Rohovec, J. The pH-dependent leaching of inorganic contaminants from secondary lead smelter fly ash. J. Hazard. Mater. 2009, 167, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ruttens, A.; Adriaensen, K.; Meers, E.; De Vocht, A.; Geebelen, W.; Carleer, R.; Mench, M.; Vangronsveld, J. Long-term sustainability of metal immobilization by soil amendments: Cyclonic ashes versus lime addition. Environ. Pollut. 2010, 158, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Leng, L.; Huang, H.; Chen, X.; Wang, H.; Xiao, Z.; Zhai, Y.; Chen, H.; Zeng, G. Speciation and environmental risk assessment of heavy metal in bio-oil from liquefaction/pyrolysis of sewage sludge. Chemosphere 2015, 120, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Prokkola, H.; Nurmesniemi, E.T.; Lassi, U. Removal of Metals by Sulphide Precipitation Using Na2S and HS—Solution. ChemEngineering 2020, 4, 51. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Liu, J.; Su, Y.; Li, Q.; Yue, Q.; Gao, B. Preparation of wheat straw based superabsorbent resins and their applications as adsorbents for ammonium and phosphate removal. Bioresour. Technol. 2013, 143, 32–39. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Yip, T.C.; Yan, D.Y.; Yui, M.M.; Tsang, D.C.; Lo, I.M. Heavy metal extraction from an artificially contaminated sandy soil under EDDS deficiency: Significance of humic acid and chelant mixture. Chemosphere 2010, 80, 416–421. [Google Scholar] [CrossRef]
- Xu, Y. Stabilization of heavy metal-contaminated sediment with a chelator and humic acid mixture. Water Air Soil Pollut. 2017, 228, 1–11. [Google Scholar] [CrossRef]
- Skłodowski, P.; Maciejewska, A.; Kwiatkowska, J. The effect of organic matter from brown coal on bioavailability of heavy metals in contaminated soils. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2006; pp. 299–307. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Notices 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Pachana, K.; Wattanakornsiri, A.; Nanuam, J. Heavy metal transport and fate in the environmental compartments. Int. J. Sci. 2010, 7, 1–11. [Google Scholar]
- Zhang, H.; Tian, Y.; Guan, B.; Zhou, D.; Sun, Z.; Baskin, C.C. The best salt solution parameter to describe seed/seedling responses to saline and sodic salts. Plant Soil 2018, 426, 313–325. [Google Scholar] [CrossRef]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.; Evans, L.; Lambert, R. Effects of cement or lime on Cd, Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils. J. Hazard. Mater. 2012, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Rinklebe, J. Impact of emerging and low cost alternative amendments on the (im) mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Solidification/stabilization of arsenic containing solid wastes using portland cement, fly ash and polymeric materials. J. Hazard. Mater. 2006, 131, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012089. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.U.; Khan, M.Z.; Khan, A.; Saba, S.; Hussain, F.; Jan, I.U. Effect of humic acid on growth and crop nutrient status of wheat on two different soils. J. Plant Nutr. 2018, 41, 453–460. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Melis, P. Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 2005, 60, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Gutierrez, J.; Yun, S.W.; Lee, Y.B.; Yu, C.; Kim, P.J. Heavy metal contamination of arable soil and corn plant in the vicinity of a zinc smelting factory and stabilization by liming. Arch. Environ. Contam. Toxicol. 2009, 56, 190–200. [Google Scholar] [CrossRef]
- Lagerwerff, J.V.; Biersdorf, G.T.; Milberg, R.P.; Brower, D.L. Effects of Incubation and Liming on Yield and Heavy Metal Uptake by Rye from Sewage-Sludged Soil. J. Environ. Qual. 1977, 6, 427–431. [Google Scholar] [CrossRef]
- Hooda, P.S.; Alloway, B.J. The effect of liming on heavy metal concentrations in wheat, carrots and spinach grown on previously sludge-applied soils. J. Agric. Sci. 1996, 127, 289–294. [Google Scholar] [CrossRef]
- Silva, H.F.; Silva, N.F.; Oliveira, C.M.; Matos, M.J. Heavy Metals Contamination of Urban Soils—A Decade Study in the City of Lisbon, Portugal. Soil Syst. 2021, 5, 27. [Google Scholar] [CrossRef]
- Nayak, A.K.; Kumar, A.; Raja, R.; Rao, K.S.; Mohanty, S.; Shahid, M.; Tripathy, R.; Panda, B.B.; Bhattacharyya, P. Fly ash addition affects microbial biomass and carbon mineralization in agricultural soils. Bull. Environ. Contam. Toxicol. 2014, 92, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.K.; Singh, N.; Singh, S.B. Effect of fly ash amendment on metolachlor and atrazine degradation and microbial activity in two soils. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef]
- Doğan, K.; Sarıoğlu, A.; Coşkan, A. Contribution of Green Manure, Rhizobium and Humic + Fulvic Acid on Recovering Soil Biologic Activity of Olive Mill Wastewater Contaminated Soil. Sci. Pap. Ser. A Agron. 2016, 59, 63–68. [Google Scholar]
- Xue, D.; Huang, X.; Yao, H.; Huang, C. Effect of lime application on microbial community in acidic tea orchard soils in comparison with those in wasteland and forest soils. J. Environ. Sci. 2010, 22, 1253–1260. [Google Scholar] [CrossRef]
- Inagaki, T.M.; de Moraes Sá, J.C.; Caires, E.F.; Gonçalves, D.R.P. Why does carbon increase in highly weathered soil under no-till upon lime and gypsum use? Sci. Total Environ. 2017, 599, 523–532. [Google Scholar] [CrossRef] [PubMed]

| HM Fractions | Bio-Available to Total HM Ratios | |||
|---|---|---|---|---|
| Pb | Cd | Ni | Cr | |
| Bio-available | 0.76 ** | 0.30 | 0.97 ** | 0.86 ** |
| Total | 0.46 | 0.36 | 0.70 ** | 0.35 |
| Fly Ash-Soil-Ameliorant Combinations | PC1 | PC2 | PC3 |
|---|---|---|---|
| A1T0 | −1.705 | −0.676 | 0.749 |
| A1T1 | 2.328 | −0.478 | −0.142 |
| A1T2 | 0.608 | −0.909 | 0.292 |
| A1T3 | 1.634 | 0.111 | −0.209 |
| A1T4 | −0.276 | −0.886 | −0.312 |
| A2T0 | −2.705 | −0.711 | 0.225 |
| A2T1 | 2.132 | −0.326 | 0.238 |
| A2T2 | 1.041 | −1.041 | 0.359 |
| A2T3 | 1.418 | 1.195 | 0.292 |
| A2T4 | −0.423 | −0.095 | −1.364 |
| A3T0 | −3.622 | 0.644 | 0.024 |
| A3T1 | 0.732 | 0.892 | 0.087 |
| A3T2 | −0.452 | 0.914 | −0.256 |
| A3T3 | 0.0749 | 1.600 | 0.398 |
| A3T4 | −0.784 | −0.231 | −0.383 |
| Standard deviation | 1.71 | 0.84 | 0.49 |
| Proportion of Variance (%) | 73.36 | 18.05 | 6.02 |
| Cumulative Proportion (%) | 73.36 | 91.41 | 97.43 |
| Fly Ash-Soil-Ameliorant Combinations | MBC | Dehydrogenase |
|---|---|---|
| A1T0 | 34.34 e ± 1.2 | 4.35 f ± 1.26 |
| A1T1 | 65.84 b ± 0.81 | 11.92 b ± 1.16 |
| A1T2 | 55.45 c ± 0.81 | 7.64 d ± 0.81 |
| A1T3 | 56.82 c ± 0.21 | 8.80 c ± 0.65 |
| A1T4 | 79.53 a ± 0.99 | 13.8 a ± 1.24 |
| A2T0 | 18.39 g ±1.63 | 1.77 g ± 0.31 |
| A2T1 | 31.24 f ± 1.65 | 5.07 e ± 0.11 |
| A2T2 | 33.89 ef ± 1.85 | 5.06 e ± 0.60 |
| A2T3 | 33.86 ef ± 1.34 | 5.11 e ± 0.77 |
| A2T4 | 45.09 d ± 0.66 | 7.69 d ± 1.10 |
| A3T0 | 4.88 i ± 0.43 | 0.47 h ± 0.11 |
| A3T1 | 4.84 i ± 0.99 | 0.47 h ± 0.07 |
| A3T2 | 6.78 i ± 0.94 | 0.50 h ± 0.01 |
| A3T3 | 4.68 i ± 1.23 | 0.29 h ± 0.02 |
| A3T4 | 11.63 h ± 2.22 | 0.63 h ± 0.12 |
| Parameters | Fly Ash | Soil | Referenced Methods |
|---|---|---|---|
| Physical properties | |||
| Sand (%) | 56.66 | 29.47 | Bouyoucos [20] |
| Silt (%) | 38.45 | 52.20 | |
| Clay (%) | 4.32 | 18.33 | |
| Textural Class | Sandy silty loam | Silty loam | |
| Bulk density (g cm−3) | 1.01 | 1.35 | |
| Physio-chemical and chemical properties | |||
| pH (1:2.5, H2O) | 7.91 | 7.01 | Jackson [21] |
| EC (dsm−1) | 0.41 | 0.21 | |
| Organic C (%) | 0.004 | 0.61 | Walkley and Black [22] |
| Available N (mg kg−1) | - | 92.31 | Subbai and Asija [23] |
| Olsen-P (mgkg−1) | 46.21 | 16.38 | Olsen et al. [24] |
| NH4OAc-K | 85.32 | 80.51 | Hanway and Heidel [25] |
| Fe (mgkg−1) | 12.11 | 7.45 | Lindsay and Norvell [26] |
| Mn (mgkg−1) | 9.15 | 14.21 | |
| Zn (mgkg−1) | 1.98 | 1.08 | |
| Cu (mgkg−1) | 2.49 | 1.66 | |
| Biological properties | |||
| MBC (µg g−1) | 15.21 | 188.61 | Vance et al. [27] |
| Dehydrogenase (µg TPF g−1 h−1) | 2.14 | 19.21 | Klein et al. [28] |
| Different Combinations of Treatments | Notations |
|---|---|
| 50% fly ash + 50% soil + no ameliorant | A1T0 |
| 50% fly ash + 50% soil + lime at 5 Mg ha−1 | A1T1 |
| 50% fly ash + 50% soil + sodium sulphide at 2 Mg ha−1 | A1T2 |
| 50% fly ash + 50% soil + di-ammonium phosphate at 0.5 Mg ha−1 | A1T3 |
| 50% fly ash + 50% soil + humic acid at 4 Mg ha−1 | A1T4 |
| 75% fly ash + 25% soil + no ameliorant | A2T0 |
| 75% fly ash + 25% soil + lime at 5 Mg ha−1 | A2T1 |
| 75% fly ash + 25% soil + sodium sulphide at 2 Mg ha−1 | A2T2 |
| 75% fly ash + 25% soil + di-ammonium phosphate at 0.5 Mg ha−1 | A2T3 |
| 75% fly ash + 25% soil + humic acid at 4 Mg ha−1 | A2T4 |
| 100% fly ash + no ameliorant | A3T0 |
| 100% fly ash + lime at 5 Mg ha−1 | A3T1 |
| 100% fly ash + sodium sulphide at 2 Mg ha−1 | A3T2 |
| 100% fly ash + di-ammonium phosphate at 0.5 Mg ha−1 | A3T3 |
| 100% fly ash + humic acid at 4 Mg ha−1 | A3T4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, J.K.; Mukherjee, S.; Saha, N.; Halder, N.; Biswas, T.; Chakraborty, S.; Hassan, S.; Hassan, M.M.; Abo-Shosha, A.A.; Hossain, A. Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil. Molecules 2021, 26, 7019. https://doi.org/10.3390/molecules26227019
Mandal JK, Mukherjee S, Saha N, Halder N, Biswas T, Chakraborty S, Hassan S, Hassan MM, Abo-Shosha AA, Hossain A. Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil. Molecules. 2021; 26(22):7019. https://doi.org/10.3390/molecules26227019
Chicago/Turabian StyleMandal, Joy Kumar, Siddhartha Mukherjee, Niharendu Saha, Nibedan Halder, Tufleuddin Biswas, Sanjoy Chakraborty, Sabry Hassan, Mohamed M. Hassan, Ali A. Abo-Shosha, and Akbar Hossain. 2021. "Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil" Molecules 26, no. 22: 7019. https://doi.org/10.3390/molecules26227019
APA StyleMandal, J. K., Mukherjee, S., Saha, N., Halder, N., Biswas, T., Chakraborty, S., Hassan, S., Hassan, M. M., Abo-Shosha, A. A., & Hossain, A. (2021). Assessing the Capability of Chemical Ameliorants to Reduce the Bioavailability of Heavy Metals in Bulk Fly Ash Contaminated Soil. Molecules, 26(22), 7019. https://doi.org/10.3390/molecules26227019









