Bioactive Carbohydrate Polymers—Between Myth and Reality
Abstract
:1. Introduction—What Is a Bioactive Polysaccharide?
2. Structure-Function Relationships
2.1. Antioxidant Function of Polysaccharides
2.2. Immunomodulatory Function of Polysaccharides
2.3. Antitumor Function of Polysaccharides
3. Elicitation and Biostimulation
3.1. Polysaccharides as Inducers of Plant Defenses
3.2. Polysaccharides as Plant Growth Stimulator
4. Medicine
5. Food and Feed
5.1. Polysaccharides in Food Field
5.2. Polysaccharides in Feed Field
6. Antimicrobial/Antiviral Agents
7. Chemical, Chemo-Enzymatic, and Enzymatic Functionalization of Polysaccharides
7.1. Chemical Functionalization
7.2. Chemo-Enzymatic Functionalization
7.3. Enzymatic Functionalization
8. Current Markets
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Sili, C.; Paperi, R.; Vincenzini, M. Exopolysaccharide-producing cyanobacteria and their possible exploitation: A review. J. Appl. Phycol. 2001, 13, 293–299. [Google Scholar] [CrossRef]
- Raposo, M.P.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.H.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates—Comprehensive Studies on GlycoBiology and Glycotechnology; Chang, C.-F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 489–532. [Google Scholar]
- Arad, S.M.; Levy-Ontman, O. Red microalgae cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Misurcova, L.; Orsavova, J.; Ambrozova, J.V. Algal Polysaccharides and Health. Polysacch. Bioactivity Biotechnol. 2015, 110–144. [Google Scholar]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Kakar, M.U.; Naveed, M.; Saeed, M.; Zhao, S.; Rasheed, M.; Firdos, S.; Dai, R. A review on structure, extraction, and Biol.ogical activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. Int. J. Biol. Macromol. 2020, 156, 420–429. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ye, J.; Zhao, Y.; Su, W. Partial characterization, antioxidant and antitumor activities of polysaccharides from Philomycus bilineatus. Int. J. Biol. Macromol. 2014, 65, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, J.; Zhang, L.; Hu, M.; Xu, Y.; Su, W. Extraction, characterization, and Biol.ogical activity of polysaccharides from Sophora flavescens Ait. Int. J. Biol. Macromol. 2016, 93, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of in vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Radzki, W.; Ziaja-Sołtys, M.; Nowak, J.; Rzymowska, J.; Dominik, J.; Sławińska, A.; Michalak-Majewska, M.; Zalewska-Korona, M.; Kuczumow, A. Effect of processing on the content and Biol.ogical activity of polysaccharides from Pleurotus ostreatus mushroom. LWT Food Sci. Technol. 2016, 66, 27–33. [Google Scholar] [CrossRef]
- Han, L.; Suo, Y.; Yang, Y.; Meng, J.; Hu, N. Optimization, characterization, and Biol.ogical activity of polysaccharides from Berberis dasystachya Maxim. Int. J. Biol. Macromol. 2016, 85, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Zhang, D.; Yu, B.; Zhao, S.-P.; Wang, J.-W.; Yao, L.; Cao, W.-G. Antioxidant activity and optimization of extraction of polysaccharide from the roots of Dipsacus asperoides. Int. J. Biol. Macromol. 2015, 81, 332–339. [Google Scholar] [CrossRef]
- Yao, Y.L.; Shu, C.; Feng, G.; Wang, Q.; Yan, Y.-Y.; Yi, Y.; Wang, H.-X.; Zhang, X.-F.; Wang, L.-M. Polysaccharides from Pyracantha fortuneana and its Biol.ogical activity. Int. J. Biol. Macromol. 2020, 150, 1162–1174. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models In Vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Meng, L.; Sun, S.; Li, R.; Shen, Z.; Wang, P.; Jiang, X. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr. Polym. 2015, 117, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Nandi, A.K.; Sen, I.K.; Maity, P.; Pattanayak, M.; Devi, K.S.P.; Khatua, S.; Maiti, T.K.; Acharya, K.; Islam, S.S. Studies on antioxidative and immunostimulating fucogalactan of the edible mushroom Macrolepiota dolichaula. Carbohydr. Res. 2015, 413, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Zhao, Y.; Tian, Y.; Miao, S.; Zheng, B. Extraction optimization, structure and antioxidant activities of Fortunella margarita Swingle polysaccharides. Int. J. Biol. Macromol. 2015, 74, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Xu, W.; Liang, J.-W.; Wang, C.-S.; Kang, Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization, and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Ren, X.; Li, B.; Zhang, Q. Extraction, preliminary characterization and In Vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Faulkner, C.L.; Nelson-Overton, L.K.; Wiley, J.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int. Immunopharmacol. 2005, 5, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Yang, H.R.; Wu, X.L.; Peng, F.; Huang, Z.; Pu, L.; Lou, W.Y. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int. J. Biol. Macromol. 2019, 137, 568–575. [Google Scholar] [CrossRef]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Shao, J.; Ren, X.; Jia, J.; Li, B. Study on the immunomodulatory activity of a novel polysaccharide from the lichen Umbilicaria esculenta. Int. J Biol. Macromol. 2019, 121, 846–851. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef]
- Ma, L.; Jiao, K.; Luo, L.; Xiang, J.; Fan, J.; Zhang, X.; Zhu, W. Characterization and macrophage immunomodulatory activity of two polysaccharides from the flowers of Paeonia suffruticosa Andr. Int. J Biol. Macromol. 2019, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Gu, D.; Wang, X.; Shen, X.; Yan, L.; Zhang, W.; Fan, J. Purification, characterization and immunomodulatory activity of polysaccharides from Leccinum crocipodium (Letellier.) Watliag. Int. J. Biol. Macromol. 2020, 148, 647–656. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, characterization and Biol.ogical activity of polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Xing, X.; Cheng, T.J.R.; Liang, P.H.; Bulone, V.; Hsieh, Y.S. Structural analysis and Biol.ogical activity of cell wall polysaccharides extracted from Panax ginseng marc. Int. J. Biol. 2019, 135, 29–37. [Google Scholar]
- Li, S.; Gao, A.; Dong, S.; Chen, Y.; Sun, S.; Lei, Z.; Zhang, Z. Purification, antitumor and immunomodulatory activity of polysaccharides from soybean residue fermented with Morchella esculenta. Int. J. Biol. Macromol. 2017, 96, 26–34. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef]
- Yang, X.; Ji, H.; Feng, Y.; Yu, J.; Liu, A. Structural Characterization and Antitumor Activity of Polysaccharides from Kaempferia galanga L. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.; Yang, Z.; Liu, A. Relationship between structural properties and antitumor activity of Astragalus polysaccharides extracted with different temperatures. Int. J. Biol. Macromol. 2019, 124, 469–477. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate Polymers Exhibit Great Potential as Effective Elicitors in Organic Agriculture: A Review. Carbohydr. Polym. 2020, 230, 115637. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, M.M.A.; Jahan, A.; Shabbir, A.; Jaleel, H. Increased Production of Valuable Secondary Products in Plants by Leaf Applied Radiation-Processed Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection against Pathogens in Plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed Oligosaccharides Stimulate Plant Growth by Enhancing Carbon and Nitrogen Assimilation, Basal Metabolism, and Cell Division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Drira, M.; Ben Mohamed, J.; Ben Hlima, H.; Hentati, F.; Michaud, P.; Abdelkafi, S.; Fendri, I. Improvement of Arabidopsis thaliana salt tolerance using a polysaccharidic extract from the brown algae Padina pavonica. Algal. Res. 2021, 56, 102324. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of Carbohydrate Additives on the Growth Rate of Microalgae Biomass with an Increased Carbohydrate Content. Mar. Drugs 2021, 19, 381. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Andreeva, A.; Budenkova, E.; Sukhikh, S.; Babich, O.; Ivanova, S.; Prosekov, A.; Ulrikh, E. Study of Morphological Features and Determination of the Fatty Acid Composition of the Microalgae Lipid Complex. Biomolecules 2020, 10, 1571. [Google Scholar] [CrossRef]
- Dey, P.; Ramanujam, R.; Venkatesan, G.; Nagarathnam, R. Sodium Alginate Potentiates Antioxidant Defense and PR Proteins against Early Blight Disease Caused by Alternaria Solani in Solanum Lycopersicum Linn. PLoS ONE 2019, 14, 1–26. [Google Scholar]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of Alginate Extracted from Moroccan Brown Algae to Stimulate Natural Defense in Date Palm Roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef]
- Mani, S.D.; Govindan, M.; Muthamilarasan, M.; Nagarathnam, R. A Sulfated Polysaccharide κ-Carrageenan Induced Antioxidant Defense and Proteomic Changes in Chloroplast against Leaf Spot Disease of Tomato. J. Appl. Phycol. 2021, 33, 2667–2681. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of Local Plant Immunity against Tobacco Mosaic Virus Infection after Treatment with Sulphated-Carrageenan from Red Alga (Hypnea musciformis). Physiol. Mol. Plant. Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kandasamy, S.; Khan, W.; Bahia, N.S.; Singh, R.P.; Critchley, A.T.; Prithiviraj, B. λ-Carrageenan Suppresses Tomato Chlorotic Dwarf Viroid (TCDVd) Replication and Symptom Expression in Tomatoes. Mar. Drugs 2015, 13, 2875–2889. [Google Scholar] [CrossRef]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.C.; Kloareg, B.; Fritig, B. Sulfated Fucan Oligosaccharides Elicit Defense Responses in Tobacco and Local and Systemic Resistance against Tobacco Mosaic Virus. Mol. Plant Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.El. Evaluation of Microalgae Polysaccharides as Biostimulants of Tomato Plant Defense Using Metabolomics and Biochemical Approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Drira, M.; Elleuch, J.; Hlima, H.B.; Hentati, F.; Gardarin, C.; Rihouey, C.; Cerf, D.L.; Michaud, P.; Abdelkafi, S.; Fendri, I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to Induce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.H.; Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Alginate Oligosaccharides Enhanced Triticum Aestivum L. Tolerance to Drought Stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Zhuo, R.; Li, B.; Tian, S. Alginate Oligosaccharide Improves Resistance to Postharvest Decay and Quality in Kiwifruit (Actinidia deliciosa Cv. Bruno). Hortic. Plant J. 2021. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.; Jiang, L.; Hou, Y.; Yang, F.; Chen, W.; Li, X. Elicitor Activity of Algino-Oligosaccharide and Its Potential Application in Protection of Rice Plant (Oryza Saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015, 29, 646–652. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Noormohammadi, A. Effects of Sodium Alginate Elicitation on Secondary Metabolites and Antioxidant Activity of Safflower Genotypes under in Vitro Salinity Stress. In Vitro Cell. Dev. Biol. Plant 2019, 55, 527–538. [Google Scholar] [CrossRef]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate Oligosaccharide (AOS) Induced Resistance to Pst DC3000 via Salicylic Acid-Mediated Signaling Pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115221. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, X.; Yan, G.; Liu, L.; Zheng, H.; Zhao, B.; Tang, J.; Guo, Y.D. Alginate-Derived Oligosaccharides Promote Water Stress Tolerance in Cucumber (Cucumis Sativus L.). Plant Physiol. Biochem. 2018, 130, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Microalgae: New Source of Plant Biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- El Arroussi, H.; Elmernissi, N.; Benhima, R.; El Kadmiri, I.M.; Bendaou, N.; Smouni, A.; Wahby, I. Microalgae Polysaccharides a Promising Plant Growth Biostimulant. J. Algal Biomass. Utln. 2016, 7, 55–63. [Google Scholar]
- Yang, J.; Shen, Z.; Sun, Z.; Wang, P.; Jiang, X. Growth Stimulation Activity of Alginate-Derived Oligosaccharides with Different Molecular Weights and Mannuronate/Guluronate Ratio on Hordeum vulgare L. J. Plant Growth Regul. 2021, 40, 91–100. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Soból, M. Oligo-Alginate with Low Molecular Mass Improves Growth and Physiological Activity of Eucomis autumnalis under Salinity Stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-Carrageenans Stimulate Growth by Enhancing Photosynthesis, Basal Metabolism, and Cell Cycle in Tobacco Plants (Var. Burley). J. Plant Growth Regul. 2012, 31, 173–185. [Google Scholar] [CrossRef]
- Saucedo, S.; Contreras, R.A.; Moenne, A.; Oligo-Carrageenan Kappa Increases, C.N.S. Assimilation, Auxin and Gibberellin Contents, and Growth in Pinus Radiata Trees. J. For. Res. 2015, 26, 635–640. [Google Scholar] [CrossRef]
- Albuquerque, I.R.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.A.; Leite, E.L.; Nader, H.B.; Rocha, H.A.O. Evaluation of Anti-Nociceptive and Anti-Inflammatory Activities of a Heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Chapter 5—Algal polysaccharides, novel application, and outlook. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Chen, H.; Zhang, S.; Yu, S.; Li, Y.; Cui, M.; Pan, W.; Yang, X. A novel ion-activated in situ gelling ophthalmic delivery system based on κ-carrageenan for acyclovir. Drug Dev. Ind. Pharm. 2018, 44, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, R.M.; Alnaief, M.; Mashaqbeh, H. Investigation of Carrageenan Aerogel Microparticles as a Potential Drug Carrier. AAPS Pharm. Sci. Tech. 2018, 19, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.S.; Moghannem, S.A.M.; Shehabeldine, A.M.; Azab, M.S. Antitumor effect of exopolysaccharide produced by Bacillus mycoides. Microb. Pathog. 2020, 140, 103947. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-P.; Ooi, C.-W.; Tey, B.-T.; Chan, E.-S. Controlled delivery of oral insulin aspart using pH-responsive alginate/κ-carrageenan composite hydrogel beads. React. Funct. Polym. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate—carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Yermak, I.M.; Gorbach, V.I.; Karnakov, I.A.; Davydova, V.N.; Pimenova, E.A.; Chistyulin, D.A.; Isakov, V.V.; Glazunov, V.P. Carrageenan gel beads for echinochrome inclusion: Influence of structural features of carrageenan. Carbohydr. Polym. 2021, 272, 118479. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lam, U.-I. Chitosan/Fucoidan pH Sensitive Nanoparticles for Oral Delivery System. J. Chin. Chem. Soc. 2011, 58, 779–785. [Google Scholar] [CrossRef]
- Don, T.-M.; Chang, W.-J.; Jheng, P.-R.; Huang, Y.-C.; Chuang, E.-Y. Curcumin-laden dual-targeting fucoidan/chitosan nanocarriers for inhibiting brain inflammation via intranasal delivery. Int. J. Biol. Macromol. 2021, 181, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Enomoto, A.; Todoh, H.; Ametani, A.; Kaminogawa, S. Activation of Murine Macrophages by Polysaccharide Fractions from Marine Algae (Porphyra yezoensis). Biosci. Biotechnol. Biochem. 1993, 57, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.-T.; Jeon, Y.-J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocoll. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Barahona, T.; Chandía, N.P.; Encinas, M.V.; Matsuhiro, B.; Zúñiga, E.A. Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocoll. 2011, 25, 529–535. [Google Scholar] [CrossRef]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and Antithrombotic Properties in Vitro and in Vivo of a Novel Sulfated Polysaccharide from Marine Green Alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef]
- Ciancia, M.; Quintana, I.; Cerezo, A.S. Overview of Anticoagulant Activity of Sulfated Polysaccharides from Seaweeds in Relation to their Structures, Focusing on those of Green Seaweeds. Curr. Med. Chem. 2010, 17, 2503–2529. [Google Scholar] [CrossRef] [PubMed]
- Gheda, S.; El-Sheekh, M.; Abou-Zeid, A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pac. J. Trop. Med. 2018, 11, 583–589. [Google Scholar]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Barboríková, J.; Šutovská, M.; Kazimierová, I.; Jošková, M.; Fraňová, S.; Kopecký, J.; Capek, P. Extracellular polysaccharide produced by Chlorella vulgaris—Chemical characterization and anti-asthmatic profile. Int. J. Biol. Macromol. 2019, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy Drug Targets 2012, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Dalavi, P.A.; Venkatesan, J.; Rani, V.; Anil, S. Marine Polysaccharides Systems for Drug Delivery Applications BT—Advanced Biopolymeric Systems for Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 373–386. [Google Scholar]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Sarıyer, S.; Duranoğlu, D.; Doğan, Ö.; Küçük, İ. pH-responsive double network alginate/kappa-carrageenan hydrogel beads for controlled protein release: Effect of pH and crosslinking agent. J. Drug Deliv. Sci. Technol. 2020, 56, 101551. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, A.A.; Sayed, S.M.; Abou-Okeil, A. K-carrageenan/Na-alginate wound dressing with sustainable drug delivery properties. Polym. Adv. Technol. 2021, 32, 1793–1801. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.-O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Chiang, C.-S.; Hsu, C.-H.; Cheng, H.-W.; Chen, S.-Y. Development and Characterization of a Fucoidan-Based Drug Delivery System by Using Hydrophilic Anticancer Polysaccharides to Simultaneously Deliver Hydrophobic Anticancer Drugs. Biomolecules 2020, 10, 970. [Google Scholar] [CrossRef]
- Wang, P.; Kankala, R.K.; Chen, B.; Long, R.; Cai, D.; Liu, Y.; Wang, S. Poly-allylamine hydrochloride and fucoidan-based self-assembled polyelectrolyte complex nanoparticles for cancer therapeutics. J. Biomed. Mater. Res. Part. A 2019, 107, 339–347. [Google Scholar] [CrossRef]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; Le Visage, C. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2015, 5, 187–197. [Google Scholar] [CrossRef]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Usha, S.K.; Arumugam, M. Bioactive and thermostable sulphated polysaccharide from Sargassum swartzii with drug delivery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar]
- Phan, N.H.; Ly, T.T.; Pham, M.N.; Luu, T.D.; Vo, T.V.; Tran, P.H.; Tran, T.T. A Comparison of Fucoidan Conjugated to Paclitaxel and Curcumin for the Dual Delivery of Cancer Therapeutic Agents. Anticancer. Agents Med. Chem. 2018, 18, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Frias, A.M.; Carida, M.; Cancedda, R.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Carrageenan-Based Hydrogels for the Controlled Delivery of PDGF-BB in Bone Tissue Engineering Applications. Biomacromolecules 2009, 10, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Popa, E.; Reys, L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carrageenans. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; AACC International Press: London, UK, 2019; pp. 279–291. [Google Scholar]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Purwanti, T.; Maulydia, D.; Estherline, C.A.; Hendradi, E.; Rahmadi, M. Performance and drug deposition of kappa-carrageenan microspheres encapsulating ciprofloxacin HCl: Effect of polymer concentration. J. Adv. Pharm. Technol. Res. 2021, 12, 242–249. [Google Scholar] [PubMed]
- Yuan, H.; Song, J.; Zhang, W.; Li, X.; Li, N.; Gao, X. Antioxidant activity and cytoprotective effect of kappa-carrageenan oligosaccharides and their different derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, B.D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar]
- Hoffman, R.C. arrageenans inhibit growth-factor binding. Biochem. J. 1993, 289, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Gao, Y.; Yan, X.-J. Carrageenan oligosaccharides inhibit growth-factor binding and heparanase activity. Yao Xue Xue Bao 2011, 46, 280–284. [Google Scholar]
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother. Res. 2004, 18, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.D.; Dave, A.M.; Devi, S. Entrapment of lipase into K-carrageenan beads and its use in hydrolysis of olive oil in biphasic system. J. Mol. Catal. B Enzym. 2004, 31, 143–150. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Patwa, R.; Zandraa, O.; Saha, N.; Saha, P. Preparation and characterization of injectable self-antibacterial gelatin/carrageenan/bacterial cellulose hydrogel scaffolds for wound healing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102415. [Google Scholar] [CrossRef]
- Popa, E.G.; Rodrigues, M.T.; Coutinho, D.F.; Oliveira, M.B.; Mano, J.F.; Reisab, R.L.; Gomes, M.E. Cryopreservation of cell laden natural origin hydrogels for cartilage regeneration strategies. Soft Matter 2013, 9, 875–885. [Google Scholar] [CrossRef]
- Popa, E.G.; Carvalho, P.P.; Dias, A.F.; Santos, T.C.; Santo, V.E.; Marques, A.P.; Viegas, C.A.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Evaluation of the in vitro and in vivo biocompatibility of carrageenan-based hydrogels. J. Biomed. Mater. Res. 2014, 102, 4087–4097. [Google Scholar] [CrossRef]
- Popa, E.; Reis, R.; Gomes, M. Chondrogenic phenotype of different cells encapsulated in κ-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol. Appl. Biochem. 2012, 59, 132–141. [Google Scholar] [CrossRef]
- Bornhöft, M.; Thommes, M.; Kleinebudde, P. Preliminary assessment of carrageenan as excipient for extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2005, 59, 127–131. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Meyer, D.E.; Shin, B.C.; Kong, G.A.; Dewhirst, M.W.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Maciel, D.J.; de Mello Ferreira, I.L.; da Costa, G.M.; da Silva, M.R. Nanocomposite hydrogels based on iota-carrageenan and maghemite: Morphological, thermal and magnetic properties. Eur. Polym. J. 2016, 76, 147–155. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Yılmaz, S. Microgels Derived from Different Forms of Carrageenans, Kappa, Iota, and Lambda for Biomedical Applications. MRS Adv. 2017, 2, 2521–2527. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A.S. Structure, Biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Lewis, L.A.; McCourt, R.M. Green algae and the origin of land plants. Am. J. Bot. 2004, 91, 1535–1556. [Google Scholar] [CrossRef]
- Domozych, D.S.; Stewart, K.D.; Mattox, K.R. The comparative aspects of cell wall chemistry in the green algae (Chlorophyta). J. Mol. Evol. 1980, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Tuohy, M.G. Beyond the Green: Understanding the Evolutionary Puzzle of Plant and Algal Cell Walls. Plant. Physiol. 2010, 153, 373–383. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva Rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.A.; Reis, R.L. Processing ulvan into 2D structures: Cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Morelli, A.; Chiellini, F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. 2013, 101, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Pina, C.D.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Pippa, N.; Georgantea, P.; Ioannou, E.; Demetzos, C.; Roussis, V. Marine sulfated polysaccharides as versatile polyelectrolytes for the development of drug delivery nanoplatforms: Complexation of ulvan with lysozyme. Int. J. Biol. Macromol. 2018, 118, 69–75. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Bhatt, H.; Khandelwal, A.; Vasu, V.T.; Reddy, C.R.K.; Lakshmi, D.S. A facile chemical cross-linking approach toward the fabrication of a sustainable porous ulvan scaffold. J. Bioact. Compat. Polym. 2020, 35, 301–313. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Morelli, A.; Bartoli, C.; Declercq, H.A.; Douglas, T.E.L.; Dubruel, P.; Chiellini, F. Ulvan-chitosan polyelectrolyte complexes as matrices for enzyme induced biomimetic mineralization. Carbohydr. Polym. 2018, 182, 254–264. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Bartoli, C.; Morelli, A.; Smet, P.F.; Dubruel, P.; Chiellini, F. Biofunctionalization of Ulvan Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Bang, T.H.; Van, T.T.; Hung, L.X.; Ly, B.M.; Nhut, N.D.; Thuy, T.T. Nanogels of acetylated ulvan enhance the solubility of hydrophobic drug curcumin. Bull. Mater. Sci. 2019, 42, 1. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Aggelidou, E.; Tziveleka, L.-A.; Demiri, E.; Bakopoulou, A.; Zinelis, S.; Kritis, A.; Roussis, V. The Marine Polysaccharide Ulvan Confers Potent Osteoinductive Capacity to PCL-Based Scaffolds for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 3086. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biol.ogical activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Kesavan, S.; Smeen, K.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and D-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Kim, K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- Madub, K.; Goonoo, N.; Gimié, F.; Arsa, I.A.; Schönherr, H.; Bhaw-Luximon, A. Green seaweeds ulvan-cellulose scaffolds enhance in vitro cell growth and in vivo angiogenesis for skin tissue engineering. Carbohydr. Polym. 2021, 251, 117025. [Google Scholar] [CrossRef]
- Molino, P.J.; Garcia, L.; Stewart, E.M.; Lamaze, M.; Zhang, B.; Harris, A.R.; Winbergde, P.; Wallace, G.G. PEDOT doped with algal, mammalian and synthetic dopants: Polymer properties, protein and cell interactions, and influence of electrical stimulation on neuronal cell differentiation. Biomater. Sci. 2018, 6, 1250–1261. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid Sponge-Like Scaffolds Based on Ulvan and Gelatin: Design, Characterization and Evaluation of Their Potential Use in Bone Tissue Engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Zelinsky, N.D. Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; (Woodhead Publishing Series in Food Science, Technology and Nutrition), Domínguez, F.N., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 23–86. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, J.-K.; Cho, T.-S. Applications of ophthalmic biomaterials embedded with fucoidan. J. Ind. Eng. Chem. 2012, 18, 1197–1201. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Piccoli, A.; Totani, L.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Grachev, A.A.; et al. Fucans, but not fucomannoglucuronans, determine the Biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS ONE 2011, 6, e17283. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 2003, 136, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Yang, Y.; Wei, H.; Liu, Z.; Liu, Z.; Ma, Y.; Gao, Z.; Hou, L.; Zou, X. Fucoidan Suppresses Hypoxia-Induced Lymphangiogenesis and Lymphatic Metastasis in Mouse Hepatocarcinoma. Mar. Drugs 2015, 13, 3514–3530. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-K.; Lee, M.; Kwon, H.O.; Lee, D.; Park, J.; Kim, E.; You, Y.; Lim, Y.T.; Jun, W.; Lee, J. Costaria costata Extract Suppresses Development of Atopic Dermatitis in chloro-2,4-dinitrobenzene-treated NC/Nga Mice. Skin Pharmacol. Physiol. 2018, 31, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef]
- Rui, X.; Pan, H.-F.; Shao, S.-L.; Xu, X.-M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kwon, M.-J.; Nam, T.-J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017, 15, 4116–4122. [Google Scholar] [CrossRef]
- Park, H.Y.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.H.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Kim, D.H.; et al. Induction of p53-Independent Apoptosis and G1 Cell Cycle Arrest by Fucoidan in HCT116 Human Colorectal Carcinoma Cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef]
- Yu, S.-H.; Wu, S.-J.; Wu, J.-Y.; Wen, D.-Y.; Mi, F.-L. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 2015, 126, 97–107. [Google Scholar] [CrossRef]
- Wu, S.-J.; Don, T.-M.; Lin, C.-W.; Mi, F.-L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.D.; Ximenes, R.M.; Pessoa, O.D.; Magalhães, N.S.; de Britto Lira-Nogueira, M.C. Fucoidan-coated PIBCA nanoparticles containing oncocalyxone A: Activity against metastatic breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 65, 102698. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y. Preparation and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs. 2014, 12, 4379–4398. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yang, Y.-T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Liu, T.-J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef]
- Nakamura, S.; Nambu, M.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Takase, B.; Kishimoto, S.; Amano, Y.; Aoki, H.; Kiyosawa, T.; et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J. Biomed. Mater. Res. 2008, 85, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Gonçalves, C.S.; Martins, E.P.; Neves, N.M.; Reis, R.L.; Costa, B.M.; Silva, T.H.; Martins, A. Fucoidan/chitosan nanoparticles functionalized with anti-ErbB-2 target breast cancer cells and impair tumor growth in vivo. Int. J. Pharm. 2021, 600, 120548. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.A.M.; Guezennec, J.; Bowman, J.P. Bacterial exopolysaccharides from extreme marine environments with special consideration of the Southern Ocean, sea ice, and deep-sea hydrothermal vents: A review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Two new exopolysaccharides from a thermophilic bacterium Geobacillus sp. WSUCF1: Characterization and bioactivities. N. Biotechnol. 2021, 61, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Pichyangkura, R.; Nepogodiev, S.A.; Wonganan, P.; Mahalapbutr, P.; Field, R.A. Characterization of a nanoparticulate exopolysaccharide from Leuconostoc holzapfelii KM01 and its potential application in drug encapsulation. Int. J. Biol. Macromol. 2021, 187, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, P.; Kołodziejczyk, A.; Makowski, K.; Kotarba, S.; Walkowiak, B. In situ-formed bacterial exopolysaccharide (EPS) as a potential carrier for anchorage-dependent cell cultures. Eng. Biomater. 2021, 159, 18–23. [Google Scholar]
- Sun, C.; Wang, J.-W.; Fang, L.; Gao, X.-D.; Tan, R.-X. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 2004, 75, 1063–1073. [Google Scholar] [CrossRef]
- Sun, C.; Shan, C.Y.; Gao, X.D.; Tan, R.X. Protection of PC12 cells from hydrogen peroxide-induced injury by EPS2, an exopolysaccharide from a marine filamentous fungus Keissleriella sp. YS4108. J. Biotechnol. 2005, 115, 137–144. [Google Scholar] [CrossRef]
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I.; et al. Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Mar. Drugs 2021, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Murillo, M.A.; Ascencio, F. Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of red sore disease-associated bacteria and helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, Y.; Iqbal, N.M.; Yang, X.; Zhang, X. Sulfitobacter alexandrii sp. nov., a new microalgae growth-promoting bacterium with exopolysaccharides bioflocculanting potential isolated from marine phycosphere. Antonie Van Leeuwenhoek 2021, 114, 1091–1106. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735. [Google Scholar] [CrossRef]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; Mordujovich de Buschiazzo, P.; Ríos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Sun, H.-H.; Mao, W.-J.; Chen, Y.; Guo, S.-D.; Li, H.-Y.; Qi, X.-H.; Chen, Y.-L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chung, B.H. Antioxidant activity of levan coated cerium oxide nanoparticles. Carbohydr. Polym. 2016, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wang, W.-H.; Yang, Y.; Zhang, H.-N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of Biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Popoola, A.O. Biogenic synthesis and antimicrobial activity of silver nanoparticle using exopolysaccharides from Lactic Acid bacteria. Int. J. Nano Dimens. 2017, 8, 61–69. [Google Scholar]
- Sun, M.L.; Zhao, F.; Chen, X.L.; Zhang, X.Y.; Zhang, Y.Z.; Song, X.Y.; Sun, C.Y.; Yang, J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Kanti Bandyopadhyay, T.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, characterization and Biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Sharmili, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Bacterial exopolysaccharide (EPS)-coated ZnO nanoparticles showed high antibiofilm activity and larvicidal toxicity against malaria and Zika virus vectors. J. Trace Elem. Med. Biol. 2018, 45, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and Purification of the Polysaccharides with Marked Antitumor Activity, Especially Lentinan, from “Lentinus edodes” (Berk.) Sing. (an Edible Mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar]
- Matsuda, M.; Yamori, T.; Naitoh, M.; Okutani, K. Structural Revision of Sulfated Polysaccharide B-1 Isolated from a Marine Pseudomonas Species and Its Cytotoxic Activity Against Human Cancer Cell Lines. Mar. Biotechnol. 2003, 5, 13–19. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zanchetta, P.; Helley, D.; Ratiskol, J.; Sinquin, C.; Fischer, A.M.; Guezennec, J. Les polysaccharides microbiens d’origine marine et leur potentiel en thérapeutique humaine. Pathol. Biol. 2004, 52, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Aslim, B. Characterization of lactic acid bacteria derived exopolysaccharides for use as a defined neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells. Sci. Rep. 2020, 10, 8124. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, J.; Pignet, P.; Lijour, Y.; Gentric, E.; Ratiskol, J.; Colliec-Jouault, S. Sulfation and depolymerization of a bacterial exopolysaccharide of hydrothermal origin. Carbohydr. Polym 1998, 37, 19–24. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- De Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Al-Qaysi, S.A.S.; Thabit, Z.A.; Kadhem, S.B. Microbial levan from Brachybacterium phenoliresistens: Characterization and enhancement of production. Process. Biochem. 2017, 57, 9–15. [Google Scholar] [CrossRef]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Rederstorff, E.; Weiss, P.; Sourice, S.; Pilet, P.; Xie, F.; Sinquin, C.; Colliec-Jouault, S.; Guicheux, J.; Laïb, S. An in vitro study of two GAG-like marine polysaccharides incorporated into injectable hydrogels for bone and cartilage tissue engineering. Acta Biomater. 2011, 7, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.R.; Baud’Huin, M.; Sinquin, C.; Maillasson, M.; Heymann, D.; Colliec-Jouault, S.; Padrines, M. Effects of a sulfated exopolysaccharide produced by Altermonas infernus on bone Biology. Glycobiology 2011, 21, 781–795. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Zhang, X.-K.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Chen, X.-L. Improvement of the production of an Arctic bacterial exopolysaccharide with protective effect on human skin cells against UV-induced oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Chapman, V. Seaweeds and Their Uses; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Physiol 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Mahmut, M.K. Experience dependent changes in odour–viscosity perception. Acta Psychol. 2011, 136, 60–66. [Google Scholar] [CrossRef]
- Kuo, W.Y.; Lee, Y. Effect of food matrix on saltiness perception-implications for sodium reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 906–923. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Moroney, N.C.; O’Grady, M.N.; Lordan, S.; Stanton, C.; Kerry, J.P. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs 2015, 13, 2447–2464. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.G.; Knudsen, J.C.; Viereck, N.; Kristensen, M.; Astrup, A. Functionality of alginate-based supplements for application in human appetite regulation. Food Chem. 2012, 132, 823–829. [Google Scholar] [CrossRef]
- Mattes, R.D. Effects of a combination fiber system on appetite and energy intake in overweight humans. Physiol. Behav. 2007, 90, 705–711. [Google Scholar] [CrossRef]
- Albert, A.; Salvador, A.; Fiszman, S.M. A film of alginate plus salt as an edible susceptor in microwaveable food. Food Hydrocoll. 2012, 27, 421–426. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Juszczak, L.; Sikora, M. Sensory and textural profile of confectionery masses produced using natural honey and selected polysaccharide hydrocolloids as the basis. Zywnosc Nauka Technologia Jakosc 2011, 3, 40–52. [Google Scholar] [CrossRef]
- Piculell, L. Gelling carrageenans. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor and Francis: London, UK, 2006; p. 239. Available online: http://www.taylorandfrancis.com (accessed on 4 September 2021).
- Haghighimanesh, S.; Farahnaky, A. Ice cream powder production and investigation of its rheological and organoleptic properties. Int. J. Food Eng. 2011, 7. [Google Scholar] [CrossRef]
- Černíková, M.; Buňka, F.; Pospiech, M.; Tremlová, B.; Hladká, K.; Pavlínek, V.; Březina, P. Replacement of traditional emulsifying salts by selected hydrocolloids in processed cheese production. Int. Dairy J. 2010, 20, 336–343. [Google Scholar] [CrossRef]
- Ayub, M. Influence of different types of milk and stabilizers on sensory evaluation and whey separation of yoghurt. Biol. Sci. PJSIR 2004, 47, 398–402. [Google Scholar]
- Bixler, H.J.; Johndro, K.; Falshaw, R. Kappa-2 carrageenan: Structure and performance of commercial extracts: II. Performance in two simulated dairy applications. Food Hydrocoll. 2001, 15, 619–630. [Google Scholar] [CrossRef]
- Shon, J.; Yun, Y.; Shin, M.; Chin, K.B.; Eun, J.B. Effects of milk proteins and gums on quality of bread made from frozen dough. J. Sci. Food Agric. 2009, 89, 1407–1415. [Google Scholar] [CrossRef]
- Seol, K.H.; Lim, D.G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry FAO Fisheries; Technical Paper 441; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2003. [Google Scholar]
- Kumar, M.; Sharma, B.D. The storage stability and textural, physico-chemical and sensory quality of low-fat ground pork patties with Carrageenan as fat replacer. Int. J. Food Sci. Tech. 2004, 39, 31–42. [Google Scholar] [CrossRef]
- Plotto, A.; Narciso, J.A.; Rattanapanone, N.; Baldwin, E.A. Surface treatments and coatings to maintain fresh-cut mango quality in storage. J. Sci. Food Agric. 2010, 90, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kandasamy, S.; Zhang, J.; Kirby, C.W.; Karakach, T.; Hafting, J.; Prithiviraj, B. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement. Altern. Med. 2015, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yu, D.Y.; Kim, J.A.; Choi, E.Y.; Lee, C.Y.; Hong, Y.H.; Cho, K. Effects of Undaria pinnatifida and Laminaria japonica on rat’s intestinal microbiota and metabolite. Int. J. Nutr. Food Sci. 2016, 6, 502. [Google Scholar]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan extracts ameliorate acute colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef]
- Kuda, T.; Kosaka, M.; Hirano, S.; Kawahara, M.; Sato, M.; Kaneshima, T.; Kimura, B. Effect of sodium-alginate and laminaran on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Carbohydr. Polym 2015, 125, 113–119. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef]
- Carse, S.; Bergant, M.; Schäfer, G. Advances in targeting hpv infection as potential alternative prophylactic means. Int. J. Mol. Sci. 2021, 22, 2201. [Google Scholar] [CrossRef]
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications. In Advances in Cyanobacterial Biology; Academic Press: London, UK, 2020; pp. 173–194. [Google Scholar]
- Bouaziz, F.; Koubaa, M.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Biological properties of water-soluble polysaccharides and hemicelluloses from almond gum. Int. J. Biol. Macromol. 2017, 95, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 12, 100826. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef]
- Moga, M.A.; Dima, L.; Balan, A.; Blidaru, A.; Dimienescu, O.G.; Podasca, C.; Toma, S. Are bioactive molecules from seaweeds a novel and challenging option for the prevention of HPV infection and cervical cancer therapy? A review. Int. J. Mol. Sci. 2021, 22, 629. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Sen, I.K.; Chakraborty, I.; Mandal, A.K.; Bhanja, S.K.; Patra, S.; Maity, P. A review on antiviral and immunomodulatory polysaccharides from Indian medicinal plants, which may be beneficial to COVID-19 infected patients. Int. J. Biol. Macromol. 2021, 181, 462–470. [Google Scholar] [CrossRef]
- Hetta, M.; Mahmoud, R.; El-Senousy, W.; Ibrahim, M.; El-Taweel, G.; Ali, G. Antiviral and antimicrobial activities of Spirulina platensis. World J. Pharm. Sci. 2014, 3, 31–39. [Google Scholar]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef]
- Liu, J.; Obaidi, I.; Nagar, S.; Scalabrino, G.; Sheridan, H. The antiviral potential of algal-derived Macromolecules. Curr. Res. Biotechnol. 2021, 3, 120–134. [Google Scholar] [CrossRef]
- Satpati, G.G. Algal Sulfated Polysaccharides: Potent Immunomodulators against COVID-19 in Pandemic 2020. Biosci. Biotechnol. Res. Asia. 2020, 17, 601–605. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.P.; Jia, S.R.; Chu, L.Q.; Xie, Y.Y.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, M.R.; Choi, S.M.; Na, S.S.; Cha, J.D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef]
- Phull, A.; Ali, A.; Ahmed, M.; Zia, M.; Haq, I.; Kim, S.J. In vitro antileishmanial, antibacterial, antifungal and anticancer activity of fucoidan from Undaria pinnatifida. Int. J. Biosci. 2017, 11, 219–227. [Google Scholar]
- Ratan, Z.A.; Youn, S.H.; Kwak, Y.S.; Han, C.K.; Haidere, M.F.; Kim, J.K.; Cho, J. Y Adaptogenic effects of Panax ginseng on modulation of immune functions. J. Ginseng Res. 2021, 45, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Y.; Yin, G.; Wang, J.; Wang, P.; Chen, Z.Y.; Ren, G. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother. Res. 2020, 34, 1226–1236. [Google Scholar] [CrossRef]
- Akshay Kumar, K.P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Makvandi, P.; Černík, M.; Varma, R.S. Electrospun fibers based on botanical, seaweed, microbial, and animal sourced biomacromolecules and their multidimensional applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langout-Astri, C.J.; Schmidt, E.P. Heparin as a therapy for COVID-19: Current evidence and future possibilities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L211–L217. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tingting, W.; Li, J.P.; Sullivan, M.A.; Wang, C.; Wang, H.; Zhang, Y. Comprehensive landscape of heparin therapy for COVID-19. Carbohydr. Polym. 2021, 254, 117232. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.; Hatmal, M.M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 2, 5017. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silveira, R.F.; Fidelis, G.P.; Pereira Costa, M.S.S.; Telles, C.B.S.; Dantas-Santos, N.; de Oliveira Elias, S.; Rocha, H.A.O. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Biol. Macromol. 2012, 13, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Castillo, G.D.C.; Rodrigo, D.; Martínez, A.; Pina-Pérez, M.C. Bioactivity of Fucoidan as an antimicrobial agent in a new functional beverage. Beverages 2018, 4, 64. [Google Scholar] [CrossRef]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and analysis of compounds with antibacterial potential from the red alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van Den Ende, W. Fructans as immunomodulatory and antiviral agents: The case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Shalaby, A.S.G.; Awdan, S.A.E.; El-Bassyouni, G.T.; Salama, B.M.; Helmy, W.A.; Esawy, M.A. Role of levan extracted from bacterial honey isolates in curing peptic ulcer: In vivo. Int. J. Biol. Macromol. 2020, 142, 564–573. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. The derivatization and antitumor mechanisms of polysaccharides. Future Med. Chem. 2017, 9, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, J.; Hou, G.; Shi, F.; Ye, M. Cardioprotective effect of an exopolysaccharide from Lachnum YM130 and its derivatives on diabetic mice. Proc. Biochem. 2017, 58, 333–340. [Google Scholar] [CrossRef]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264. 7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jia, S.; Huang, L.; Wang, Z.; Li, C.; Xie, M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264. 7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, F.; Luo, P. Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation In Vitro. Mar. Drugs 2019, 17, 626. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Gabriel, L.; Tied, A.; Heinze, T. Carboxymethylation of polysaccharides—A comparative study. Cellul. Chem. Technol. 2020, 54, 82. [Google Scholar]
- Suflet, D.M.; Chitanu, G.C.; Desbrières, J. Phosphorylated polysaccharides: 2: Synthesis and properties of phosphorylated dextran. Carbohydr. Polym. 2010, 82, 1271–1277. [Google Scholar] [CrossRef]
- Silva, D.A.; de Paula, R.C.M.; Feitosa, J.; de Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Brumer, H. Enzymatic functionalization of cellulosic fibres for textile and other applications: Xyloglucan as a molecular anchor. In Advances in Textile Biotechnology; Woodhead Publishing: Cambridge, UK, 2010; pp. 266–287. [Google Scholar]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef]
- Bozic, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Tegl, G.; Stagl, V.; Mensah, A.; Huber, D.; Somitsch, W.; Grosse-Kracht, S.; Guebitz, G.M. The chemo enzymatic functionalization of chitosan zeolite particles provides antioxidant and antimicrobial properties. Eng. Life Sci. 2018, 18, 334–340. [Google Scholar] [CrossRef]
- Vuillemin, M.E.; Muniglia, L.; Linder, M.; Bouguet-Bonnet, S.; Poinsignon, S.; dos Santos Morais, R.; Simard, B.; Paris, C.; Michaux, F.; Jasniewski, J. Polymer functionalization through an enzymatic process: Intermediate products characterization and their grafting onto gum Arabic. Int. J. Biol. Macromol. 2021, 169, 480–491. [Google Scholar] [CrossRef]
- Yin, Y.; Lucia, L.A.; Pal, L.; Jiang, X.; Hubbe, M.A. Lipase-catalyzed laurate esterification of cellulose nanocrystals and their use as reinforcement in PLA composites. Cellulose 2020, 27, 6263–6273. [Google Scholar] [CrossRef]
- Marjamaa, K.; Kruus, K. Enzyme Biotechnology in degradation and modification of plant cell wall polymers. Physiol. Plant. 2018, 164, 106–118. [Google Scholar] [CrossRef]
- Kaewprapan, K.; Wongkongkatep, J.; Panbangred, W.; Phinyocheep, P.; Marie, E.; Durand, A.; Inprakhon, P. Lipase-catalyzed synthesis of hydrophobically modified dextrans: Activity and regioselectivity of lipase from Candida rugosa. J. Biosci. Bioeng. 2011, 112, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Ayala, M. Biocatalysis Based on Heme Peroxidases: Peroxidases as Potential Industrial Biocatalysts; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Li, X.; Li, S.; Liang, X.; McClements, D.J.; Liu, X.; Liu, F. Applications of oxidases in modification of food molecules and colloidal systems: Laccase, peroxidase and tyrosinase. Trends Food Sci. Technol. 2020, 103, 78–93. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Shang, G.; Wen, Q.; Fang, Y.P.; Nishinari, K.; Phillips, G.O.; Jiang, F.T. Emulsification properties of sugar beet pectin after modification with horseradish peroxidase. Food Hydrocoll. 2015, 43, 107–113. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Searle-Van Leeuwen, M.J.F.; Voragen, A.G.J. Effect of enzymatic deacetylation on gelation of sugar beet pectin in the presence of calcium. Carbohydr. Polym. 2000, 43, 249–256. [Google Scholar] [CrossRef]
- Nachtigall, C.; Rohm, H.; Jaros, D. Degradation of Exopolysaccharides from Lactic Acid Bacteria by Thermal, Chemical, Enzymatic and Ultrasound Stresses. Foods 2021, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lv, C.; Lu, J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: A review of isolation, structures, and bioactivities. Int. J. Biol. Macromol. 2019, 133, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Dong, F.; Zhang, Z.; Hu, B.; Chen, S.; Zhu, Z.; Li, F.; Wang, X.; Zhang, Y.; Wang, Y.; et al. Low molecular weight heparin and 28-day mortality among patients with coronavirus disease 2019: A cohort study in the early epidemic era. Thromb. Res. 2021, 198, 19–22. [Google Scholar] [CrossRef] [PubMed]
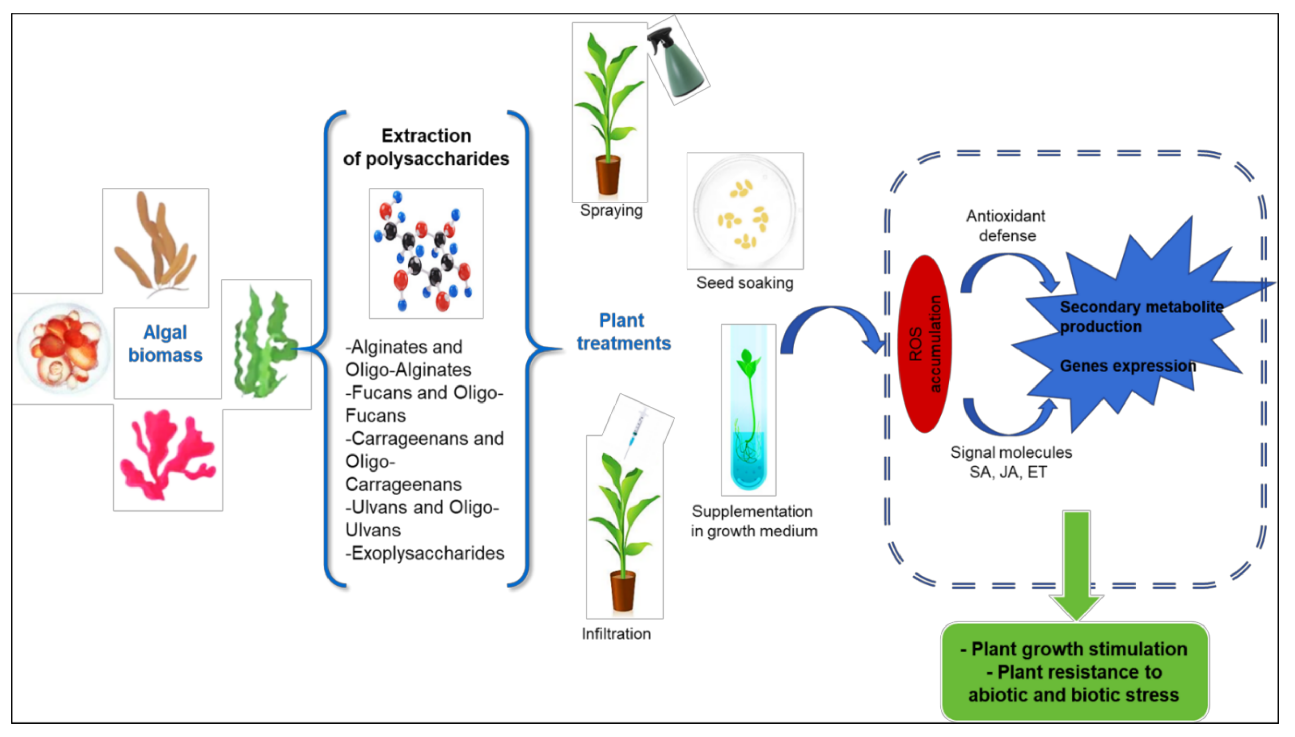


| Polysaccharide Components | Mw (kDa) | Immunomodulatory Activity | Sources |
|---|---|---|---|
| residues of arabinose, glucose, galactose, low content of protein components | 401 | did not have cytotoxicity, increased NO production, promoted the proliferation of spleen lymphocytes | [18] |
| residues of arabinose, glucose, galactose, low content of protein components | 99 | did not have cytotoxicity, increased NO production, promoted the proliferation of spleen lymphocytes | [18] |
| residues of uronic acid, arabinose, galactose, and glucose, low content of protein components | 99 | did not have cytotoxicity, increased NO production, promoted proliferation of spleen lymphocytes and proliferation of T-lymphocytes | [18] |
| residues of uronic acid, galactose, arabinose, and glucose, low content of protein components | 43 | did not have cytotoxicity, increased NO production, promoted proliferation of spleen lymphocytes and proliferation of B-lymphocytes | [18] |
| residues of glucose, mannose, ribose, galactose, xylose, and arabinose | - | increased survival of L02 cells caused by H2O2 | [22] |
| residues of fucose, galactose, and 3-O-methylgalactose | 120 | increased NO production, stimulated splenocytes | [25] |
| type II arabinogalactan, galacturonic acid residues | - | did not have cytotoxicity, increased NO production by J774. A1 macrophage cells, and increased cytokine production | [34] |
| residues of d-mannose, d-glucose | 394 | had a stimulating effect on macrophage cells RAW 264.7, at high concentrations decreased cell viability, increased NO production, stimulated splenocytes and T-lymphocytes, had a protective effect against macrophage apoptosis caused by H2O2 | [43] |
| residues of d-mannose, d-glucose | 362 | had a stimulating effect on macrophage cells RAW 264.7, at high concentrations decreased cell viability, increased NO production, stimulated splenocytes and T-lymphocytes, had a protective effect against macrophage apoptosis caused by H2O2 | [43] |
| galacturonic acid, arabinose, and galactose | - | increased the production of cytokines IL-1α and G-CSF | [46] |
| Plant | Polysaccharide | Dose | Application Mode | Effect | Metabolism | References |
|---|---|---|---|---|---|---|
| Wheat | Alginate oligosaccharides | 1000 mgL−1 | Supplementation in the growth medium | Tolerance to drought stress | -Enhancement of antioxidant system -Activation of related genes involved in ABA signal pathway (LEA1, SnRK2 and P5CS) | [67] |
| Kiwi fruit | Alginate oligosaccharides | 50 mgL−1 | Fruit soaking | Disease resistance to gray mold caused by Botrytis cinerea | -Enhancement of antioxidant system -Inducing of defense-related enzymes activities (PPO, PAL, and GLU) | [68] |
| Rice | Alginate oligosaccharides | 1 mgmL−1 | Foliar spraying | Disease resistance to Magnaporthe grisea | -Activation of PAL, POD and CAT activities | [69] |
| Safflowr | Alginate | 0.075% and 0.15% (w/v) | Supplementation in growth medium | In vitro tolerance to salt stress | -Production of secondary metabolites (TPC, TFL, TFD, and Ant) -Enhancement in the antioxidant activity (CAT, TAC, and PAL) | [70] |
| Arabidopsis thaliana | Alginate Oligosaccharide | 25 mgL−1 | Foliar spraying | Resistance to Pst DC3000 | -Production of early signal molecules (ROS, NO) - Activation of SA pathway | [71] |
| Cucumber | Alginate oligosaccharides | 0.2% (w/v) | Foliar spraying | Water stress tolerance | -Decrease on MDA and (•OH) content -Activation of SOD and POD activities -Activation of genes involved in ABA signaling pathway | [72] |
| Polysaccharides | Source | Biological Activity | Species | References | |
|---|---|---|---|---|---|
| Alginate/alginic acid | Seaweed | Antiviral | HIV-1, HPV, DENV, HSV | [257,258,259] | |
| Almond gum | Plant | Antibacterial | Bacillus thuringiensis, Klebsiella pneumonia, Bacillus subtilis, Pseudomonas aeruginosa, Listeria monocytogenes | [260] | |
| Carrageenan | Seaweed | Antibacterial | Chlamydia trachomatis, Saccharomyces cerevisiae, Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa | [257,258,261,262,263,264,265] | |
| Antiviral | HPV, HSV-1, HSV-2, HIV, HRV, Influenza A, DENV-2, DENV-3, VZV, RABV, EV71, SARS-CoV-2 | ||||
| Ulvan | Seaweed | Antibacterial | Enterobacter cloace, Escherichia coli | [262,266] | |
| Antiviral | NDV, JEV | ||||
| Rhodophyta Galactans | Seaweed | Antiviral | HSV-1 and HSV-2, DENV-2, HIV-1 and HIV-2, HAV, HPV, DENV | [257,259,267] | |
| Calcium spirulan | Cyanobacteria | Antiviral | HSV-1, HCMV, Influenza A, Coxsackie virus, MV, HIV-1, PV, Mumps virus, HPV, DENV | [257,259,268] | |
| Nostoflan | Cyanobacteria | Antiviral | HSV-1, HSV-2, HCMV, Influenza A | [257,259,269,270,271] | |
| Chitin/chitosan | Animal | Antibacterial | Escherichia coli, Vibrio cholerae, Shigella dysenteriae, Bacteroides fragilis | [272,273] | |
| Dextran | Bacteria | Antiviral | HPV | [258,266,274] | |
| Antifungal | Candida albicans | ||||
| Fucoidan | Seaweed | Antibacterial | Listeria monocytogenes, Micrococcus luteus, Staphylococcus aureus, Salmonella typhimurium, Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Strongyloides ratti, Streptococcus criceti, Streptococcus anginosus, Streptococcus gordonii, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis | [95,257,262,267,271,275,276] | |
| Antiviral | HIV, HSV-1, HSV-2, DENV, HCMV, NDV, SARS-CoV-2 | ||||
| Antifungal | Aspergillus flavus, Aspergillus fumigatus, Mucor sp. | ||||
| Ginseng’s polysaccharide | Plant | Antibacterial | Helicobacter pylori, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Listeria monocytogenes, Salmonella enteritidis, Escherichia coli, Streptococcus pneumoniae | [277,278] | |
| Antifungal | Candida albicans | ||||
| Antiviral | H1N1 Influenza virus, H5N1 Influenza vírus, HIV, HBV, RSV | ||||
| Heparin | Animal | Antiviral | HPV, SARS-CoV-2 | [258,279,280,281] | |
| Laminarin | Seaweed | Antibacterial | Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, Salmonella typhimurium | [257,259] | |
| Antiviral | HBV, HIV-1 | ||||
| Lentinan | Fungi | Antiviral | SARS-CoV-2 | [282] | |
| Levan | Bacteria | Antiviral | (HPAI) A(H5N1), ad40 | [283] | |
| Pectin | Plant | Antibacterial | Citrobacter sp., Salmonella sp., Enterobacter sp., Shigella sp., Proteus sp., Klebsiella sp. | [256] | |
| Polygonum multiflorum’s polysaccharide | Plant | Antiviral | Coronavirus | [284] | |
| Ganoderma polysaccharides | Fungi | Antibacterial | Erwinia carotovora, Bacillus cereus, Acinetobacter aerogenes, Acrobacter aerogenes, Arthrobacter citreus, Bacillus brevis, Bacillus subtilis, Corynebacterium insidiosum, Escherichia coli, Proteus vulgaris, Clostridium pasteurianum, Micrococcus roseus, Mycobacterium phlei, Staphylococcus aureus | [285] | |
| Antifungal | Penicillium digitatum, Aspergillus niger | ||||
| Xylan | Plant | Antibacterial | Klebsiella pneumoniae | [286] | |
| Antiviral | HSV | ||||
| Polysaccharide | Mainly Sold As/For |
|---|---|
| Animal Origin | |
| Heparin | Anticoagulant (medical practice) Scientific research (several novel medical uses) |
| Chondroitin sulfate | Treatment of osteoarthritis in humans and other animals Treatment of cataracts Nutraceutical for cartilage, joint, and bone health 1 |
| Hyaluronic acid 2 | Treatment of osteoarthritis and cataracts Topical pharmaceutics (e.g., dry skin, dry eyes, wounds, or oral inflammation) Plastic surgery filler Cosmetic formulations (anti-aging properties) |
| Chitin/chitosan 3 | Sizing and strengthening paper Plant priming Soil fertilizer/conditioner Wine fining Water treatment Biomaterial (e.g., food packaging or wound dressings) Nutraceutical for weight loss 1 Nutraceutical with cholesterol lowering effect Scientific research (several novel uses) |
| Plant Origin | |
| Pectin | Gelling agent Stabilizer Dietary fiber (supplement) with cholesterol lowering effect Dietary fiber (supplement) that delays gastric emptying 1 Dietary fiber (supplement) that ameliorates constipation 1 |
| Konjac glucomannan | Thickening agent Emulsifier Dietary fiber (supplement) with cholesterol lowering effect Dietary fiber (supplement) that ameliorates constipation Nutraceutical for weight loss 1 |
| Astragalus polysaccharide | Nutraceutical for immune system stimulation Nutraceutical with diuretic effect Nutraceutical with antiviral activity Nutraceutical with several other medical applications 1 |
| Xylan | Source for xylitol production Plastic additive Scientific research (novel packaging solutions and biomedical applications) Cosmetic ingredient |
| Inulin | Nutraceutical with prebiotic and gut health promoting activitiesNutraceutical with antidiabetic activity Nutraceutical with weight loss activity Dietary fiber (supplement) that ameliorates constipation Food ingredient to increase fiber content and substitute sugar, starch, and fats |
| Polygonum multiflorum polysaccharide | Nutraceutical for hair strengthening and color restoration 1 Nutraceutical for neuroprotection 1 Nutraceutical with several other medical applications 1 |
| Guar gum | Nutraceutical to increase fiber intake (also, indirect method of weight loss) Nutraceutical with cholesterol lowering effect Food ingredient to thicken without gluten, gelatin, or eggs Dietary fiber (supplement) that ameliorates constipation or diarrhea Multiple uses in paper and textile industries Thickener (multiple industries) Stabilizer (food industry) Binder (multiple industries) Laxative |
| Seaweed Origin | |
| Alginate/Alginic acid | Superabsorbent Plant priming Drug-release carrier Inoculant carrier Thickening agent (multiple industries) Stabilizer (multiple industries) Gelling agent (multiple industries) Emulsifier (multiple industries) Biomedical scaffolding Anti-poisoning 1 Nutraceutical with cholesterol lowering effect 1 Nutraceutical with anti-hypertensive effect 1 Nutraceutical to increase fiber intake (also, indirect method of weight loss) 1 Ingredient in peel-off skin masks Reflux treatment |
| Carrageenan | Gelling agent (multiple industries) Thickening agent (multiple industries) Stabilizer (multiple industries) Emulsifier (multiple industries) Lubricant Inactive excipient in pharmaceutical formulae Immobilization agent for cells/enzymes (biotechnology) Pro-inflammatory agent (scientific research) |
| Agar | Gelling agent (multiple industries) Food ingredient to solidify liquids Dietary fiber (supplement) that ameliorates constipation or diarrhea Scientific research (molecular biology and microbial/plant culture media) |
| Fucoidan | Nutraceutical with immunostimulant activity Nutraceutical with anticancer activity 1 Nutraceutical with anti-inflammatory activity 1 Nutraceutical with antihypertension and antihypercholesterolemia activity 1 Nutraceutical with anticoagulant and antithrombotic activities Nutraceutical with antioxidant activity |
| Laminarin | Reagent for scientific research (bioactivities and enzyme activity) Cosmeceutical with moisturizing capacity Dietary fiber (supplement)Nutraceutical with some health-related claims 1 |
| Caulerpa sulfated polysaccharide | Nutraceutical with immunostimulation activity Nutraceutical with anticoagulant activity |
| Bacterial Origin | |
| Dextran | Pharmaceutical for hypovolaemia treatment Antithrombotic and blood thinner Medical lubricant Pharmaceutical for parenteral nutrition/blood substituent Research (several laboratory applications) |
| Levan | Cosmeceutical ingredient with haircare and skin-whitening properties Food ingredient (dietary fiber supplement and sweetener) Scientific research (bioactivities, e.g., prebiotic, anti-inflammatory, and antimicrobial) |
| Curdlan | Gelling agent (multiple industries) Water-holding and stabilizing agent (food industry) Scientific research (several therapeutic potentials) |
| Bacterial cellulose | Thickener (multiple industries) Stabilizer (multiple industries) Modern wound dressings Several medical applications as structural material |
| Xanthan Gum | Thickener (multiple industries) Stabilizer (multiple industries) Scientific research (biomedical scaffolding) |
| Gellan gum | Thickener (multiple industries) Emulsifier and stabilizer Drug-release carrier and cell encapsulation agent Agar-substitute in culture media |
| Fungal Origin | |
| Pullulan | Edible film-forming polysaccharide (oxygen-barrier) Low-calories, tasteless food ingredient (bulking fiber, antifungal) Adhesive and binder (multiple industries) Drug-release agent |
| Scleroglucan | Thickening agent (food industry) Suspension agent (food industry) Water-holding agent (cosmeceutical industry) Immunostimulant (especially against fungal infections) Edible films Binder in tablets and drug-release carrier Nutraceutical with hypolipidemic and hypoglycemic activities Artificial tears and artificial saliva component |
| Lentinan | Anticancer pharmaceutical Anti-HIV and anti-hepatitis pharmaceutical Malignant pleural effusion treatment Nutraceutical with immunomodulation activity |
| Grifolan | Nutraceutical with immunostimulation activity (indirect antitumor activity) |
| Schizophyllan | Nutraceutical with immunomodulation activity (indirect antitumor activity) Cosmetic ingredient with soothing/anti-inflammatory properties |
| Krestin | Nutraceutical with immunomodulation activity (indirect antitumor activity) |
| Reishi polysaccharide | Nutraceutical with immunomodulation activity (indirect antitumor activity) Several nutraceutical and pharmaceutical uses |
| Lichen origin | |
| Lichenan | Scientific research (bioactivity and enzyme activity) |
| Pustulan | Scientific research (bioactivity and enzyme activity) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drira, M.; Hentati, F.; Babich, O.; Sukhikh, S.; Larina, V.; Sharifian, S.; Homaei, A.; Fendri, I.; Lemos, M.F.L.; Félix, C.; et al. Bioactive Carbohydrate Polymers—Between Myth and Reality. Molecules 2021, 26, 7068. https://doi.org/10.3390/molecules26237068
Drira M, Hentati F, Babich O, Sukhikh S, Larina V, Sharifian S, Homaei A, Fendri I, Lemos MFL, Félix C, et al. Bioactive Carbohydrate Polymers—Between Myth and Reality. Molecules. 2021; 26(23):7068. https://doi.org/10.3390/molecules26237068
Chicago/Turabian StyleDrira, Maroua, Faiez Hentati, Olga Babich, Stanislas Sukhikh, Viktoria Larina, Sana Sharifian, Ahmad Homaei, Imen Fendri, Marco F. L. Lemos, Carina Félix, and et al. 2021. "Bioactive Carbohydrate Polymers—Between Myth and Reality" Molecules 26, no. 23: 7068. https://doi.org/10.3390/molecules26237068
APA StyleDrira, M., Hentati, F., Babich, O., Sukhikh, S., Larina, V., Sharifian, S., Homaei, A., Fendri, I., Lemos, M. F. L., Félix, C., Félix, R., Abdelkafi, S., & Michaud, P. (2021). Bioactive Carbohydrate Polymers—Between Myth and Reality. Molecules, 26(23), 7068. https://doi.org/10.3390/molecules26237068











