5-Nitrotetrazol and 1,2,4-Oxadiazole Methylene-Bridged Energetic Compounds: Synthesis, Crystal Structures and Performances
Abstract
:1. Introduction
2. Results and Discussions
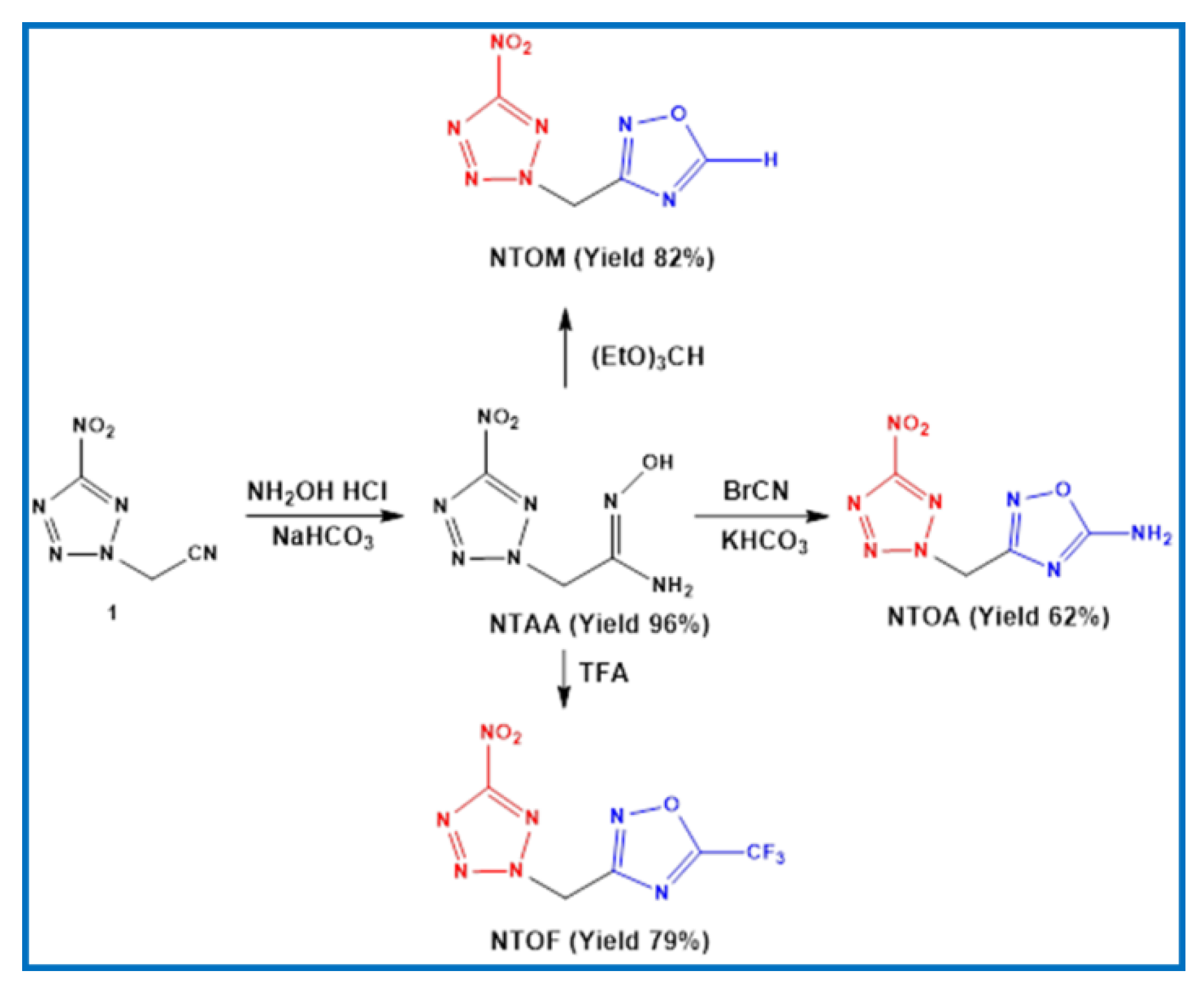
2.1. Synthesis
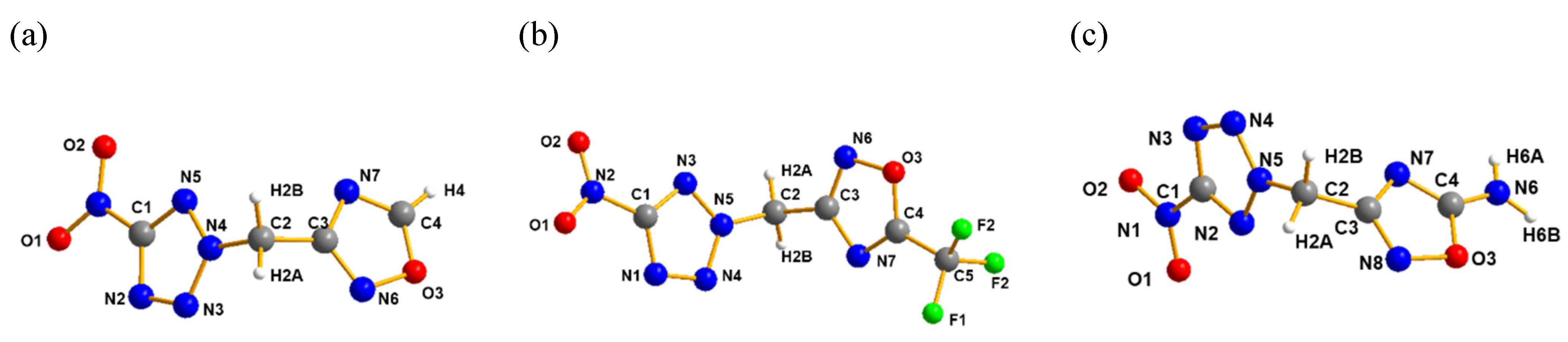
2.2. X-ray Crystallography
2.3. Thermal Stabilities
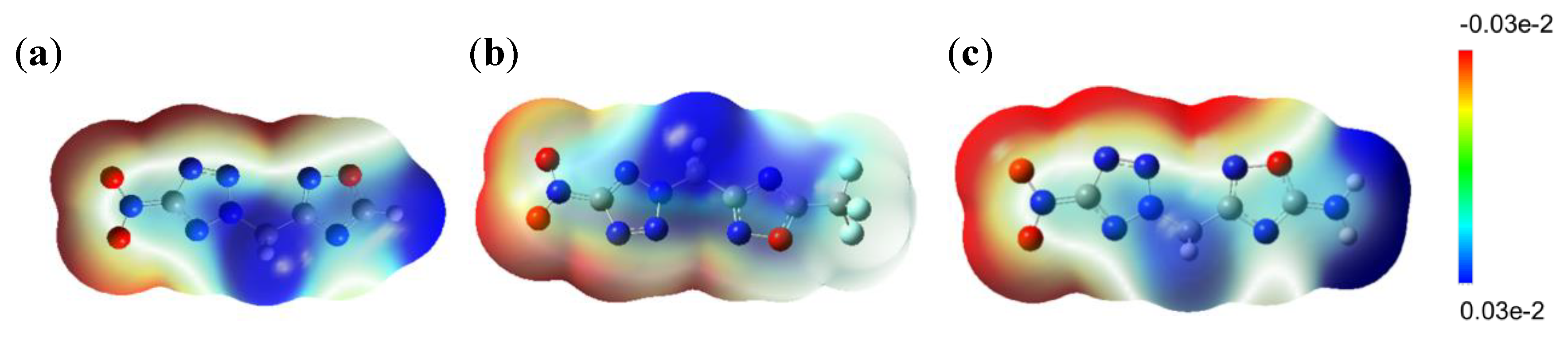
2.4. Sensitivities
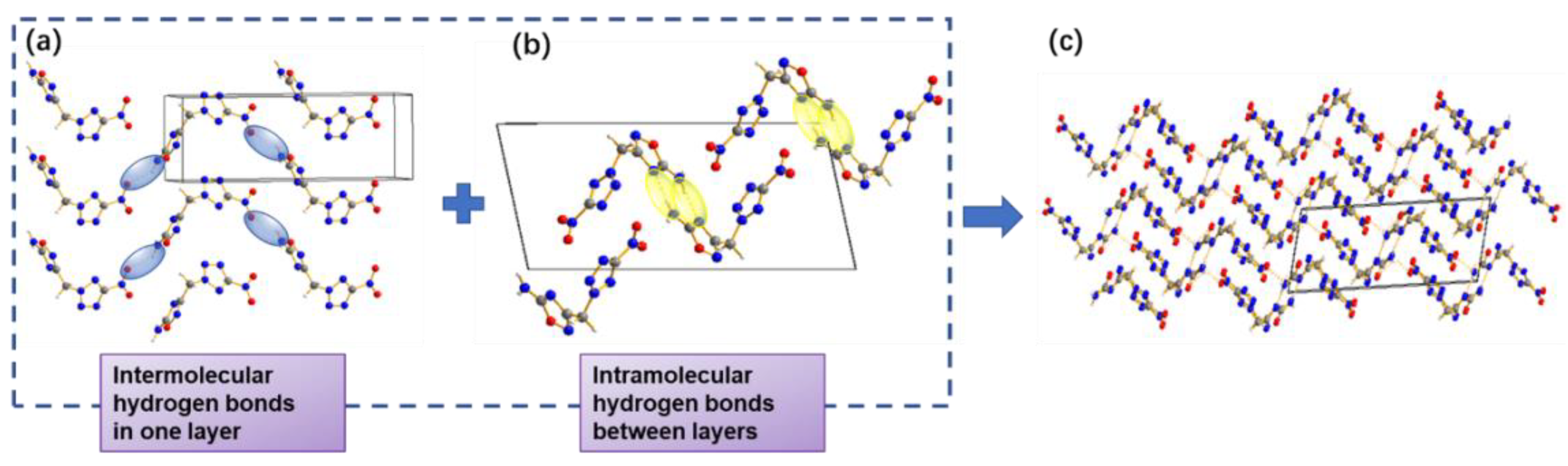
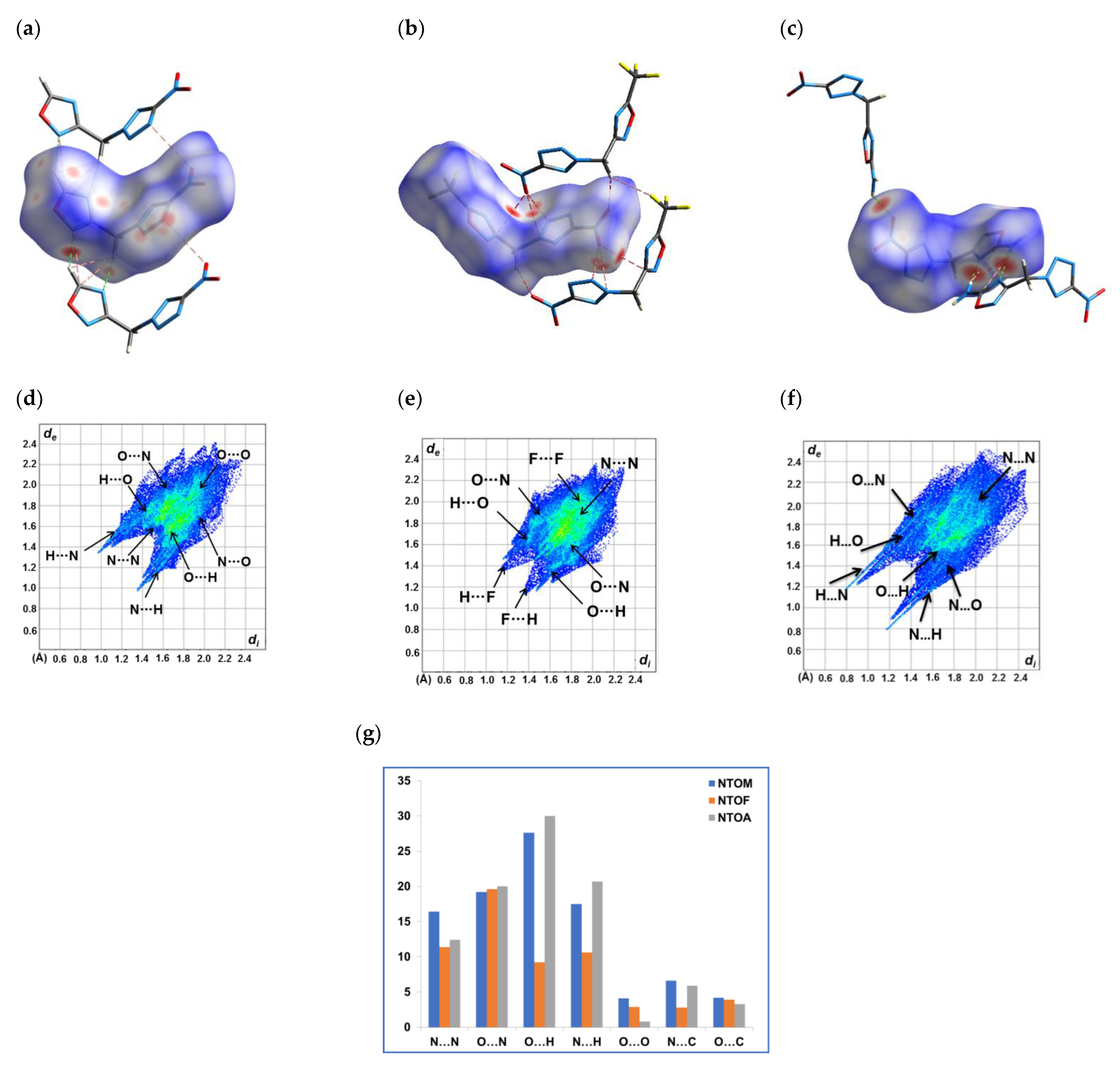
2.5. Hirshfeld Analysis
2.6. Physiochemical Properties and Detonation Performances of NTOM, NTOF and NTOA
3. Experiments
3.1. Materials and Measurements
3.2. X-ray Crystallography
3.3. Synthesis of NTAA
3.4. Synthesis of NTOA
3.5. Synthesis of NTOM
3.6. Synthesis of NTOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ravi, P.; Badgujar, D.M.; Gore, G.M.; Tewari, S.P.; Sikder, A.K. Review on Melt Cast Explosives. Propellants Explos. Pyrotech. 2011, 36, 393–403. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sabatini, J.J.; Chavez, D.E.; Wells, L.A.; Banning, J.E.; Sausa, R.C.; Byrd, E.; Orlicki, J.A. Bis(Nitroxymethylisoxazolyl) Furoxan: A Promising Standalone Melt-Castable Explosive. ChemPlusChem 2020, 85, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.W.; Wang, T.I.; Huang, D.J.; Pao, Y.J.; Lin, Y.A.; Cheng, T.W.; Liang, S.H.; Chen, C.Y.; Kao, G.M.; Sheu, Y.T.; et al. Copper promotes E. coli laccase-mediated TNT biotransformation and alters the toxicity of TNT metabolites toward Tigriopus japonicas. Ecotoxicol. Environ. Saf. 2019, 173, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Núñez, A.; Caballero, A.; Ramos, J.L. Biological Degradation of 2,4,6-Trinitrotoluene. Microbiol. Mol. Biol. Rev. 2001, 65, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, J.J.; Oyler, K.D. Recent Advances in the Synthesis of High Explosive Materials. Crystals 2015, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.C.; Bukowski, E.J.; Sabatini, J.J.; Sausa, R.C.; Byrd, E.F.; Garner, M.A.; Chavez, D.E. Bis(1,2,4-oxadiazolyl)Furoxan: A Promising Melt-Castable Eutectic Material of Low Sensitivity. ChemPlusChem 2019, 84, 319. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, K.; Liu, T.; Yang, H.; Cheng, G.; Zhang, Q. Exploiting the energetic potential of 1,2,4-oxadiazole derivatives: Combining the benefits of a 1,2,4-oxadiazole framework with various energetic functionalities. Dalton Trans. 2017, 46, 14210–14218. [Google Scholar] [CrossRef]
- Kettner, M.A.; Karaghiosoff, K.; Klapötke, T.M.; Sućeska, M.; Wunder, S. 3, 3′-Bi (1,2,4-oxadiazoles) Featuring the Fluorodinitromethyl and Trinitromethyl Groups. Chem. Eur. J. 2014, 20, 7622–7631. [Google Scholar] [CrossRef]
- Kettner, M.A.; Klapötke, T.M. 5,5′-Bis-(trinitromethyl)-3,3′-bi-(1,2,4-oxadiazole): A stable ternary CNO-compound with high density. Chem. Commun. 2014, 50, 2268–2270. [Google Scholar] [CrossRef] [Green Version]
- Kettner, M.A.; Klapötke, T.M.; Witkowski, T.G.; von Hundling, F. Synthesis, Characterisation and Crystal Structures of Two Bi-oxadiazole Derivatives Featuring the Trifluoromethyl Group. Chem. Eur. J. 2015, 21, 4238–4241. [Google Scholar] [CrossRef] [PubMed]
- Tsyshevsky, R.; Pagoria, P.F.; Zhang, M.; Racoveanu, A.; Parrish, D.A.; Smirnov, A.; Kuklja, M.M. Comprehensive End-to-End Design of Novel High Energy Density Materials: I. Synthesis and Characterization of Oxadiazole Based Heterocycles. J. Phys. Chem. C 2017, 121, 23853–23864. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Zhang, M.-X.; Zuckerman, N.B.; DeHope, A.J.; Parrish, D.A. Synthesis and characterization of multicyclic oxadiazoles and 1-hydroxytetrazoles as energetic materials. Chem. Heterocycl. Compd. 2017, 53, 760–778. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Efficient assembly of mono- and bis (1,2,4-oxadiazol-3-yl) furoxan scaffolds via tandem reactions of furoxanylamidoximes. RSC Adv. 2015, 5, 47248–47260. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sabatini, J.J.; Chavez, D.E.; Sausa, R.C.; Byrd, E.; Wingard, L.A.; Guzmàn, P.E. Bis (1,2,4-oxadiazole) bis (methylene) Dinitrate: A High-Energy Melt-Castable Explosive and Energetic Propellant Plasticizing Ingredient. Org. Process. Res. Dev. 2018, 22, 736–740. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Z.; Yang, W.; Li, W.; Ju, J.; Fan, G. Strategies for constructing melt-castable energetic materials: A critical review. Energ. Mater. Front. 2021, 2, 69–85. [Google Scholar] [CrossRef]
- Lu, T.; Wang, C.; Wang, G.; Wang, S.; Song, J.; Yin, H.; Fan, G.; Chen, F.-X. 1,2,4-Oxadiazole-derived polynitro energetic compounds with sensitivity reduced by a methylene bridge. New J. Chem. 2019, 43, 13330–13333. [Google Scholar] [CrossRef]
- Zhang, J.R.; Bi, F.Q.; Wang, B.Z.; Zhai, L.J. Synthesis and Characterization of Methylene-bridged Energetic Compound: 3-(5-nitro-2H-tetrazol-2-yl)-1,2,4- Oxadiazol-5-amine (NTOA). In Proceedings of the 50th International Annual Conference of Fraunhofer ICT, Karlsruhe, Germany, 25–28 June 2019; Volume 76. [Google Scholar]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Ren, F.-D.; Cao, D.-L.; Shi, W.-J.; Gao, H.-F. A theoretical prediction of the relationships between the impact sensitivity and electrostatic potential in strained cyclic explosive and application to H-bonded complex of nitrocyclohydrocarbon. J. Mol. Model. 2016, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, X.; Yang, J.; Gong, X.; Fan, G.; Huang, H. Synthesis and performance study of methylene-bridged bis(nitramino-1,2,4-oxadiazole) and its energetic salts. New J. Chem. 2019, 43, 5441–5447. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P.; Bolduc, P.R. A Relationship Between Impact Sensitivity and the Electrostatic Potentials at the Midpoints of C-NO2 Bonds in Nitroaromatics. Chem. Phys. Lett. 1990, 168, 135–139. [Google Scholar] [CrossRef]
- Murray, J.S.; Concha, M.C.; Politzer, P. Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C–NO2/N–NO2bond dissociation energies. Mol. Phys. 2009, 107, 89–97. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Nordheider, A.; Stierstorfer, J. Synthesis and reactivity of an unexpected highly sensitive 1-carboxymethyl-3-diazonio-5-nitrimino-1,2,4-triazole. New J. Chem. 2012, 36, 1463–1468. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Lane, P.; Politzer, P. Relationships between impact sensitivities and molecular surface electrostatic potentials of nitroaromatic and nitroheterocyclic molecules. Mol. Phys. 1995, 85, 1–8. [Google Scholar] [CrossRef]
- Chan, E.J.; Grabowsky, S.; Harrowfield, J.M.; Shi, M.W.; Skelton, B.W.; Sobolev, A.N.; White, A.H. Hirshfeld surface analysis of crystal packing in aza-aromatic picrate salts. CrystEngComm 2014, 16, 4508–4538. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Bi, F.; Luo, Y.; Sun, L.; Huo, H.; Zhang, J.; Zhang, J.; Wang, B.; Chen, S. Exploring the highly dense energetic materials via regiochemical modulation: A comparative study of two fluorodinitromethyl-functionalized herringbone trifuroxans. Chem. Eng. J. 2020, 391, 123573. [Google Scholar] [CrossRef]
- Wei, H.; He, C.; Zhang, J.; Shreeve, J.M. Combination of 1,2,4-Oxadiazole and 1,2,5-Oxadiazole Moieties for the Generation of High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2015, 54, 9367–9371. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of Detonations. I. A Simple Method for Calculating Detonation Properties of C–H–N–O Explosives. J. Chem. Phys. 1968, 48, 23–35. [Google Scholar] [CrossRef]



| β (K·min−1) | Tp (K) | E/(kJ·mol−1) | r | lg (Ak/s−1) | |||
|---|---|---|---|---|---|---|---|
| Kissinger | Ozawa | Kissinger | Ozawa | Kissinger | |||
| NTOA | 2.5 | 521.55 | 152.72 | 153.68 | 0.98549 | 0.9871 | 14.55 |
| 5 | 530.25 | ||||||
| 10 | 539.15 | ||||||
| 15 | 548.35 | ||||||
| NTOM | 2.5 | 491.05 | 121.54 | 123.56 | 0.99565 | 0.99613 | 12.2 |
| 5 | 503.65 | ||||||
| 10 | 514.25 | ||||||
| 15 | 520.35 | ||||||
| NTOF | 2.5 | 498.65 | 153.05 | 153.61 | 0.98583 | 0.98734 | 15.32 |
| 5 | 506.75 | ||||||
| 10 | 514.75 | ||||||
| 15 | 523.15 | ||||||
| Compounds | NTOA | NTOM | NTOF | TNT | BODN [14] |
|---|---|---|---|---|---|
| ρ (a) (g/cm3) | 1.66 | 1.76 | 1.87 | 1.65 | 1.83 |
| ΔfH (b) (kJ/mol) | 275.8 | 344.9 | −328.8 | −67 | −79.4 |
| Tm (c) (℃) | 131.6 | 82.6 | 71.7 | 81 | 84.5 |
| Td (d) (℃) | 256.6 | 241.1 | 261.6 | 295 | 183.4 |
| Ω (e) (%) | −60.67 | −0.53 | −0.48 | −74 | −33.3 |
| D (f) (m/s) | 7451 | 7909 | 7271 | 6881 | 8180 |
| P(g) (GPa) | 20.89 | 24.80 | 23.66 | 19.5 | 29.4 |
| IS (h) (J) | >40 | >40 | >40 | 15 | 8.7 |
| FS (i) (N) | >360 | >360 | >360 | 240 | 282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bi, F.; Yang, Z.; Xue, Q.; Wang, B. 5-Nitrotetrazol and 1,2,4-Oxadiazole Methylene-Bridged Energetic Compounds: Synthesis, Crystal Structures and Performances. Molecules 2021, 26, 7072. https://doi.org/10.3390/molecules26237072
Zhang J, Bi F, Yang Z, Xue Q, Wang B. 5-Nitrotetrazol and 1,2,4-Oxadiazole Methylene-Bridged Energetic Compounds: Synthesis, Crystal Structures and Performances. Molecules. 2021; 26(23):7072. https://doi.org/10.3390/molecules26237072
Chicago/Turabian StyleZhang, Jiarong, Fuqiang Bi, Zhi Yang, Qi Xue, and Bozhou Wang. 2021. "5-Nitrotetrazol and 1,2,4-Oxadiazole Methylene-Bridged Energetic Compounds: Synthesis, Crystal Structures and Performances" Molecules 26, no. 23: 7072. https://doi.org/10.3390/molecules26237072