Advances in Radiopharmaceutical Sciences for Vascular Inflammation Imaging: Focus on Clinical Applications
Abstract
:1. Introduction
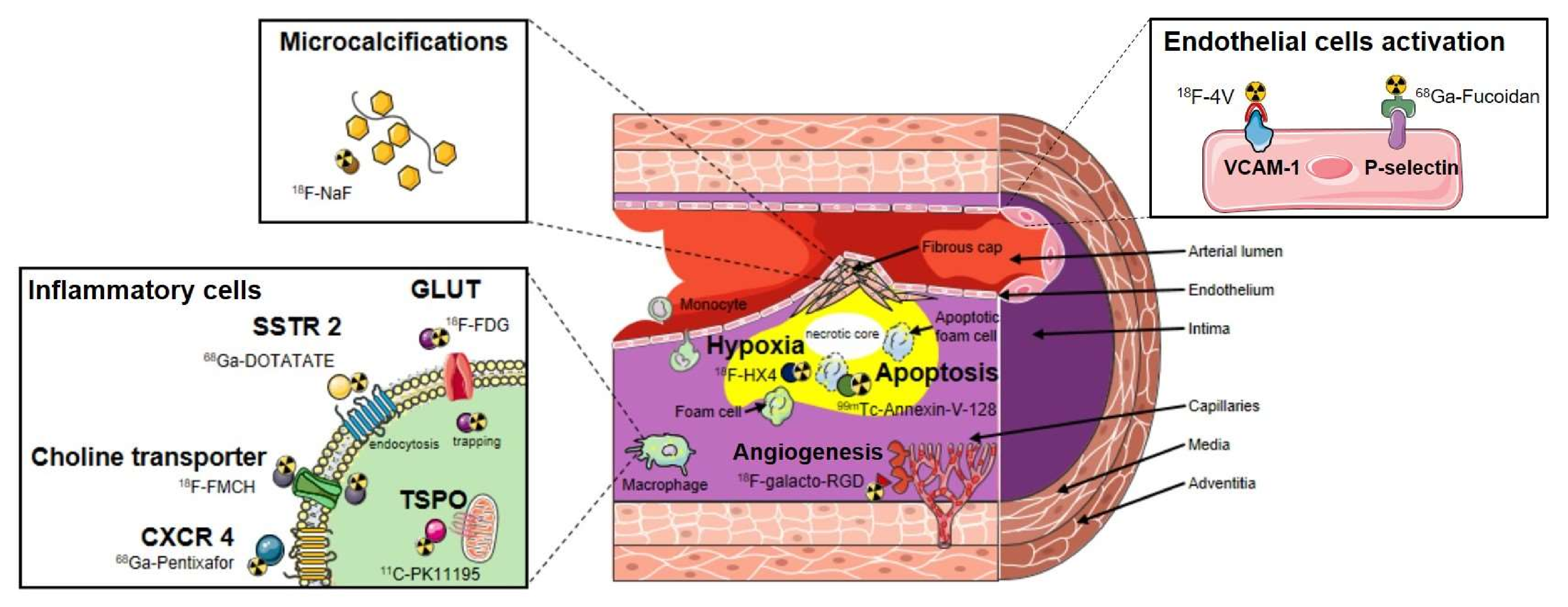
2. Current and Emerging Nuclear Probes for the In Vivo Molecular Imaging of Vascular Inflammation
2.1. Small Molecules-Based PET Probes for the In Vivo Molecular Imaging of Vascular Inflammation
2.2. Nanoparticles-Based PET Probes for the In Vivo Molecular Imaging of Vascular Inflammation
3. Clinical Findings of Nuclear Medicine to Identify and Stratify Vascular Inflammation
3.1. Atherosclerosis
3.1.1. [18F]FDG PET/CT Imaging in Atherosclerosis
3.1.2. [18F]FMCH PET/CT Imaging in Atherosclerosis
3.1.3. [68Ga]Ga-DOTA-TATE PET/CT Imaging in Atherosclerosis
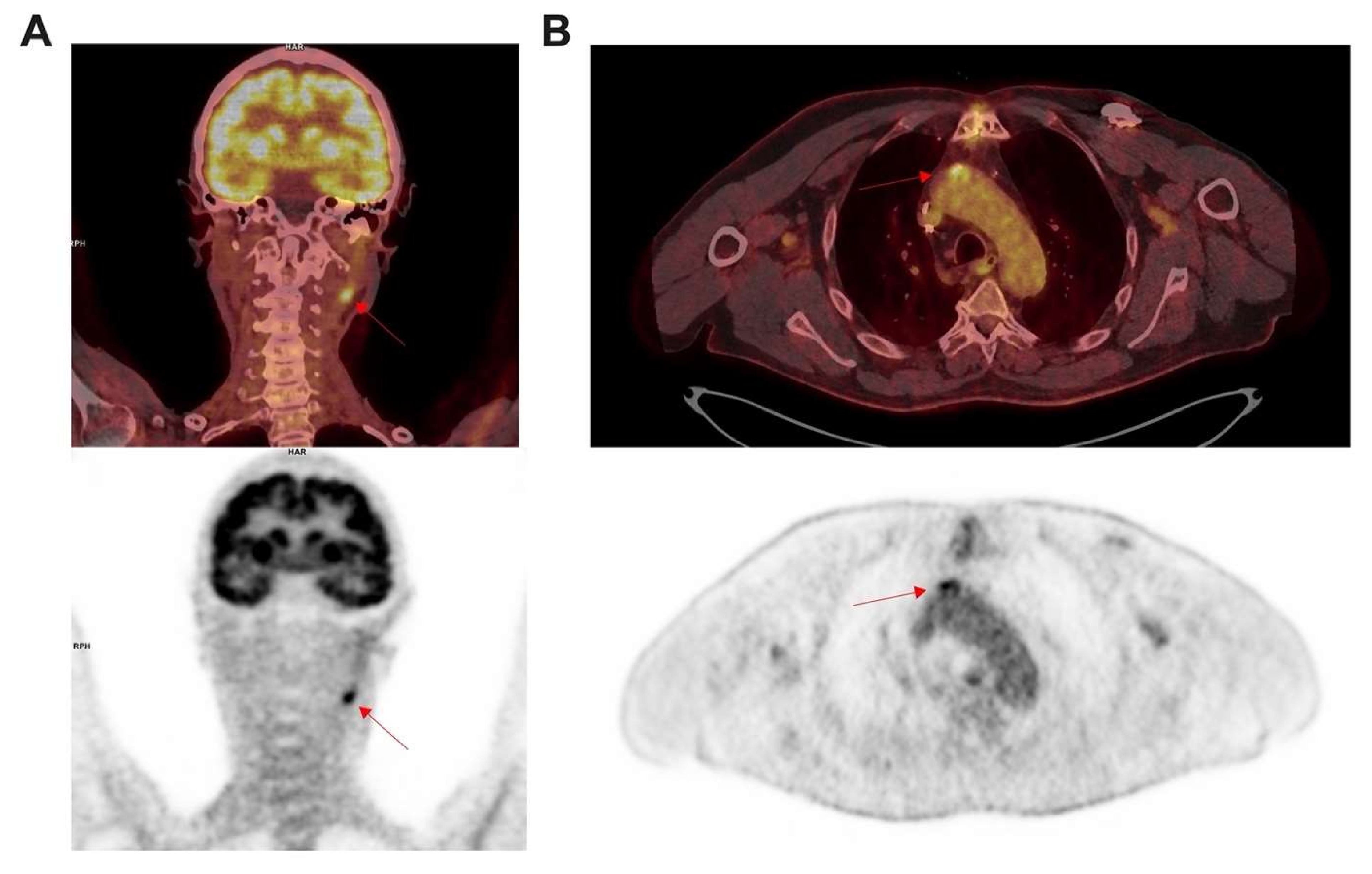
3.2. Large Vessel Vasculitis
[18F]FDG PET/CT Imaging in Large Vessel Vasculitis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammoud, D.A. Molecular Imaging of Inflammation: Current Status. J. Nucl. Med. Soc. Nucl. Med. 2016, 57, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Vigne, J.; Bay, S.; Aid-Launais, R.; Pariscoat, G.; Rucher, G.; Sénémaud, J.; Truffier, A.; Anizan, N.; Even, G.; Ganneau, C.; et al. Cleaved CD31 as a target for in vivo molecular imaging of inflammation. Sci. Rep. 2019, 9, 19560. [Google Scholar] [CrossRef] [PubMed]
- Vigne, J.; Cognet, T.; Guedj, K.; Morvan, M.; Merceron, O.; Louedec, L.; Choqueux, C.; Nicoletti, A.; Escoubet, B.; Chaubet, F.; et al. Early Detection of Localized Immunity in Experimental Autoimmune Myocarditis Using [99mTc]Fucoidan SPECT. Mol. Imaging Biol. 2019, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef]
- Herter, J.; Zarbock, A. Integrin Regulation during Leukocyte Recruitment. J. Immunol. 2013, 190, 4451–4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, A.E.; van Buul, J.D. Endothelial junction regulation: A prerequisite for leukocytes crossing the vessel wall. J. Innate Immun. 2013, 5, 324–335. [Google Scholar] [CrossRef]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Choudhury, R.P.; Fuster, V.; Fayad, Z.A. Molecular, cellular and functional imaging of atherothrombosis. Nat. Rev. Drug Discov. 2004, 3, 913–925. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Medium- and large-vessel vasculitis. N. Engl. J. Med. 2003, 349, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Dey, A.; Manyak, G.; Teklu, M.; Patel, N.; Teague, H.; Mehta, N.N. The application of molecular imaging to advance translational research in chronic inflammation. J. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Coenen, H.H.; Ermert, J. Expanding PET-applications in life sciences with positron-emitters beyond fluorine-18. Nucl. Med. Biol. 2021, 92, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.F.; Warburton, E.A.; Fryer, T.D.; Jones, H.A.; Clark, J.C.; Antoun, N.; Johnström, P.; Davenport, A.P.; Kirkpatrick, P.J.; Arch B, N.; et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002, 105, 2708–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, P.; Tawakol, A. Imaging atherosclerosis with positron emission tomography. Eur. Heart J. 2016, 37, 2974–2980. [Google Scholar] [CrossRef]
- Ćorović, A.; Wall, C.; Mason, J.C.; Rudd, J.H.F.; Tarkin, J.M. Novel Positron Emission Tomography Tracers for Imaging Vascular Inflammation. Curr. Cardiol. Rep. 2020, 22, 119. [Google Scholar] [CrossRef]
- Cheng, V.Y.; Slomka, P.J.; Meunier, L.L.; Tamarappoo, B.K.; Nakazato, R.; Dey, D.; Berman, D.S. Coronary Arterial 18F-FDG Uptake by Fusion of PET and Coronary CT Angiography at Sites of Percutaneous Stenting for Acute Myocardial Infarction and Stable Coronary Artery Disease. J. Nucl. Med. Soc. Nucl. Med. 2012, 53, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; Calvert, P.A.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Vorster, M.; Maes, A.; van de Wiele, C.; Sathekge, M. Gallium-68 PET: A Powerful Generator-based Alternative to Infection and Inflammation Imaging. Semin. Nucl. Med. 2016, 46, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Matter, C.M.; Wyss, M.T.; Meier, P.; Späth, N.; von Lukowicz, T.; Lohmann, C.; Weber, B.; de Molina, A.R.; Lacal, J.C.; Ametamey, S.M.; et al. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 584–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucerius, J.; Schmaljohann, J.; Böhm, I.; Palmedo, H.; Guhlke, S.; Tiemann, K.; Schild, H.H.; Biersack, H.-J.; Manka, C. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans—First results. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 815–820. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Vogl, E.; Übleis, C.; la Fougère, C.; Förster, S.; Haug, A.; Cumming, P.; Reiser, M.F.; Nikolaou, K.L.; et al. In Vivo Imaging of Macrophage Activity in the Coronary Arteries Using 68Ga-DOTATATE PET/CT: Correlation with Coronary Calcium Burden and Risk Factors. J. Nucl. Med. Soc. Nucl. Med. 2010, 51, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.K.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Ambrosini, V.; Zompatori, M.; Luca, F.D.; Antonia, D.; Allegri, V.; Nanni, C.; Malvi, D.; Tonveronachi, E.; Fasano, L.; Fabbri, M.; et al. 68Ga-DOTANOC PET/CT Allows Somatostatin Receptor Imaging in Idiopathic Pulmonary Fibrosis: Preliminary Results. J. Nucl. Med. Soc. Nucl. Med. 2010, 51, 1950–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yu, W.; Wollenweber, T.; Lu, X.; Wei, Y.; Beitzke, D.; Wadsak, W.; Kropf, S.; Wester, H.J.; Haug, A.R.; et al. [68Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.S.; Schirbel, A.; Buck, A.K.; Kropf, S.; Wester, H.-J.; Lapa, C. [68Ga]Pentixafor-Positron Emission Tomography/Computed Tomography Detects Chemokine Receptor CXCR4 Expression After Ischemic Stroke. Circ. Cardiovasc. Imaging 2016, 9, e005217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thackeray, J.T.; Derlin, T.; Haghikia, A.; Napp, L.C.; Wang, Y.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Wester, H.J.; Wollert, K.C.; et al. Molecular Imaging of the Chemokine Receptor CXCR4 After Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1417–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, L.; Guzner-Gur, H.; Dotan, I. Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory bowel disease. Theranostics 2013, 3, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Kim, S.; Seo, H.S.; Lee, Y.J.; Eo, J.S.; Jeong, J.M.; Lee, B.; Kim, J.Y.; Park, Y.M.; Jeong, M. Novel PET Imaging of Atherosclerosis with 68Ga-Labeled NOTA-Neomannosylated Human Serum Albumin. J. Nucl. Med. 2016, 57, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanni, M.V.; Toribio, M.; Wilks, M.Q.; Lu, M.T.; Burdo, T.H.; Walker, J.; Autissier, P.; Foldyna, B.; Stone, L.; Martin, A.; et al. Application of a Novel CD206+ Macrophage-Specific Arterial Imaging Strategy in HIV-Infected Individuals. J. Infect. Dis. 2017, 215, 1264–1269. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, N.K.; Hird, M.; Thompson, S.; Williamson, D.J.; Qiao, L.; Owen, D.R.; Brooks, A.F.; Scott, P.J.H.; Bacallado, S.; O’Brien, J.T.; et al. Preclinical evaluation of (S)-[18F]GE387, a novel 18-kDa translocator protein (TSPO) PET radioligand with low binding sensitivity to human polymorphism rs6971. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; Landau, D.B.; Odell, E.W.; Cornelius, V.R.; O’Doherty, M.J.; Marsden, P.K. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer. 2012, 48, 3499–3513. [Google Scholar] [CrossRef] [PubMed]
- Bading, J.R.; Shields, A.F. Imaging of Cell Proliferation: Status and Prospects. J. Nucl. Med. Soc. Nucl. Med. 2008, 49, 64S–80S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.-X.; Calcagno, C.; Binderup, T.; Courties, G.; Keliher, E.J.; Wojtkiewicz, G.R.; Iwamoto, Y.; Tang, J.; Pérez-Medina, C.; Mani, V.; et al. Imaging Macrophage and Hematopoietic Progenitor Proliferation in Atherosclerosis. Circ. Res. 2015, 117, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Binderup, T.; Duivenvoorden, R.; Fay, F.; van Leent, M.M.T.; Malkus, J.; Baxter, S.; Ishino, S.; Zhao, Y.; Sanchez-Gaytan, B.; Teunissen, A.J.P.; et al. Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci. Transl. Med. 2019, 11, eaaw7736. [Google Scholar] [CrossRef]
- Laitinen, I.; Saraste, A.; Weidl, E.; Poethko, T.; Weber, A.W.; Nekolla, S.G.; Leppänen, P.; Ylä-Herttuala, S.; Hölzlwimmer, G.; Walch, A.; et al. Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ. Cardiovasc. Imaging 2009, 2, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Dietz, M.; Kamani, C.H.; Deshayes, E.; Dunet, V.; Mitsakis, P.; Coukos, G.; Lalonde, M.N.; Schaefer, N.; Prior, J.O. Imaging angiogenesis in atherosclerosis in large arteries with 68Ga-NODAGA-RGD PET/CT: Relationship with clinical atherosclerotic cardiovascular disease. EJNMMI Res. 2021, 11, 71. [Google Scholar] [CrossRef]
- Wu, M.; Ning, J.; Li, J.; Lai, Z.; Shi, X.; Xing, H.; Hacker, M.; Liu, B.; Huo, L.; Li, X. Feasibility of in vivo Imaging of Fibroblast Activation Protein in Human Arterial Walls. J. Nucl. Med. Soc. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- Brokopp, C.E.; Schoenauer, R.; Richards, P.; Bauer, S.; Lohmann, C.; Emmert, M.Y.; Weber, B.; Winnik, S.; Aikawa, E.; Graves, K.; et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur. Heart J. 2011, 32, 2713–2722. [Google Scholar] [CrossRef] [Green Version]
- Hellberg, S.; Silvola, J.M.U.; Liljenbäck, H.; Kiugel, M.; Eskola, O.; Hakovirta, H.; Hörkkö, S.; Morisson-Iveson, V.; Hirani, E.; Saukko, P.; et al. Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques. Molecules 2019, 24, 1072. [Google Scholar] [CrossRef] [Green Version]
- Bucerius, J.; Barthel, H.; Tiepolt, S.; Werner, P.; Sluimer, J.C.; Wildberger, J.E.; Patt, M.; Hesse, S.; Gertz, H.-J.; Biessen, E.A.L.; et al. Feasibility of in vivo 18F-florbetaben PET/MR imaging of human carotid amyloid-β. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bauer, W.; Israel, I.; Kreissl, M.C.; Weirather, J.; Richter, D.; Bauer, E.; Herold, V.; Jakob, P.; Buck, A.; et al. Targeting P-selectin by gallium-68-labeled fucoidan positron emission tomography for noninvasive characterization of vulnerable plaques: Correlation with in vivo 17.6T MRI. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1661–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvola, J.M.U.; Virtanen, H.; Siitonen, R.; Hellberg, S.; Liljenbäck, H.; Metsälä, O.; Ståhle, M.; Saanijoki, T.; Käkelä, M.; Hakovirta, H.; et al. Leukocyte trafficking-associated vascular adhesion protein 1 is expressed and functionally active in atherosclerotic plaques. Sci. Rep. 2016, 6, 35089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haukkala, J.; Laitinen, I.; Luoto, P.; Iveson, P.; Wilson, I.; Karlsen, H.; Cuthbertson, A.; Laine, J.; Leppänen, P.; Ylä-Herttula, S.; et al. 68Ga-DOTA-RGD peptide: Biodistribution and binding into atherosclerotic plaques in mice. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, S.; Rottenburger, C.; Schegk, E.; Aschwanden, M.; Juengling, F.; Staub, D.; Recher, M.; Kyburz, D.; Berger, C.T.; Daikeler, T. [18F]FDG positron emission tomography in patients presenting with suspicion of giant cell arteritis-lessons from a vasculitis clinic. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 933–940. [Google Scholar] [CrossRef]
- Grayson, P.C.; Alehashemi, S.; Bs, A.A.B.; Civelek, A.C.; Cupps, T.R.; Kaplan, M.J.; Malayeri, A.A.; Merkel, P.A.; Rn, E.N.; Bluemke, D.A.; et al. 18F-Fluorodeoxyglucose-Positron Emission Tomography as an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients with Large Vessel Vasculitis. Arthritis Rheumatol. 2018, 70, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Blockmans, D.; Coudyzer, W.; Vanderschueren, S.; Stroobants, S.; Loeckx, D.; Heye, S.; Ceuninck, L.D.; Marchal, G.; Bobbaers, H. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatology 2008, 47, 1179–1184. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.H.; Hoffman, G.S.; Merkel, P.A.; Min, Y.I.; Uhlfelder, M.L.; Hellmann, D.B.; Specks, U.; Allen, N.B.; Davis, J.C.; Spiera, R.F.; et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001, 44, 912–920. [Google Scholar] [CrossRef]
- Tso, E.; Flamm, S.D.; White, R.D.; Schvartzman, P.R.; Mascha, E.; Hoffman, G.S. Takayasu arteritis: Utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002, 46, 1634–1642. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Merkel, P.A.; Brasington, R.D.; Lenschow, D.J.; Liang, P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum. 2004, 50, 2296–2304. [Google Scholar] [CrossRef]
- Fuchs, M.; Briel, M.; Daikeler, T.; Walker, U.A.; Rasch, H.; Berg, S.; Ng, Q.K.T.; Raatz, H.; Jayne, D.; Kötter, I.; et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Prieto-González, S.; García-Martínez, A.; Tavera-Bahillo, I.; Hernández-Rodríguez, J.; Gutiérrez-Chacoff, J.; Alba, M.A.; Murgia, G.; Espígol-Frigolé, G.; Sánchez, M.; Arguis, P.; et al. Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Medicine 2015, 94, e486. [Google Scholar] [CrossRef] [Green Version]
- Tarkin, J.M.; Wall, C.; Gopalan, D.; Aloj, L.; Manavaki, R.; Fryer, T.D.; Aboagye, E.O.; Bennett, M.R.; Peters, J.E.; Rudd, J.H.F.; et al. Novel Approach to Imaging Active Takayasu Arteritis Using Somatostatin Receptor Positron Emission Tomography/Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2020, 13, e010389. [Google Scholar] [CrossRef]
- Pugliese, F.; Gaemperli, O.; Kinderlerer, A.R.; Lamare, F.; Shalhoub, J.; Davies, A.H.; Rimoldi, O.E.; Mason, J.; Camici, P.G. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J. Am. Coll. Cardiol. 2010, 56, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhawad, M.; Rudd, J.H.; Sarov-Blat, L.; Cai, G.; Wells, R.; Davies, L.C.; Collier, D.J.; Marber, M.S.; Choudhury, R.P.; Fayad, Z.A.; et al. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc. Imaging 2012, 5, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, E.H.; Han, E.J.; Kim, C.J.; Kim, S.H.; Joo-Hyun, O.; Chang, K.; Seung, K.B. Effect of Pioglitazone in Combination with Moderate Dose Statin on Atherosclerotic Inflammation: Randomized Controlled Clinical Trial Using Serial FDG-PET/CT. Korean Circ. J. 2018, 48, 591–601. [Google Scholar] [CrossRef]
- Vöö, S.; Kwee, R.M.; Sluimer, J.C.; Schreuder, F.H.B.M.; Wierts, R.; Bauwens, M.; Heeneman, S.; Cleutjens, J.P.M.; van Oostenbrugge, R.J.; Daemen, J.-W.H.; et al. Imaging Intraplaque Inflammation in Carotid Atherosclerosis with 18F-Fluorocholine Positron Emission Tomography-Computed Tomography: Prospective Study on Vulnerable Atheroma with Immunohistochemical Validation. Circ. Cardiovasc. Imaging 2016, 9, e004467. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Schober, O.; Ikeda, M.; Schäfers, M.; Ishigaki, T.; Kies, P.; Naganawa, S.; Stegger, L. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Rominger, A.; Saam, T.; Wolpers, S.; Nikolaou, K.; Cumming, P.; Reiser, M.F.; Bartenstein, P.; Hacker, M. 18F-fluoroethylcholine uptake in arterial vessel walls and cardiovascular risk factors: Correlation in a PET-CT study. Nuklearmedizin 2010, 49, 148–153. [Google Scholar] [PubMed]
- Tarkin, J.M.; Calcagno, C.; Dweck, M.R.; Evans, N.R.; Chowdhury, M.M.; Gopalan, D.; Newby, D.E.; Fayad, Z.A.; Bennett, M.R.; Rudd, J.H.F. 68Ga-DOTATATE PET Identifies Residual Myocardial Inflammation and Bone Marrow Activation after Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2489–2491. [Google Scholar] [CrossRef]
- Senders, M.; Hernot, S.; Carlucci, G.; van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody-Facilitated Multiparametric PET/MRI Phenotyping of Atherosclerosis. JACC Cardiovasc. Imaging 2019, 12, 2015–2026. [Google Scholar] [CrossRef]
- Gaemperli, O.; Shalhoub, J.; Owen, D.; Lamare, F.; Johansson, S.; Fouladi, N.; Davies, A.H.; Rimoldi, O.E.; Camici, P.G. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur. Heart J. 2012, 33, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Luehmann, H.; Detering, L.; Gropler, R.J.; Liu, Y. Abstract 20674: C–C Chemokine Receptor Type 2 (CCR2) Targeted PET Imaging of Early Atherosclerosis. Circ. Am. Heart Assoc. 2017, 136, A20674. [Google Scholar]
- Woodard, P.K.; Liu, Y.; Pressly, E.D.; Luehmann, H.P.; Detering, L.; Sultan, D.E.; Laforest, R.; McGrath, A.J.; Gropler, R.J.; Hawker, C.J. Design and Modular Construction of a Polymeric Nanoparticle for Targeted Atherosclerosis Positron Emission Tomography Imaging: A Story of 25% (64)Cu-CANF-Comb. Pharm. Res. 2016, 33, 2400–2410. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Hoyer, F.F.; Meerwaldt, A.E.; van Leent, M.M.; Senders, M.L.; Calcagno, C.; Robson, P.M.; Soultanidis, G.; Pérez-Medina, C.; Teunissen, A.J.; et al. Imaging Cardiovascular and Lung Macrophages with the Positron Emission Tomography Sensor 64Cu-Macrin in Mice, Rabbits, and Pigs. Circ. Cardiovasc. Imaging 2020, 13, e010586. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Kooi, M.E.; Cappendijk, V.C.; Cleutjens, K.B.J.M.; Kessels, A.G.H.; Kitslaar, P.J.E.H.M.; Borgers, M.; Frederik, P.M.; Daemen, M.; Van Engelshoven, J.M.A. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 2003, 107, 2453–2458. [Google Scholar] [CrossRef] [Green Version]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; Di Carli, M.F.; et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Abendschein, D.; Woodard, G.E.; Rossin, R.; McCommis, K.; Zheng, J.; Welch, M.J.; Woodard, P.K. Molecular Imaging of Atherosclerotic Plaque with 64Cu-Labeled Natriuretic Peptide and PET. J. Nucl. Med. 2010, 51, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Detering, L.; Abdilla, A.; Luehmann, H.P.; Williams, J.W.; Huang, L.-H.; Sultan, D.; Elvington, A.; Heo, G.S.; Woodard, P.K.; Gropler, R.J.; et al. CC Chemokine Receptor 5 Targeted Nanoparticles Imaging the Progression and Regression of Atherosclerosis Using Positron Emission Tomography/Computed Tomography. Mol. Pharm. 2021, 18, 1386–1396. [Google Scholar] [CrossRef]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsan, M.-F. Mechanism of Gallium-67 Accumulation in Inflammatory Lesions. J. Nucl. Med. Soc. Nucl. Med. 1985, 26, 88–92. [Google Scholar]
- Gemmel, F.; Van den Wyngaert, H.; Love, C.; Welling, M.M.; Gemmel, P.; Palestro, C.J. Prosthetic joint infections: Radionuclide state-of-the-art imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.J.; Siegel, B.A.; Tunis, S.R.; Hillner, B.E.; Shields, A.F.; Carey, B.P.; Coleman, R.E. The National Oncologic PET Registry: Expanded medicare coverage for PET under coverage with evidence development. AJR Am. J. Roentgenol. 2007, 188, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S. Noninvasive detection of atherosclerosis. N. Engl. J. Med. 1998, 339, 2014–2015. [Google Scholar] [CrossRef]
- Kubota, R.; Yamada, S.; Kubota, K.; Ishiwata, K.; Tamahashi, N.; Ido, T. Intratumoral Distribution of Fluorine-18-Fluorodeoxyglucose In Vivo: High Accumulation in Macrophages and Granulation Tissues Studied by Microautoradiography. J. Nucl. Med. Soc. Nucl. Med. 1992, 33, 1972–1980. [Google Scholar]
- Gamelli, R.L.; Liu, H.; He, L.K.; Hofmann, C.A. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J. Leukoc. Biol. 1996, 59, 639–647. [Google Scholar] [CrossRef]
- Mochizuki, T.; Tsukamoto, E.; Kuge, Y.; Kanegae, K.; Zhao, S.; Hikosaka, K.; Hosokawa, M.; Kohanawa, M.; Tamaki, N. FDG Uptake and Glucose Transporter Subtype Expressions in Experimental Tumor and Inflammation Models. J. Nucl. Med. Soc. Nucl. Med. 2001, 42, 1551–1555. [Google Scholar]
- Tawakol, A.; Migrino, R.Q.; Hoffmann, U.; Abbara, S.; Houser, S.; Gewirtz, H.; Muller, J.E.; Brady, T.J.; Fischmanb, A.J. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J. Nucl. Cardiol. 2005, 12, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Machac, J.; Helft, G.; Worthley, S.G.; Tang, C.Y.; Zaman, A.G.; Rodriguez, O.J.; Buchsbaum, M.S.; Fuster, V.; Badimon, J.J. Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: A histopathological correlation. BMC Nucl. Med. 2006, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kerwin, W.S.; Caldwell, J.H.; Ferguson, M.S.; Hippe, D.S.; Alessio, A.M.; Martinez-Malo, V.; Pimentel, K.; Miyaoka, R.S.; Kohler, T.R.; et al. High resolution FDG-microPET of carotid atherosclerosis: Plaque components underlying enhanced FDG uptake. Int. J. Cardiovasc. Imaging 2016, 32, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef] [Green Version]
- Cocker, M.S.; Spence, J.D.; Hammond, R.; Dekemp, R.A.; Lum, C.; Wells, G.; Bernick, J.; Hill, A.; Nagpal, S.; Stotts, G.; et al. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation, Comparison to immunohistology and relationship to acuity of events. Int. J. Cardiol. 2018, 271, 378–386. [Google Scholar] [CrossRef]
- Figueroa Amparo, L.; Subramanian Sharath, S.; Cury Ricardo, C.; Truong Quynh, A.; Gardecki Joseph, A.; Tearney Guillermo, J.; Hoffmann, U.; Brady, T.J.; Tawakol, A. Distribution of Inflammation within Carotid Atherosclerotic Plaques with High-Risk Morphological Features. Circ. Cardiovasc. Imaging 2012, 5, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Hyafil, F.; Schindler, A.; Sepp, D.; Obenhuber, T.; Bayer-Karpinska, A.; Boeckh-Behrens, T.; Höhn, S.; Hacker, M.; Nekolla, S.G.; Rominger, A.; et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 270–279. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Wolpers, S.; Cyran, C.C.; Schmidt, M.; Foerster, S.; Nikolaou, K.; Reiser, M.F.; Bartenstein, P.; Hacker, M.; et al. 18F-FDG PET/CT Identifies Patients at Risk for Future Vascular Events in an Otherwise Asymptomatic Cohort with Neoplastic Disease. J. Nucl. Med. 2009, 50, 1611–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Martí-Fàbregas, J.; Murphy, S.; McNulty, J.; Barry, M.; Barry, P.; Calvet, D.; Coutts, S.B.; et al. Carotid Plaque Inflammation Imaged by 18F-Fluorodeoxyglucose Positron Emission Tomography and Risk of Early Recurrent Stroke. Stroke 2019, 50, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Mb, M.M.; Merwick, A.; Mb, O.C.S.; Hannon, N.; Foran, P.; Grant, T.; Dolan, E.; Moroney, J.; Murphy, S.; O’Rourke, K.; et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann. Neurol. 2012, 71, 709–718. [Google Scholar]
- Wu, Y.-W.; Kao, H.-L.; Huang, C.-L.; Chen, M.-F.; Lin, L.-Y.; Wang, Y.-C.; Lin, Y.-H.; Lin, H.-J.; Tzen, K.-Y.; Yen, R.-F.; et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 399–407. [Google Scholar] [CrossRef]
- Ishii, H.; Nishio, M.; Takahashi, H.; Aoyama, T.; Tanaka, M.; Toriyama, T.; Tamaki, T.; Yoshikawa, D.; Hayashi, M.; Amano, T.; et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: A randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin. Ther. 2010, 32, 2337–2347. [Google Scholar]
- Hyafil, F.; Feldman, L.; Le Guludec, D.; Fayad, Z.A. Evaluating efficacy of pharmaceutical interventions in atherosclerosis: Role of magnetic resonance imaging and positron emission tomography. Mt. Sinai J. Med. 2012, 79, 689–704. [Google Scholar] [CrossRef]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef] [Green Version]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Tawakol, A.; Fayad, Z.A.; Mogg, R.; Alon, A.; Klimas, M.T.; Dansky, H.; Subramanian, S.S.; Abdelbaky, A.; Rudd, J.H.F.; Farkouh, M.E.; et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J. Am. Coll. Cardiol. 2013, 62, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Tomas, L.; Edsfeldt, A.; Mollet, I.; Matic, L.P.; Prehn, C.; Adamski, J.; Paulsson-Berne, G.; Hedin, U.; Nilsson, J.; Bengtsson, E.; et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur. Heart J. 2018, 39, 2301–2310. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qian, P.; Cen, D.; Hong, W.; Peng, Q.; Xue, M. Synthesis of phosphatidylcholine in rats with oleic acid-induced pulmonary edema and effect of exogenous pulmonary surfactant on its De Novo synthesis. PLoS ONE 2018, 13, e0193719. [Google Scholar] [CrossRef] [Green Version]
- Insull, W.; Bartsch, G.E. Cholesterol, triglyceride, and phospholipid content of intima, media, and atherosclerotic fatty streak in human thoracic aorta. J. Clin. Investig. 1966, 45, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boggs, K.P.; Rock, C.O.; Jackowski, S. Lysophosphatidylcholine and 1-O-Octadecyl-2-O-Methyl-rac-Glycero-3-Phosphocholine Inhibit the CDP-Choline Pathway of Phosphatidylcholine Synthesis at the CTP, Phosphocholine Cytidylyltransferase Step (∗). J. Biol. Chem. 1995, 270, 7757–7764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeffner, E.W. Studies on choline permeation through the plasma membrane and its incorporation into phosphatidyl choline of Ehrlich-Lettré-ascites tumor cells in vitro. Eur. J. Biochem. 1975, 51, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.T.; John, H.; Zweifel, R.; Cservenyak, T.; Westera, G.; Goerres, G.W.; Von Schulthess, G.K.; Hany, T. FFluorocholine PET/CT in patients with prostate cancer: Initial experience. Radiology 2005, 235, 623–628. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Waki, A.; Obata, A.; Furukawa, T.; Yonekura, Y.; Fujibayashi, Y. Radiolabeled choline as a proliferation marker: Comparison with radiolabeled acetate. Nucl. Med. Biol. 2004, 31, 859–865. [Google Scholar] [CrossRef]
- Wyss, M.T.; Weber, B.; Honer, M.; Späth, N.; Ametamey, S.M.; Westera, G.; Bode, B.; Kaim, A.H.; Buck, A. 18F-choline in experimental soft tissue infection assessed with autoradiography and high-resolution PET. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 312–316. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar]
- Velikyan, I. Prospective of 68Ga Radionuclide Contribution to the Development of Imaging Agents for Infection and Inflammation. Contrast Media Mol. Imaging 2018, 2018, 9713691. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.-G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of Somatostatin Receptor 2-Targeting PET Tracers in the Detection of Mouse Atherosclerotic Plaques. Mol. Imaging Biol. 2016, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bauer, W.; Kreissl, M.C.; Weirather, J.; Bauer, E.; Israel, I.; Richter, D.; Riehl, G.; Buck, A.; Samnick, S. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis 2013, 230, 33–39. [Google Scholar] [CrossRef]
- Armani, C.; Catalani, E.; Balbarini, A.; Bagnoli, P.; Cervia, D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J. Leukoc. Biol. 2007, 81, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Dalm, V.A.S.H.; Van Hagen, P.M.; Van Koetsveld, P.M.; Achilefu, S.; Houtsmuller, A.B.; Pols, D.; Van Der Lely, A.-J.; Lamberts, S.W.J.; Hofland, L.J. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am. J. Physiol.-Endocrinol. Metab. Am. Physiol. Soc. 2003, 285, E344–E353. [Google Scholar] [CrossRef]
- Li, X.; Samnick, S.; Lapa, C.; Israel, I.; Buck, A.K.; Kreissl, M.C.; Bauer, W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012, 2, 52. [Google Scholar] [CrossRef] [Green Version]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.; Loft, A.; Hag, A.M.; Kjaer, A. 64Cu-DOTATATE for Noninvasive Assessment of Atherosclerosis in Large Arteries and Its Correlation with Risk Factors: Head-to-Head Comparison with 68Ga-DOTATOC in 60 Patients. J. Nucl. Med. Soc. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Mojtahedi, A.; Alavi, A.; Thamake, S.; Amerinia, R.; Ranganathan, D.; Tworowska, I.; Delpassand, E.S. Assessment of vulnerable atherosclerotic and fibrotic plaques in coronary arteries using (68)Ga-DOTATATE PET/CT. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 65–71. [Google Scholar]
- Schatka, I.; Wollenweber, T.; Haense, C.; Brunz, F.; Gratz, K.F.; Bengel, F.M. Peptide receptor-targeted radionuclide therapy alters inflammation in atherosclerotic plaques. J. Am. Coll. Cardiol. 2013, 62, 2344–2345. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Sandholt, B.V.; Keller, S.H.; Hansen, A.E.; Clemmensen, A.E.; Sillesen, H.; Højgaard, L.; Ripa, R.S.; Kjær, A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques: Studies in Patients Undergoing Endarterectomy. Arter. Thromb. Vasc. Biol. 2015, 35, 1696–1703. [Google Scholar] [CrossRef] [Green Version]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arter. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Lee, R.; Kim, J.; Paeng, J.C.; Byun, J.W.; Cheon, G.J.; Lee, D.S.; Chung, J.-K.; Kang, K.W. Measurement of 68Ga-DOTATOC Uptake in the Thoracic Aorta and Its Correlation with Cardiovascular Risk. Nucl. Med. Mol. Imaging 2018, 52, 279–286. [Google Scholar] [CrossRef]
- Velikyan, I. Prospective of 68Ga-radiopharmaceutical development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Do, L.H.C.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990, 33, 1122–1128. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Writing group; Reviewer group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees; SNMMI Cardiovascular; SNMMI Cardiovascular; Members of Council, PET Interest Group; Members of ASNC; et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef] [Green Version]
- Kerr, G.S.; Hallahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hoffman, G.S. Takayasu arteritis. Ann. Intern. Med. 1994, 120, 919–929. [Google Scholar] [CrossRef]
- Cheng, Y.; Lv, N.; Wang, Z.; Chen, B.; Dang, A. 18-FDG-PET in assessing disease activity in Takayasu arteritis: A meta-analysis. Clin. Exp. Rheumatol. 2013, 31, S22–S27. [Google Scholar]
- Besson, F.L.; Parienti, J.-J.; Bienvenu, B.; Prior, J.O.; Costo, S.; Bouvard, G.; Agostini, D. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Soussan, M.; Nicolas, P.; Schramm, C.; Katsahian, S.; Pop, G.; Fain, O.; Mekinian, A. Management of large-vessel vasculitis with FDG-PET: A systematic literature review and meta-analysis. Medicine 2015, 94, e622. [Google Scholar] [CrossRef]
- Tatò, F.; Hoffmann, U. Giant cell arteritis: A systemic vascular disease. Vasc. Med. 2008, 13, 127–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessi, M.C.; Juhan-Vague, I.; Declerck, P.J.; Collen, D. Molecular forms of plasminogen activator inhibitor-1 (PAI-1) and tissue-type plasminogen activator (t-PA) in human plasma. Thromb. Res. 1991, 62, 275–285. [Google Scholar] [CrossRef]
- Löffler, C.; Hoffend, J.; Benck, U.; Krämer, B.K.; Bergner, R. The value of ultrasound in diagnosing extracranial large-vessel vasculitis compared to FDG-PET/CT: A retrospective study. Clin. Rheumatol. 2017, 36, 2079–2086. [Google Scholar] [CrossRef]
- Einspieler, I.; Thürmel, K.; Pyka, T.; Eiber, M.; Wolfram, S.; Moog, P.; Reeps, C.; Essler, M. Imaging large vessel vasculitis with fully integrated PET/MRI: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1012–1024. [Google Scholar] [CrossRef]
- Chatterjee, S.; Flamm, S.D.; Tan, C.D.; Rodriguez, E.R. Clinical diagnosis and management of large vessel vasculitis: Giant cell arteritis. Curr. Cardiol. Rep. 2014, 16, 498. [Google Scholar] [CrossRef]
- Schmall, J.P.; Karp, J.S.; Alavi, A. The Potential Role of Total Body PET Imaging in Assessment of Atherosclerosis. PET Clin. 2019, 14, 245–250. [Google Scholar] [CrossRef]
- De Boysson, H.; Dumont, A.; Liozon, E.; Lambert, M.; Boutemy, J.; Maigné, G.; Silva, N.M.; Sultan, A.; Ly, K.H.; Aide, N.; et al. Giant-cell arteritis: Concordance study between aortic CT angiography and FDG-PET/CT in detection of large-vessel involvement. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2274–2279. [Google Scholar] [CrossRef]
- Misra, D.P.; Shenoy, S.N. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol. Int. 2017, 37, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, A.M.; Verberne, H.J.; Budde, R.P.J.; Lam, M.G.E.H. Additional Heparin Preadministration Improves Cardiac Glucose Metabolism Suppression over Low-Carbohydrate Diet Alone in 18F-FDG PET Imaging. J. Nucl. Med. Soc. Nucl. Med. 2016, 57, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorbala, S.; Di Carli, M.F.; Delbeke, D.; Abbara, S.; DePuey, E.G.; Dilsizian, V.; Forrester, J.; Janowitz, W.; Kaufmann, P.A.; Mahmarian, J.; et al. SNMMI/ASNC/SCCT Guideline for Cardiac SPECT/CT and PET/CT 1.0. J. Nucl. Med. Soc. Nucl. Med. 2013, 54, 1485–1507. [Google Scholar] [CrossRef] [Green Version]
- Ben-Haim, S.; Kupzov, E.; Tamir, A.; Israel, O. Evaluation of 18F-FDG Uptake and Arterial Wall Calcifications Using 18F-FDG PET/CT. J. Nucl. Med. Soc. Nucl. Med. 2004, 45, 1816–1821. [Google Scholar]
- Nielsen, B.D.; Gormsen, L.C.; Hansen, I.T.; Keller, K.K.; Therkildsen, P.; Hauge, E.-M. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1119–1128. [Google Scholar] [CrossRef]
- Stellingwerff, M.D.; Brouwer, E.; Lensen, K.-J.D.F.; Rutgers, A.; Arends, S.; van der Geest, K.S.M.; Glaudemans, A.W.J.M.; Slart, R.H.J.A. Different Scoring Methods of FDG PET/CT in Giant Cell Arteritis: Need for Standardization. Medicine 2015, 94, e1542. [Google Scholar] [CrossRef]


| Molecular Target | Nuclear Probes | Information | National Clinical Trial Number | References |
|---|---|---|---|---|
| Large vessel vasculitis | ||||
| GLUT transporters | [18F]FDG | Macrophage metabolism | NCT03914248 NCT04204876 NCT03765424 NCT03550781 NCT04888221 NCT01588483 NCT00744952 NCT03285945 | [11,45,46,47,48,49,50,51,52] |
| SSTR2 | [68Ga]Ga-DOTA-TATE [18F]fluoroethyltriazole-octreotate | Macrophage activity | NCT04071691 | [53] |
| [68Ga]Ga-DOTA-TATE | NCT03812302 | |||
| TSPO | [11C]PK11195 | Macrophage activity | NCT01878721 | [54] |
| Atherosclerosis | ||||
| GLUT transporters | [18F]FDG | Macrophage metabolism | NCT04181996 NCT00633022 NCT01341730 NCT01186666 NCT02162303 NCT03215550 NCT04505865 NCT04350216 | [55,56,57,58] |
| Choline transporter | [18F]FMCH | Macrophage activity | NCT03252990 NCT02640313 | [59,60,61] |
| SSTR2 | [68Ga]Ga-DOTA-TATE | Macrophage activity | NCT04043377 NCT04073810 NCT02021188 | [22,23,62] |
| Mannose receptors | [68Ga]Ga-NOTA-MSA [99mTc]Tc-Tilmanocept [68Ga]Ga-NOTA-anti-MMR-VHH2 | Macrophage activity | NCT01893489 NCT01889693 NCT02542371 NCT04758650 | [29,63] |
| TSPO | [11C]PBR28 [11C]PK11195 | Macrophage activity | NCT00547976 | [64] |
| Integrins | [18F]RGD-K5 [68Ga]Ga-NOTA-RGD | Neoangiogenesis and macrophage activity | NCT03364270 | [37] |
| FAP | [68Ga]Ga-DOTA-FAPI-04 | Proinflammatory macrophages and type I collagen breakdown in fibrous caps | NCT05036759 | [39] |
| CCR2 | [64Cu]Cu-DOTA-ECL1i | Pro-inflammatory macrophages | NCT04537403 | [65] |
| Aβ | [18F]flutemetamol | Aβ deposition in human atherosclerotic plaques | NCT03291093 | [40] |
| NPR-C | [64Cu]Cu-DOTA-CANF-Comb | Endothelial and vascular smooth muscle cells activation | NCT02498379 NCT02417688 | [66] |
| - | [64Cu]Cu-macrin | Macrophage phagocytic activity | NCT04843891 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigent, K.; Vigne, J. Advances in Radiopharmaceutical Sciences for Vascular Inflammation Imaging: Focus on Clinical Applications. Molecules 2021, 26, 7111. https://doi.org/10.3390/molecules26237111
Prigent K, Vigne J. Advances in Radiopharmaceutical Sciences for Vascular Inflammation Imaging: Focus on Clinical Applications. Molecules. 2021; 26(23):7111. https://doi.org/10.3390/molecules26237111
Chicago/Turabian StylePrigent, Kevin, and Jonathan Vigne. 2021. "Advances in Radiopharmaceutical Sciences for Vascular Inflammation Imaging: Focus on Clinical Applications" Molecules 26, no. 23: 7111. https://doi.org/10.3390/molecules26237111
APA StylePrigent, K., & Vigne, J. (2021). Advances in Radiopharmaceutical Sciences for Vascular Inflammation Imaging: Focus on Clinical Applications. Molecules, 26(23), 7111. https://doi.org/10.3390/molecules26237111





