Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. FO Led to Increased Nrf2/HO-1, Decreased NADPH Activity, and Decreased OXIDATIVE Stress in Muscle of Dexa-Treated Animals
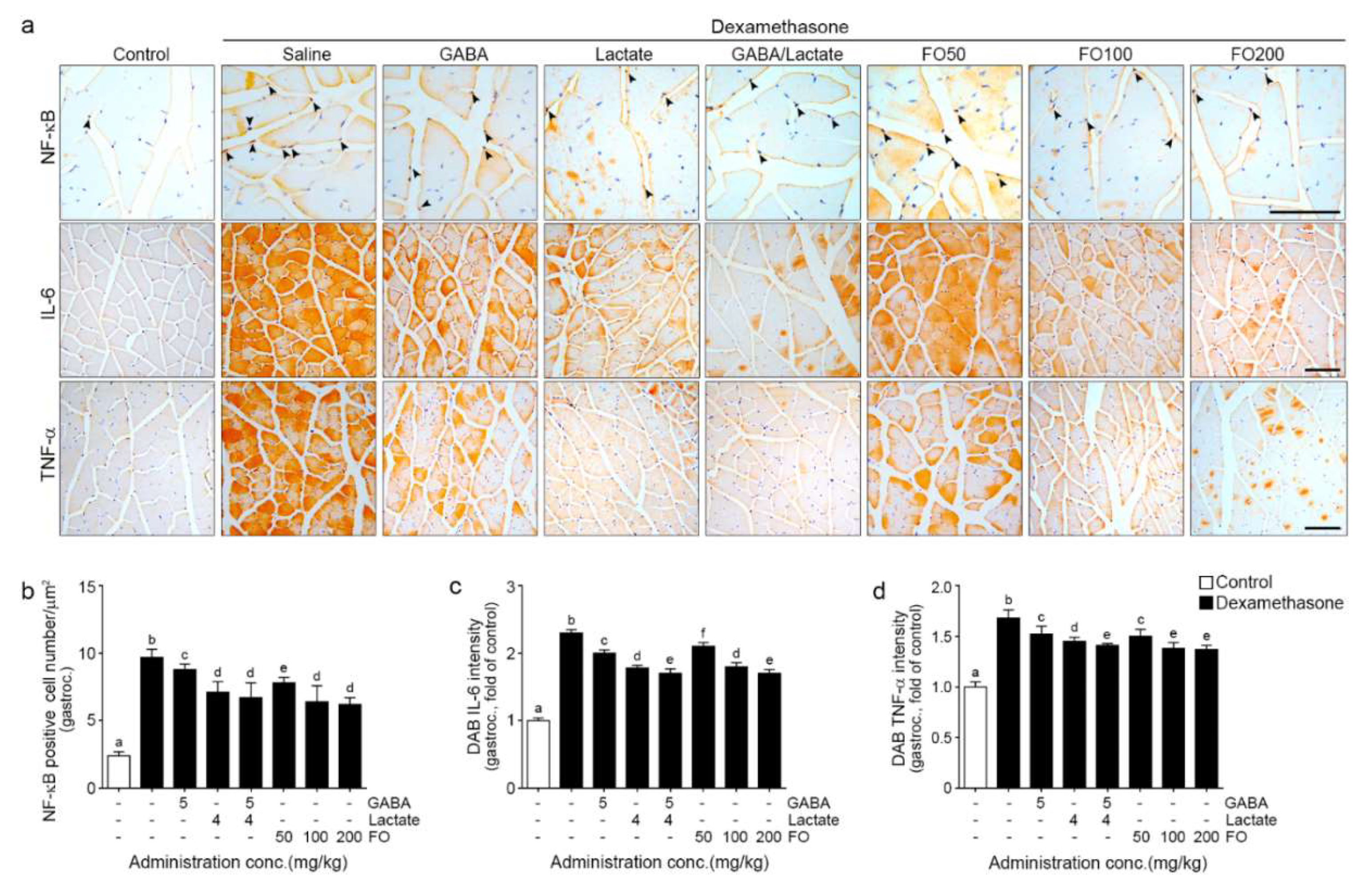
2.2. FO Decreased Expression of NF-κB/IL-6/TNF-α
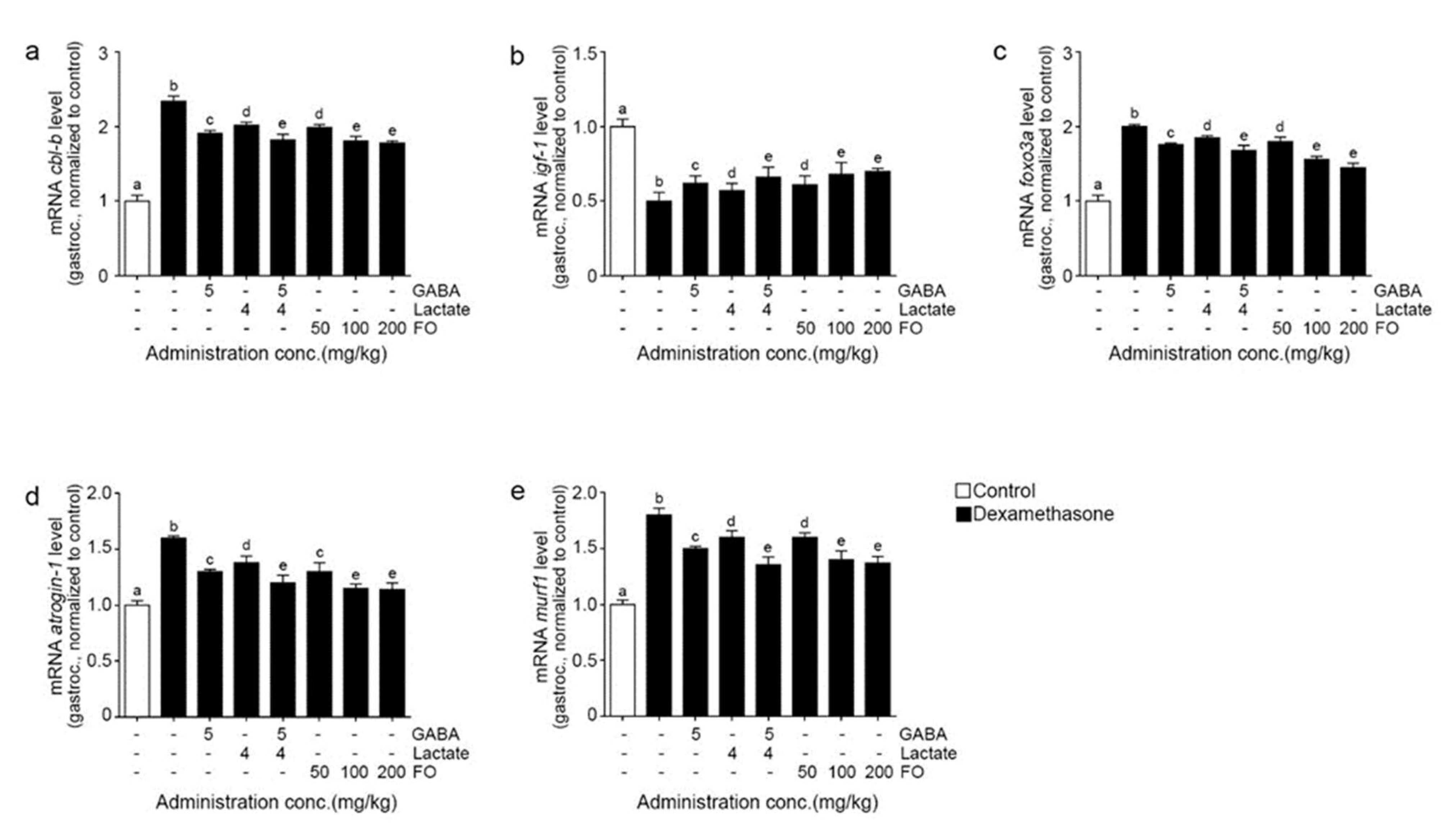
2.3. FO Leads to Decreased Expression of Cbl-b, Increased IGF-1, and Decreased FoxO3/Atrogin-1/Murf-1 in the Muscle of Dexa-Treated Animals
2.4. FO Attenuated Muscle Atrophy and Improved Grip Strength
3. Discussion
4. Materials and Methods
4.1. Preparation of FO, GABA, and Lactate
4.2. Dexa Incued Muscle Atrophy Mice Model
- (i)
- Control group: mice were orally administered with saline.
- (ii)
- Dexa/Saline group: mice were orally administered with saline, and muscle atrophy was induced with dexamethasone.
- (iii)
- Dexa/GABA group: mice were orally administered with 5 mg/kg of GABA, and muscle atrophy was induced with dexamethasone.
- (iv)
- Dexa/Lactate group: mice were orally administered with 4 mg/kg of GABA, and muscle atrophy was induced with dexamethasone.
- (v)
- Dexa/GABA+Lactate group: mice were orally administered with 5 mg/kg of GABA + 4 mg/kg of GABA, and muscle atrophy was induced with dexamethasone.
- (vi)
- Dexa/FO50: mice were orally administered with 50 mg/kg of FO, and muscle atrophy was induced with dexamethasone.
- (vii)
- Dexa/FO100: mice were orally administered 100 mg/kg of FO, and muscle atrophy was induced with dexamethasone.
- (viii)
- exa/FO200: mice were orally administered 200 mg/kg of FO, and muscle atrophy was induced with dexamethasone.
4.3. RNA Extraction and Complementary DNA (cDNA) Synthesis and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Immunohistochemistry (3,3-Diaminobenzidine: DAB)
4.6. Hematoxylin and Eosin (H&E) Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Jackson, M.J. Reactive oxygen species in sarcopenia: Should we focus on excess oxidative damage or defective redox signalling? Mol. Asp. Med. 2016, 50, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Oshima, Y.; Kuroda, Y.; Kunishige, M.; Matsumoto, T.; Mitsui, T. Oxidative stress-associated mitochondrial dysfunction in corticosteroid-treated muscle cells. Muscle Nerve 2004, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, A.; Kojima, H.; Ohtani, T.; Hayashi, K. Vitamin E reduces glucocorticoid-induced oxidative stress in rat skeletal muscle. J. Nutr. Sci. Vitaminol. 1998, 44, 779–786. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.S.; Lu, C.C.; Chiu, Y.J.; Chen, H.C.; Chung, M.I.; Wu, Y.T.; Chen, F.A. Effect of Quercetin on Dexamethasone-Induced C2C12 Skeletal Muscle Cell Injury. Molecules 2020, 25, 3267. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Ambrogini, E.; Weinstein, R.S.; Manolagas, S.C. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J. Biol. Chem. 2011, 286, 44326–44335. [Google Scholar] [CrossRef] [Green Version]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Sakashita, Y.; Kitahata, K.; Yamashita, Y.; Tomida, C.; Kimori, Y.; Komatsu, A.; Hirasaka, K.; Ohno, A.; Nakao, R.; et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2018, 314, C721–C731. [Google Scholar] [CrossRef]
- Nakao, R.; Hirasaka, K.; Goto, J.; Ishidoh, K.; Yamada, C.; Ohno, A.; Okumura, Y.; Nonaka, I.; Yasutomo, K.; Baldwin, K.M.; et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol. Cell Biol. 2009, 29, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660–2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.J.; Kim, H.S. Deregulation of Nrf2/ARE signaling pathway causes susceptibility of dystrophin-deficient myotubes to menadione-induced oxidative stress. Exp. Cell Res. 2018, 364, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Kim, D.J.; Kim, H.S. Sulforaphane ameliorates serum starvation-induced muscle atrophy via activation of the Nrf2 pathway in cultured C2C12 cells. Cell. Biol. Int. 2020, 44, 1831–1839. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, C.; Chio, I.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Steffen, B.T.; Lees, S.J.; Booth, F.W. Anti-TNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J. Appl. Physiol. 2008, 105, 1950–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yama, K.; Sato, K.; Abe, N.; Murao, Y.; Tatsunami, R.; Tampo, Y. Epalrestat increases glutathione, thioredoxin, and heme oxygenase-1 by stimulating Nrf2 pathway in endothelial cells. Redox Biol. 2015, 4, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Zhao, X.; Kelly, M.R.; Kandhi, S.; Perez, O.; Abraham, N.G.; Wolin, M.S. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1453–H1461. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, M.; Savitskaya, A.; Steiner, C.W.; Rath, E.; Bilban, M.; Wagner, O.; Bach, F.H.; Smolen, J.S.; Scheinecker, C. Heme oxygenase-1 end-products carbon monoxide and biliverdin ameliorate murine collagen induced arthritis. Clin. Exp. Rheumatol. 2012, 30, 73–78. [Google Scholar]
- Yu, Z.Y.; Ma, D.; He, Z.C.; Liu, P.; Huang, J.; Fang, Q.; Zhao, J.Y.; Wang, J.S. Heme oxygenase-1 protects bone marrow mesenchymal stem cells from iron overload through decreasing reactive oxygen species and promoting IL-10 generation. Exp. Cell Res. 2018, 362, 28–42. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Takeshige, K.; Cheung, B.S.; Minakami, S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta 1991, 1076, 369–373. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Liu, B.; Liang, C.; Li, Y.; Song, Y.H. Cytokine Signaling in Skeletal Muscle Wasting. Trends in endocrinology and metabolism. Trends Endocrinol. Metab. 2016, 27, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Balasubramanian, T. Isolation and structural elucidation of antioxidant peptides from oyster (Saccostrea cucullata) protein hydrolysate. Protein Pept. Lett. 2014, 21, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and characterization of a phenolic antioxidant from the Pacific oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Hui, S.P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. A phenolic antioxidant from the Pacific oyster (Crassostrea gigas) inhibits oxidation of cultured human hepatocytes mediated by diphenyl-1-pyrenylphosphine. Food Chem. 2012, 134, 2086–2089. [Google Scholar] [CrossRef]
- Fuda, H.; Watanabe, M.; Hui, S.P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef]
- Lee, N.K.; Paik, H.D. Bioconversion Using Lactic Acid Bacteria: Ginsenosides, GABA, and Phenolic Compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Han, M.H.; Jeong, J.W.; Kim, S.O.; Jeong, S.J.; Lee, B.J.; Kim, G.Y.; Park, E.K.; Jeon, Y.J.; et al. Cytoprotective effects of fermented oyster extracts against oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in MC3T3-E1 osteoblasts. EXCLI J. 2020, 19, 1102–1119. [Google Scholar]
- Ingawale, D.K.; Mandlik, S.K.; Patel, S.S. An emphasis on molecular mechanisms of anti-inflammatory effects and glucocorticoid resistance. J. Complement. Integr. Med. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2016, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 12, 422. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr. Hypertens Rep. 2010, 12, 433–912. [Google Scholar] [CrossRef]
- Marzetti, E.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Dupont-Versteegden, E.E.; Carter, C.S.; Bernabei, R.; Leeuwenburgh, C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim. Biophys. Acta 2010, 1800, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Molecular mechanisms controlling skeletal muscle mass. Muscle Cell Tissue 2015, 484. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic. Biol. Med. 2013, 65, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Ulla, A.; Uchida, T.; Miki, Y.; Sugiura, K.; Higashitani, A.; Kobayashi, T.; Ohno, A.; Nakao, R.; Hirasaka, K.; Sakakibara, I.; et al. Morin attenuates dexamethasone-mediated oxidative stress and atrophy in mouse C2C12 skeletal myotubes. Arch. Biochem. Biophys. 2021, 704, 108873. [Google Scholar] [CrossRef]
- Espinoza, M.B.; Aedo, J.E.; Zuloaga, R.; Valenzuela, C.; Molina, A.; Valdés, J.A. Cortisol Induces Reactive Oxygen Species Through a Membrane Glucocorticoid Receptor in Rainbow Trout Myotubes. J. Cell Biochem. 2017, 118, 718–725. [Google Scholar] [CrossRef]
- Bai, S.C.; Xu, Q.; Li, H.; Qin, Y.F.; Song, L.C.; Wang, C.G.; Cui, W.H.; Zheng, Z.; Yan, D.W.; Li, Z.J.; et al. NADPH Oxidase Isoforms Are Involved in Glucocorticoid-Induced Preosteoblast Apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 9192413. [Google Scholar] [CrossRef]
- Volonte, D.; Liu, Z.; Musille, P.M.; Stoppani, E.; Wakabayashi, N.; Di, Y.P.; Lisanti, M.P.; Kensler, T.W.; Galbiati, F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol. Biol. Cell 2013, 24, 1852–1862. [Google Scholar] [CrossRef]
- Xie, Z.X.; Xia, S.F.; Qiao, Y.; Shi, Y.H.; Le, G.W. Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet. J. Anim. Physiol. Anim. Nutr. 2015, 99, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote cellular stress resistance and longevity through ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef]
- Reid, S.; Park, J.H.; Kim, Y.; Kwak, Y.S.; Jeon, B.H. In Vitro and In Vivo Effects of Fermented Oyster-Derived Lactate on Exercise Endurance Indicators in Mice. Int. J. Environ. Res. Public Health 2020, 17, 8811. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jung, Y.; Nam, M.; Sun Kang, M.; Lee, M.K.; Cho, Y.; Choi, E.K.; Hwang, G.S.; Soo, K.H. Angiotensin II affects inflammation mechanisms via AMPK-related signalling pathways in HL-1 atrial myocytes. Sci. Rep. 2017, 7, 10328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef]
- Smith, C.V.; Jones, D.P.; Guenthner, T.M.; Lash, L.H.; Lauterburg, B.H. Compartmentation of glutathione: Implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 1996, 140, 1–12. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Hikim, I.; Sinha-Hikim, A.P.; Parveen, M.; Shen, R.; Goswami, R.; Tran, P.; Crum, A.; Norris, K.C. Long-term supplementation with a cystine-based antioxidant delays loss of muscle mass in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 8, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Frantz, J.D.; Tawa, N.E., Jr.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourkioti, F.; Kratsios, P.; Luedde, T.; Song, Y.H.; Delafontaine, P.; Adami, R.; Parente, V.; Bottinelli, R.; Pasparakis, M.; Rosenthal, N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 2006, 116, 2945–2954. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef] [Green Version]
- Mitin, N.; Kudla, A.J.; Konieczny, S.F.; Taparowsky, E.J. Differential effects of Ras signaling through NFkappaB on skeletal myogenesis. Oncogene 2001, 20, 1276–1286. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis protein protects against TNF-α-induced myotube atrophy in C2C12 myotubes via the NF-κB signaling pathway. Mol. Med. Rep. 2021, 24, 486. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Rizzuto, E.; Musarò, A. Counteracting muscle wasting in aging and neuromuscular diseases: The critical role of IGF-1. Aging 2009, 1, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Molagoda, I.; Jayasingha, J.; Choi, Y.H.; Park, E.K.; Jeon, Y.J.; Lee, B.J.; Kim, G.Y. Fermented oyster extract promotes insulin-like growth factor-1-mediated osteogenesis and growth rate. Mar. Drugs 2020, 18, 472. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.W.; Choi, S.H.; Han, M.H.; Kim, G.Y.; Park, C.; Hong, S.H.; Lee, B.J.; Park, E.K.; Kim, S.O.; Leem, S.H.; et al. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in RAW 264.7 cells. Int. J. Mol. Sci. 2019, 20, 1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Woo, M.; Keum, Y.S.; Noh, J.S.; Park, J.H.; Lee, B.J.; et al. Effect of fermented oyster extract on growth promotion in sprague-dawley rats. Integr. Med. Res. 2020, 9, 100412. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Kim, D.H.; Hong, S.H.; Lee, S.J.; Assefa, F.; Kim, G.Y.; et al. Gamma aminobutyric acid-enriched Fermented Oyster (Crassostrea gigas) increases the length of the growth plate on the proximal tibia bone in sprague-dawley rats. Molecules 2020, 25, 4375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Yang, J.; Park, C.; Son, K.; Byun, K. Dieckol attenuated glucocorticoid-induced muscle atrophy by decreasing NLRP3 inflammasome and pyroptosis. Int. J. Mol. Sci. 2021, 22, 8057. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Choi, C.H.; Lee, B.-J.; Park, J.-H.; Son, K.-H.; Byun, K. Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress. Molecules 2021, 26, 7128. https://doi.org/10.3390/molecules26237128
Oh S, Choi CH, Lee B-J, Park J-H, Son K-H, Byun K. Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress. Molecules. 2021; 26(23):7128. https://doi.org/10.3390/molecules26237128
Chicago/Turabian StyleOh, Seyeon, Chang Hu Choi, Bae-Jin Lee, Joung-Hyun Park, Kuk-Hui Son, and Kyunghee Byun. 2021. "Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress" Molecules 26, no. 23: 7128. https://doi.org/10.3390/molecules26237128





