Bioactive Molecules of Mandarin Seed Oils Diminish Mycotoxin and the Existence of Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Hydrodistillation of Seeds’ Volatile Content
2.3. Oil Extraction
2.4. Estimation for Mandarin Fatty Acids in the Seed Oil
2.5. Determination of Tocopherol Content for Mandarin Oil
2.6. Carotenoid and Sterol Content Determination
2.7. Phenolic Compound Extraction from Mandarin Oil
2.8. Determination of Phenolic and Flavonoid Fractions
2.9. Determination of Mandarin Seeds’ Volatile Content
2.10. Determination of Antimicrobial Activity
2.11. Simulation Experiment for the Evaluation of Mycotoxin Inhibition
2.12. Determination of Mycotoxin
2.13. Prevention Effect of Mandarin Oil against Mycotoxin Cytotoxicity
- ODs: optical density of sample evaluated.
- ODb: optical density of blank evaluated.
- ODc: optical density of the control evaluated.
2.14. Statistical Analysis
3. Results
3.1. Mandarin Fatty Acid Composition in Seed Oil
3.2. Carotenoid and Sterol Fractions of the Mandarin Oil
3.3. Mandarin Oil Content of Tocopherol
3.4. Phenolic and Flavonoid Fractions of Mandarin
3.5. Volatile Content of Mandarin Seeds
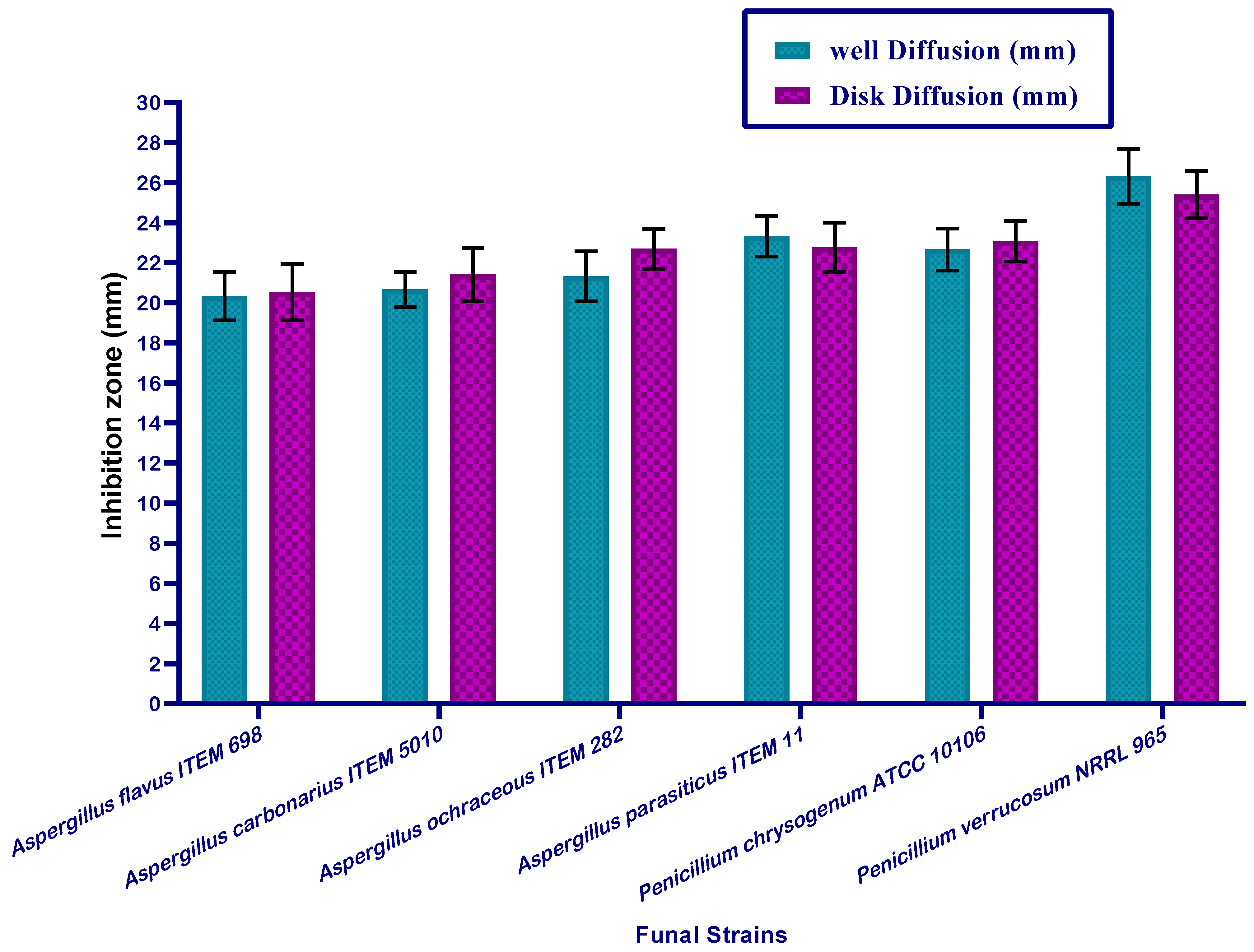
3.6. Antimicrobial Activity of Mandarin Seed Oil
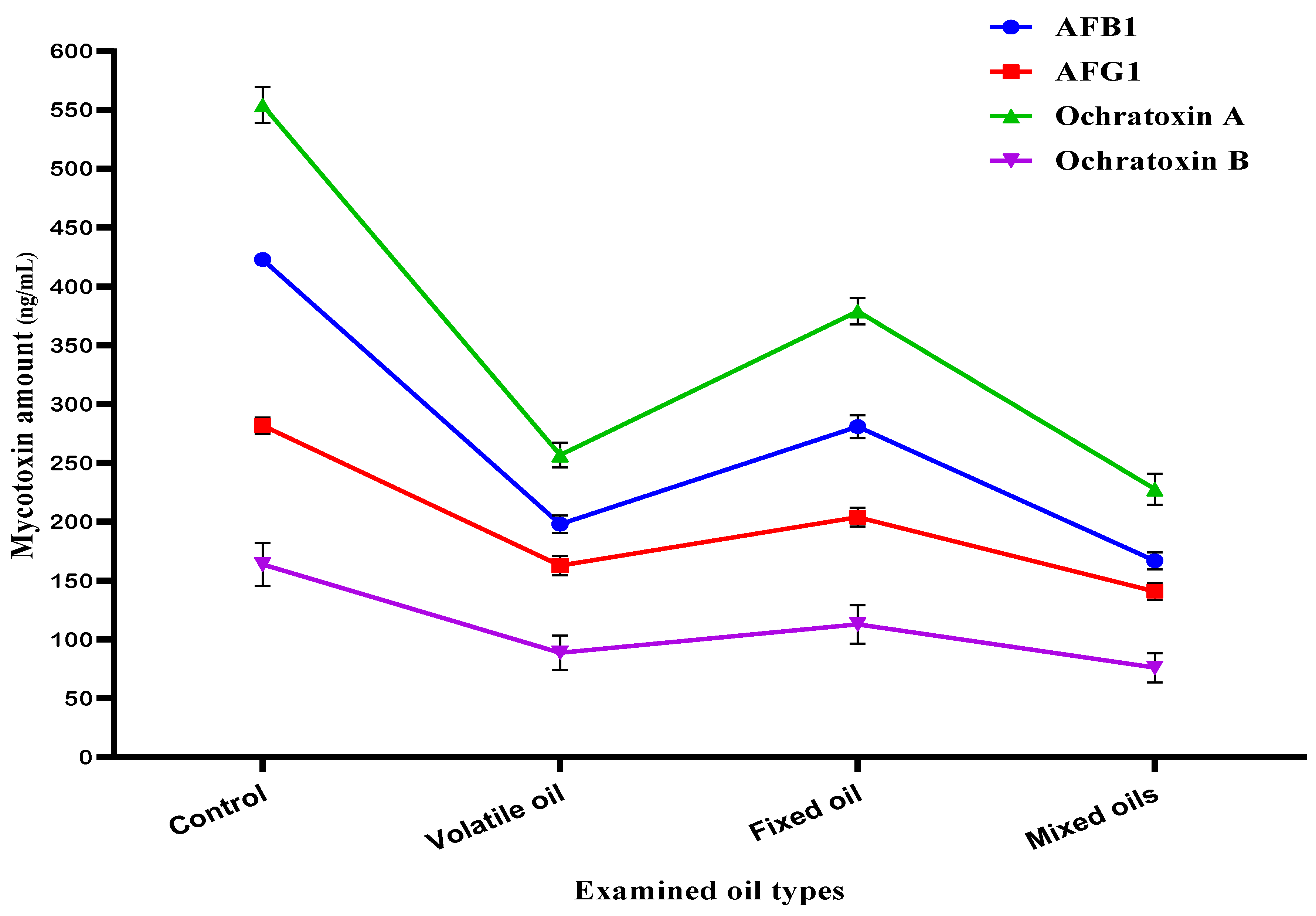
3.7. Mycotoxin Inhibition Simulation
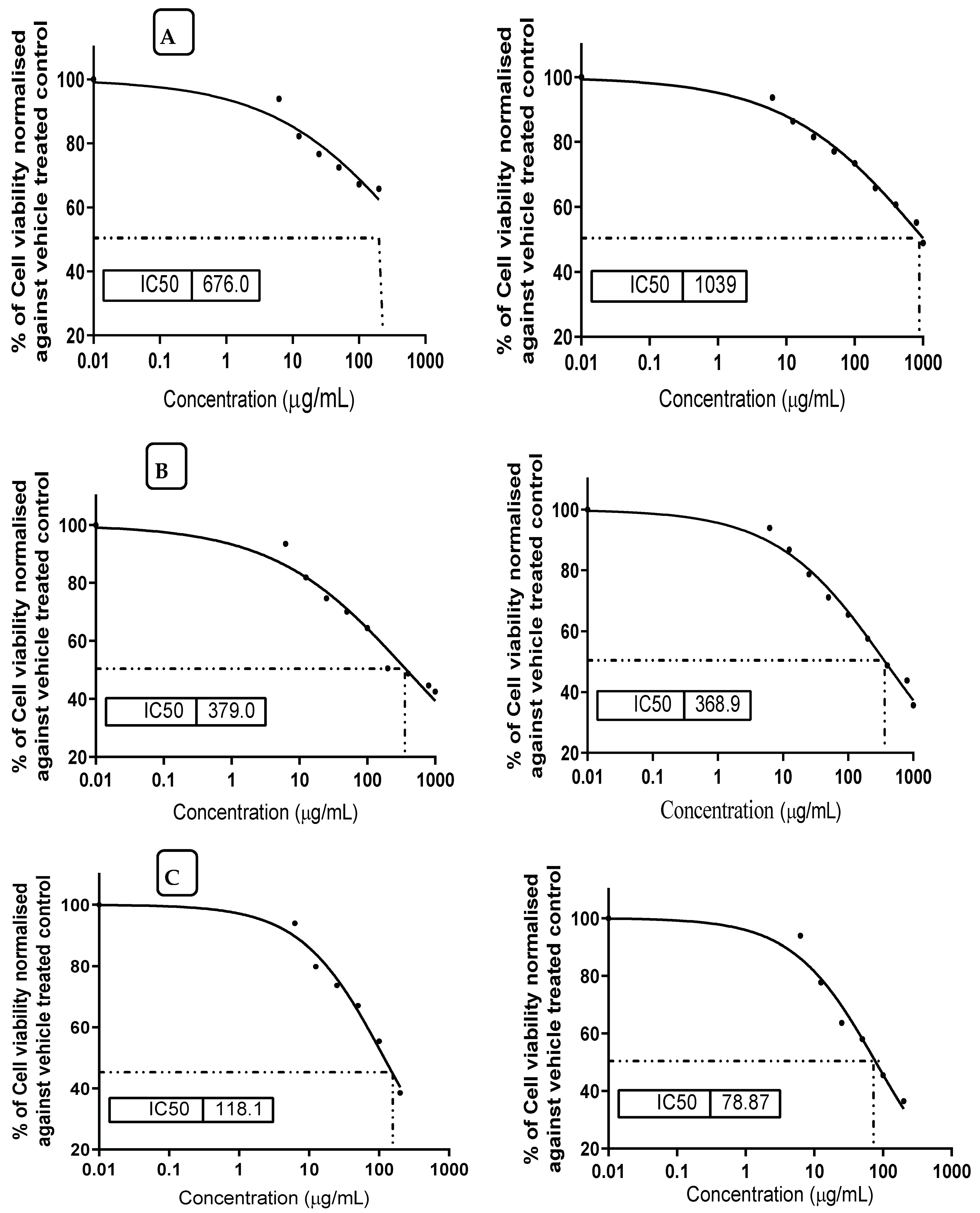
3.8. Prevention Effect of MMOS against Mycotoxin Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tietel, Z.; Plotto, A.; Fallik, E.; Lewinsohn, E.; Porat, R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2011, 91, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhaimi, F.A.L.; Matthäus, B.; Özcan, M.M.; Ghafoor, K. The physico-chemical properties of some citrus seeds and seed oils. Z. Nat. C 2016, 71, 79–85. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G. Prospective antimycotoxigenic action of wild Opuntia ficus-indica by-products. Czech J. Food Sci. 2020, 38, 308–314. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Singh, B. Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. J. Food Biochem. 2019, 43, e12706. [Google Scholar] [CrossRef]
- Abu-Sree, Y.H.; Abdel-Fattah, S.M.; Abdel-Razek, A.G.; Badr, A.N. Neoteric approach for peanuts biofilm using the merits of Moringa extracts to control aflatoxin contamination. Toxicol. Rep. 2021, 8, 1685–1692. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Shehata, M.G.; Badr, A.N.; Gromadzka, K.; Stępień, L. The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Sabry, B.A.; Hathout, A.S.; Nooh, A.; Aly, S.E.; Shehata, M.G. The prevalence of aflatoxin and Aspergillus parasiticus in Egyptian sesame seeds. Int. J. ChemTech Res. 2016, 9, 308–319. [Google Scholar]
- Sabry, B.A.; Farouk, A.; Badr, A.N. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Heliyon 2021, 7, e07721. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Shahat, M.S.; Badr, A.N.; Hegaziy, A.I.; Ramzy, S.; Samie, M.A. Reducing the histopathological and biochemical toxicity of aflatoxins contaminated soybean using ozone treatment. Annu. Res. Rev. Biol. 2017, 15, 1–16. [Google Scholar] [CrossRef]
- Savi, G.D.; Scussel, V.M. Effects of Ozone Gas Exposure on Toxigenic Fungi Species from Fusarium, Aspergillus, and Penicillium Genera. Ozone Sci. Eng. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Microbiology 2020, 11, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Naeem, M.A. Protective efficacy using Cape- golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Jalali Heravi, M.; Sereshti, H. Determination of essential oil components of Artemisia haussknechtii Boiss. using simultaneous hydrodistillation-static headspace liquid phase microextraction-gas chromatography mass spectrometry. J. Chromatogr. A 2007, 1160, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cert, A.; Moreda, W.; Pérez-Camino, M.C. Chromatographic analysis of minor constituents in vegetable oils. J. Chromatogr. A 2000, 881, 131–148. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Rogoziński, T.; Perkowski, J. Contamination of pine and birch wood dust with microscopic fungi and determination of its sterol contents. Arch. Ind. Hyg. Toxicol. 2017, 68, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical Scavenging Activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; Shehata, M.G. Characterization of olive oil by-products: Antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Badr, A.N.; Abdel-Razek, A.G.; Hassanein, M.M.; Amra, H.A. Oil-bioactive films as an antifungal application to save post-harvest food crops. Annu. Res. Rev. Biol. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; el Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Badr, A.N.; Logrieco, A.F.; Amra, H.A.; Hussein, T. Ochratoxin a occurrence on Egyptian wheat during seasons (2009–2014). Asian J. Sci. Res. 2017, 10, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Vajrabhaya, L.-O.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qin, S.; Liu, Z.; Zheng, Y.; Han, L.; Zhang, M.; Luo, N.; Gu, Y.; Zhang, Y.; Gu, N. Synergistic Anti-Proliferation and Anti-Angiogenesis Effects of Sevacizumab on Hepatocellular Carcinoma Cells in Combination with Chemotherapy. Res. Sq. 2021, 3, 1–24. [Google Scholar] [CrossRef]
- Abdel-Fattah, S.M.; Badr, A.N.; Seif, F.A.H.A.; Ali, S.M.; Hassan, R.A. Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J. Biol. Sci. 2018, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, A.N.; Youssef, M.; Abdel-Razek, A.G.; Shehata, M.G.; Hassanien, M.M.; Amra, H. Natural antioxidants: Preservation roles and mycotoxicological safety of food. Egypt. J. Chem. 2021, 64, 285–298. [Google Scholar] [CrossRef]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. South Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, R.S.; Sciences, P. Antifertility activity of β-sitosterol isolated from Barleria prionitis (L.) Roots in male albino rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 88–96. [Google Scholar]
- Harper, R.A.; Saleh, M.M.; Carpenter, G.; Abbate, V.; Proctor, G.; Harvey, R.D.; Gambogi, R.J.; Geonnotti, A.; Hider, R.; Jones, S.A. Soft, adhesive (+) alpha tocopherol phosphate planar bilayers that control oral biofilm growth through a substantive antimicrobial effect. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2307–2316. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Mariod, A.A.; Matthäus, B.; Idris, Y.M.A.; Abdelwahab, S.I. Fatty Acids, Tocopherols, Phenolics and the Antimicrobial Effect of Sclerocarya birrea Kernels with Different Harvesting Dates. J. Am. Oil Chem. Soc. 2010, 87, 377–384. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process Preserv. 2021, 45, e15112. [Google Scholar] [CrossRef]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Zahi, M.R.; el Hattab, M.; Liang, H.; Yuan, Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Chidi, F.; Bouhoudan, A.; Khaddor, M. Antifungal effect of the tea tree essential oil (Melaleuca alternifolia) against Penicillium griseofulvum and Penicillium verrucosum. J. King Saud Univ.—Sci. 2020, 32, 2041–2045. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.-Z.; Xiao, Z.-Q.; Fan, G.-R.; Chen, S.-X.; Liao, S.-L.; Luo, H.; Wang, Z.-D. Design, Synthesis, Antibacterial, Antifungal and Anticancer Evaluations of Novel β-Pinene Quaternary Ammonium Salts. Int. J. Mol. Sci. 2021, 22, 11299. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Si, H.; Wang, P.; Chen, S.; Shang, S.; Song, Z.; Wang, Z.; Liao, S. Derivatization of Natural Compound β-Pinene Enhances Its In Vitro Antifungal Activity against Plant Pathogens. Molecules 2019, 24, 3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finotti, E.; Parroni, A.; Zaccaria, M.; Domin, M.; Momeni, B.; Fanelli, C.; Reverberi, M. Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 2021, 11, 16024. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; El-Said, M.M.; El-Messery, T.M.; Abdel-Razek, A.G. Non-traditional oils encapsulation as novel food additive enhanced yogurt safety against aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef] [Green Version]

| Carbon Number | Fatty Acids | Concentration (%) |
|---|---|---|
| C12:0 | Lauric | 0.08 ± 0.001 |
| C14:0 | Mayristic | 0.02 ± 0.001 |
| C14:1 | Myristoleic | 22.9 ± 0.96 |
| C16:0 | Palmetic | 0.51 ± 0.09 |
| C16:1 ω-7 | Palmitoleic | 6.21 ± 0.22 |
| C18:0 | Stearic | 0.03 ± 0.005 |
| C18:1 ω-6 | Oleic | 7.87 ± 0.67 |
| C18:1 ω-9 | Oleic | 15.12 ± 0.97 |
| C18:2 ω-3 | Linoleic | 40.55 ± 1.08 |
| C18:3 ω-6 | Linolenic | 6.13 ± 0.34 |
| C20:0 | Arachidic | 0.06 ± 0.002 |
| C20:1 ω-9 | Gadoleic | 0.04 ± 0.004 |
| C20:2 ω-6 | Eicosadienoic | 0.02 ± 0.006 |
| C20:3 ω-9 | Mead | 0.17 ± 0.02 |
| C20:4 ω-6 | Arachidonic | 0.09 ± 0.003 |
| C20:5 ω-3 | Eicosapentanoic | 0.01 ± 0.002 |
| C22:0 | Behenic | 0.03 ± 0.005 |
| C22:1 ω-9 | Erucic | 0.01 ± 0.001 |
| C22:2 ω-3 | Docosadienoic | 0.02 ± 0.001 |
| C22:6 ω-3 | Docosahexanoic | ND |
| C24:0 | Lignoseric | 0.05 ± 0.002 |
| C24:1 ω-9 | Nervonic | 0.08 ± 0.005 |
| Significant Oil Parameters | ||
| 1 | SFA | 0.78 |
| 2 | MUFA | 52.15 |
| 3 | PUFA | 46.99 |
| 4 | SFA/MUFA | 0.015 |
| 5 | SFA/PUFA | 0.017 |
| 6 | MUFA/PUFA | 1.11 |
| 7 | SFA/MUFA/PUFA | 0.02:1.56:1.41 |
| Carotenoids (mg/100 g) | Lutein | Xanthine | β-Carotene | Violaxanthin | β-Cryptoxanthin | β,β-Xanthophyll | Phytoene | α-Carotene |
|---|---|---|---|---|---|---|---|---|
| Mean | 504.3 | 41.3 | 8.1 | 4.72 | 0.61 | 1.74 | 0.07 | 0.04 |
| SEM | ±2.17 | ±1.24 | ±0.9 | ±0.8 | ±0.4 | ±0.6 | ±0.03 | ±0.01 |
| Sterols (mg/100 g) | Campesterol | Stigmasterol | β-Sitosterol | δ-5 Avenasterol | Brassikasterol | |||
| Mean | 31.45 | 8.04 | 294.6 | 5.33 | 0.04 | |||
| SEM | ±0.94 | ±0.55 | ±3.89 | ±0.41 | ±0.002 | |||
| Tocopherols (mg/100 g) | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | ||||
| mean | 27.41 | ND | 14.85 | 0.09 | ||||
| SEM | ±0.81 | ND | ±1.02 | ±0.001 | ||||
| Phenolic Acids | Concentrations (mg/Kg) | Flavonoids | Concentrations (mg/Kg) |
|---|---|---|---|
| Gallic acid | 97.70 ± 4.57 | Catechin | 94.13 ± 5.81 |
| Chlorogenic acid | ND | Catechol | 133.81 ± 8.21 |
| Protocatechuic acid | ND | Epicatechins | 2.74± 0.89 |
| Trans-ferulic acid | 63.17 ± 1.69 | Rutin trihydrate | 32.42± 5.84 |
| Trans-cinnamic acid | 4.43 ± 1.57 | Apigenin 7 glucoside | 43.33 ± 2.98 |
| Syringic acid | 66.14 ± 3.58 | Quercetin | 73.47 ± 1.38 |
| Caffeic acid | 35.42 ± 4.66 | Luteolin | ND |
| Ferulic acid | 1.48 ± 0.34 | Hesperidin | 4.38 ± 0.51 |
| p-Hydroxybenzoic acid | 2.94 ± 0.67 | Naringenin | 35.13 ± 1.78 |
| p-Coumaric acid | 2.72 ± 0.18 | Kaempferol | 11.24 ± 1.34 |
| Resveratrol | 18.47 ± 2.44 | Isorhamnetin | 92.81 ± 2.78 |
| Vanillic acid | 0.44 ± 0.02 | Chrysin | ND |
| Compound | RI | % Volatile Fraction | Identification |
|---|---|---|---|
| Hexanal | 801 | 0.26 ± 0.08 | MS & RI |
| α-Thujene | 928 | 0.45 ± 0.05 | MS & RI |
| α-Pinene | 939 | 6.82 ± 0.73 | MS, RI & ST |
| Sabinene | 972 | 0.33 ± 0.02 | MS & RI |
| β-Pinene | 981 | 4.32 ± 0.24 | MS, RI & ST |
| β-Myrcene | 993 | 1.37 ± 0.54 | MS & RI |
| Octanal | 1006 | 1.04 ± 0.28 | MS & RI |
| α-Terpinene | 1012 | 0.32 ± 0.05 | MS, RI & ST |
| β-Phellandrene | 1030 | 3.93 ± 0.03 | MS & RI |
| Limonene | 1033 | 66.05 ± 1.41 | MS & RI |
| γ-Terpinene | 1074 | 12.31 ± 0.67 | MS, RI & ST |
| α-Terpinolene | 1096 | 0.08 ± 0.01 | MS & RI |
| Linalool | 1100 | 0.11 ± 0.03 | MS, RI & ST |
| Nonanal | 1104 | 0.09 ± 0.01 | MS & RI |
| Geranyl | 1149 | 0.05 ± 0.02 | MS & RI |
| Citronellal | 1159 | 0.23 ± 0.04 | MS, RI & ST |
| Decanal | 1234 | 0.04 ± 0.01 | MS & RI |
| Ethanone | 1274 | 0.57 ± 0.05 | MS & RI |
| Cadinene | 1275 | 0.48 ± 0.06 | MS, RI & ST |
| α-Cubebene | 1345 | 0.09 ± 0.03 | MS & RI |
| Isopiperitone | 1473 | 0.59 ± 0.11 | MS & RI |
| α-Sinensal | 1526 | 0.1 ± 0.03 | MS & RI |
| β-Sinensal | 1675 | 0.4 ± 0.02 | MS & RI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, S.S.; Badr, A.N.; Gromadzka, K.; Stuper-Szablewska, K.; Abdel-Razek, A.G.; Selim, K. Bioactive Molecules of Mandarin Seed Oils Diminish Mycotoxin and the Existence of Fungi. Molecules 2021, 26, 7130. https://doi.org/10.3390/molecules26237130
Alharthi SS, Badr AN, Gromadzka K, Stuper-Szablewska K, Abdel-Razek AG, Selim K. Bioactive Molecules of Mandarin Seed Oils Diminish Mycotoxin and the Existence of Fungi. Molecules. 2021; 26(23):7130. https://doi.org/10.3390/molecules26237130
Chicago/Turabian StyleAlharthi, Salman S., Ahmed Noah Badr, Karolina Gromadzka, Kinga Stuper-Szablewska, Adel Gabr Abdel-Razek, and Khaled Selim. 2021. "Bioactive Molecules of Mandarin Seed Oils Diminish Mycotoxin and the Existence of Fungi" Molecules 26, no. 23: 7130. https://doi.org/10.3390/molecules26237130
APA StyleAlharthi, S. S., Badr, A. N., Gromadzka, K., Stuper-Szablewska, K., Abdel-Razek, A. G., & Selim, K. (2021). Bioactive Molecules of Mandarin Seed Oils Diminish Mycotoxin and the Existence of Fungi. Molecules, 26(23), 7130. https://doi.org/10.3390/molecules26237130








