Evaluation of Traumatic Spinal Cord Injury in a Rat Model Using 99mTc-GA-5 as a Potential In Vivo Tracer
Abstract
:1. Introduction
2. Results
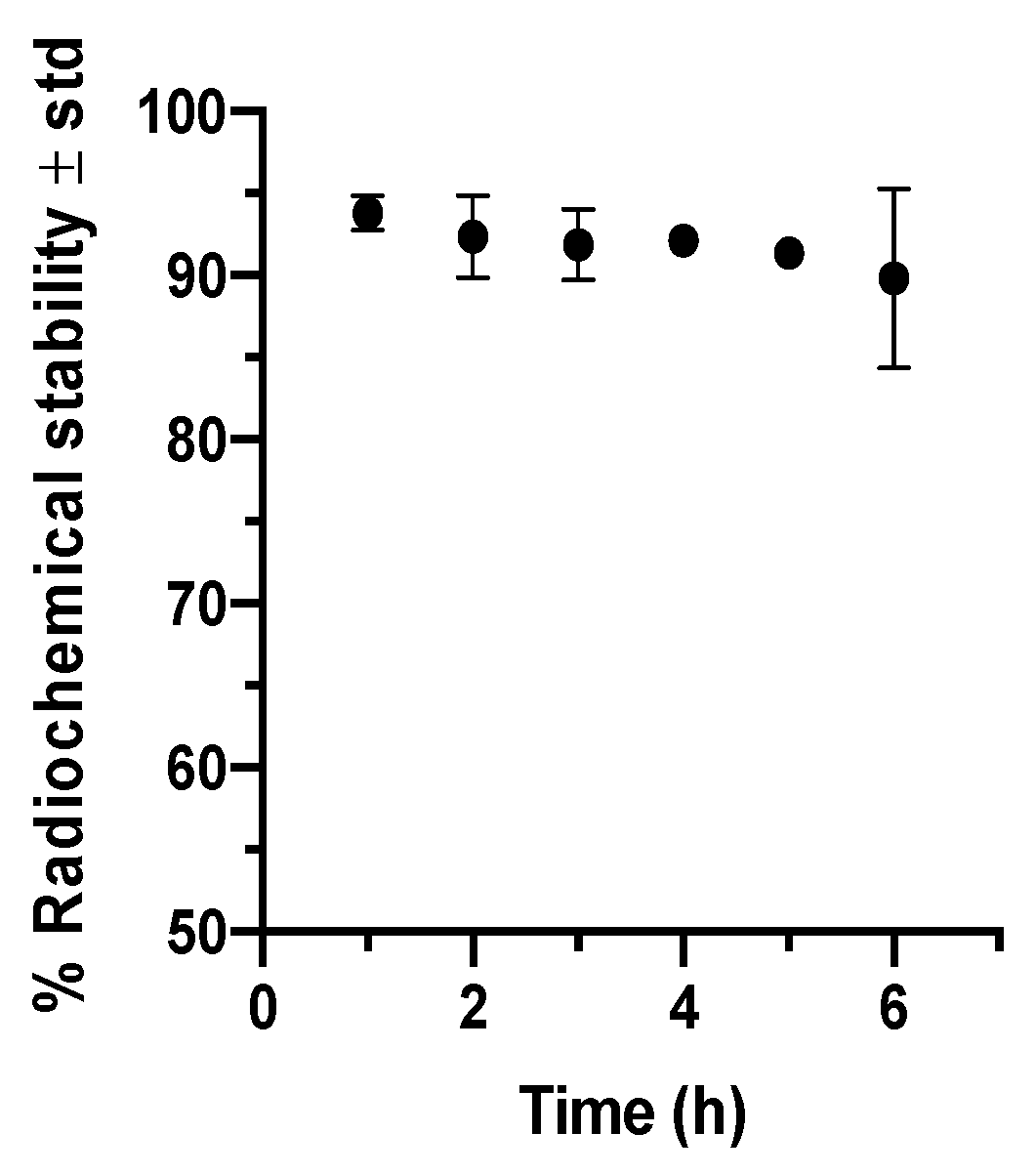
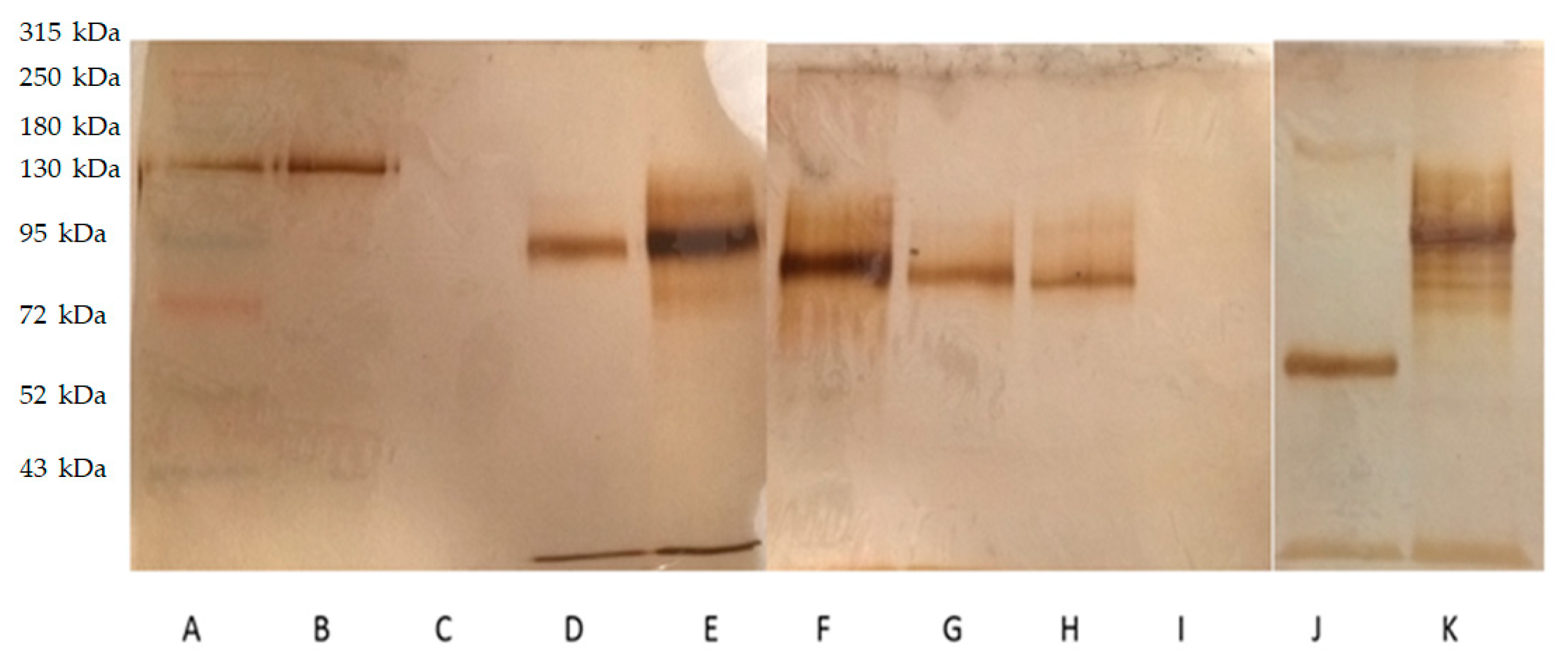
2.1. Anti-GFAP Radiolabeling and Integrity
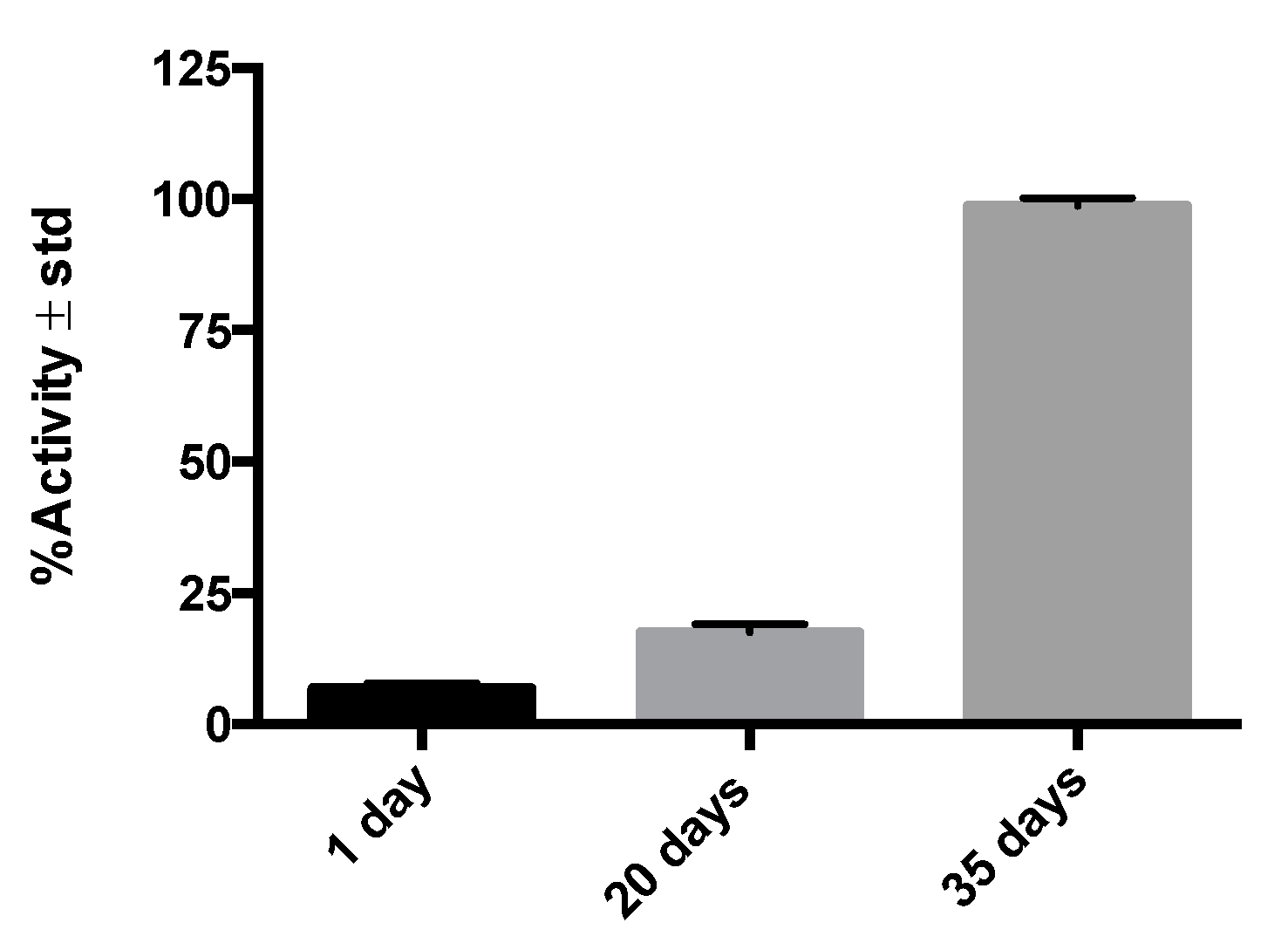
2.2. Radioimmunohistochemistry Results
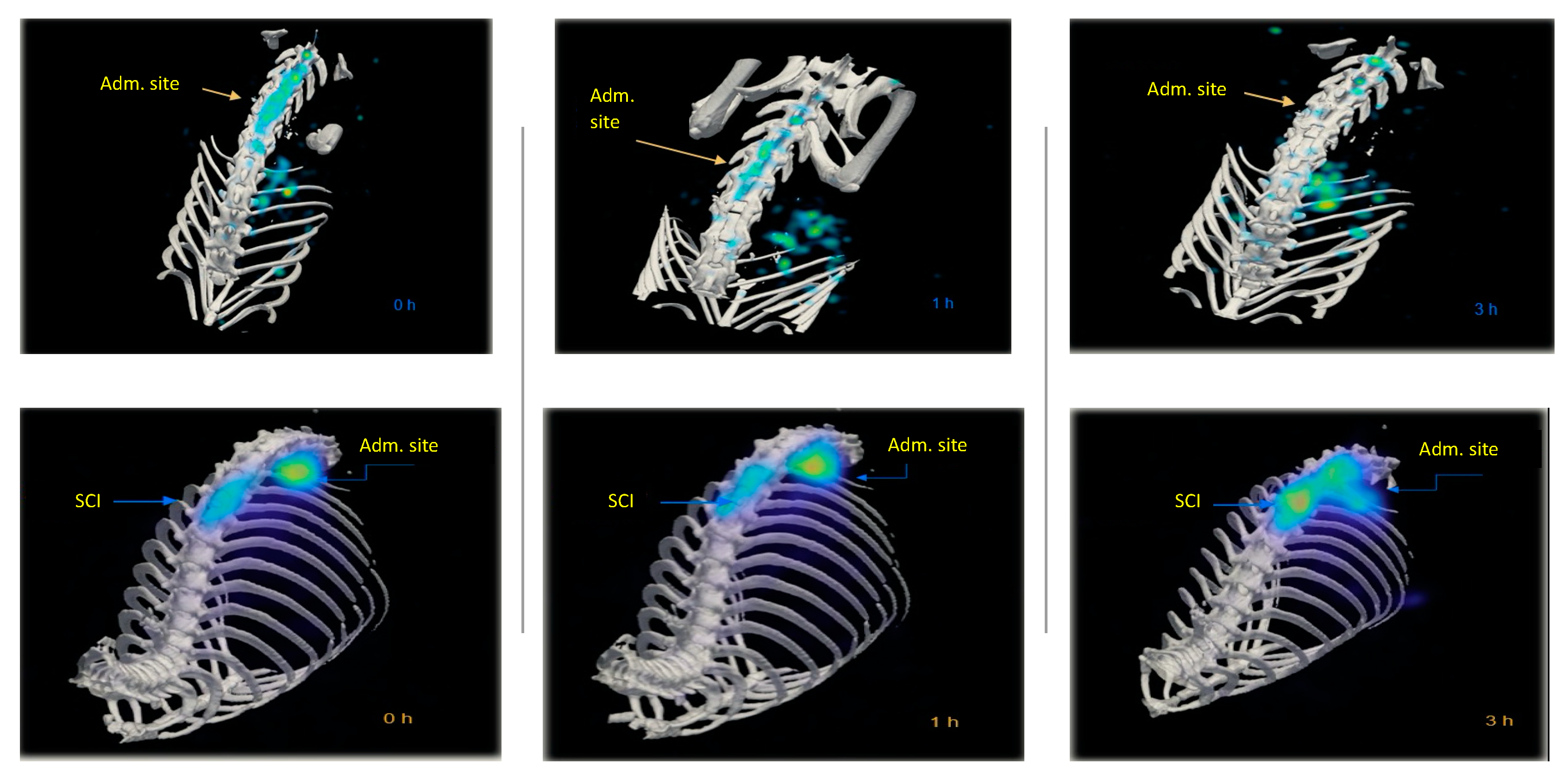
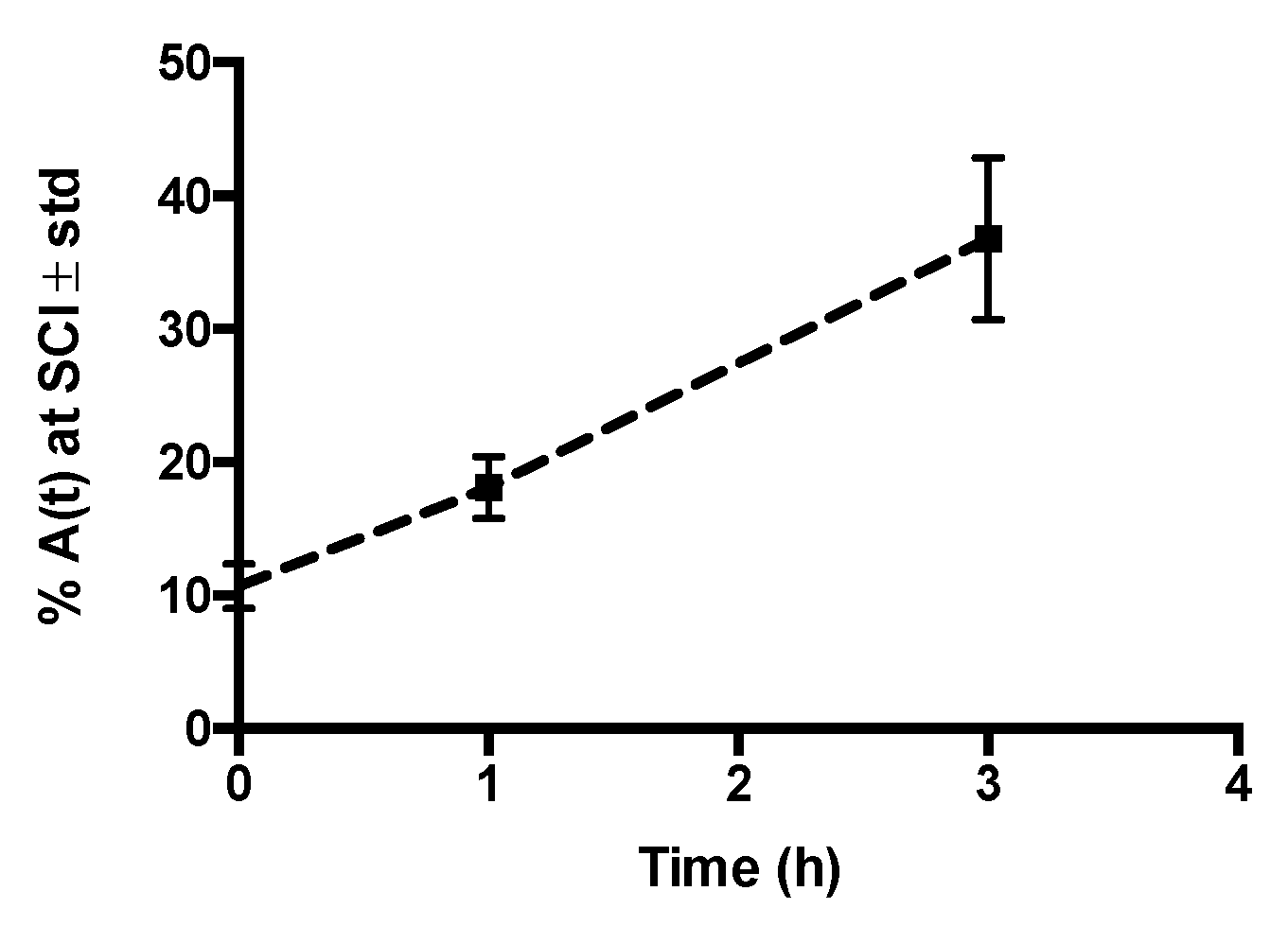
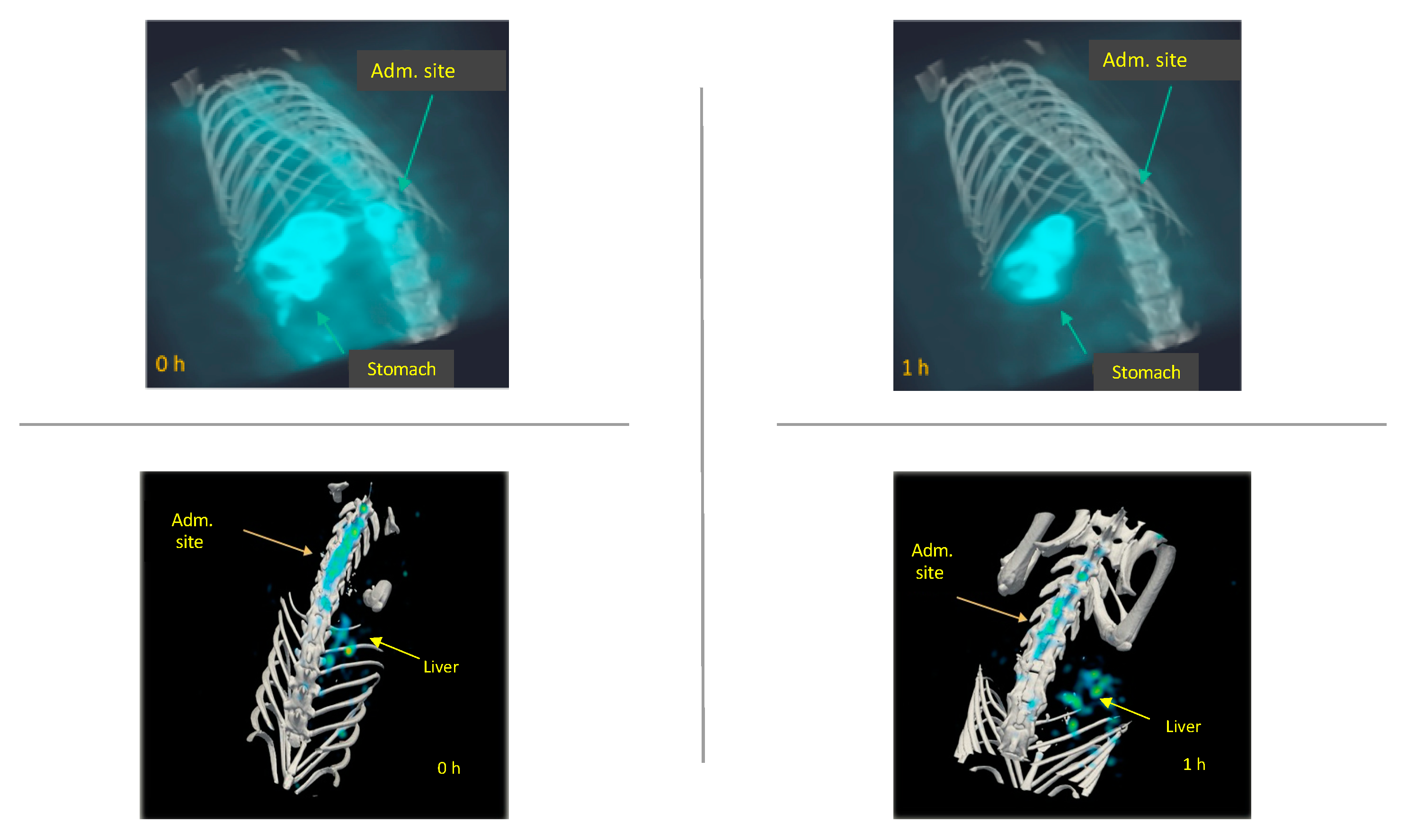
2.3. In Vivo Biodistribution of 99mTc-GA-5 in a Model of SCI
3. Discussion
4. Materials and Methods
4.1. Anti-GFAP Radiolabeling
4.2. GA-5 Integrity Assay
4.3. Animals
4.4. Traumatic Spinal Cord Injury
4.5. Radioimmunohistochemistry Assay
4.6. MicroSPECT Imaging Procedures
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- OMS Lesiones Medulares. Available online: http://www.who.int/mediacentre/factsheets/fs384/es/ (accessed on 25 February 2018).
- NINDS Spinal Cord Injury Information Page. Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Spinal-Cord-Injury-Information-Page#disorders-r1 (accessed on 28 August 2017).
- Ruiz, A.D.; Sahagún, G.G.; Castañeda, C.R. Estrategias neuroprotectoras después de una lesión traumática de la médula espinal. Rev. Med. IMSS 2002, 40, 437–455. [Google Scholar]
- NSCISC National Spinal Cord Injury Statistical Center. Available online: https://www.nscisc.uab.edu/ (accessed on 24 February 2018).
- Hernández, J.; Torres-Espín, A.N. Adult stem cell transplants for spinal cord injury repair: Current state in preclinical research. Curr. Stem Cell Res. Ther. 2011, 6, 273–287. [Google Scholar] [CrossRef]
- Cizkova, D.; Murgoci, A.N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal cord injury: Animal models, imaging tools and the treatment strategies. Neurochem. Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Wang, K.K.W. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Magaki, S.D.; Williams, C.K.; Vinters, H.V. Glial function (and dysfunction) in the normal & ischemic brain. Neuropharmacology 2018, 134, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Billakanti, R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol. Neurobiol. 2012, 46, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Maecke, H.R. Peptide-based probes for cancer imaging. J. Nucl. Med. 2008, 49, 17351738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, W.C.; Hsu, C.H.; Wu, A.T.; Yang, L.Y.; Chen, W.H.; Chiu, W.T.; Lai, W.F.; Wu, C.H.; Gelovani, J.G.; Deng, W.P. A novel cell-based therapy for contusion spinal cord injury using GDNF-delivering NIH3T3 cells with dual reporter genes monitored by molecular imaging. J. Nucl. Med. 2008, 49, 1512–1519. [Google Scholar] [CrossRef]
- Song, F.; Tian, M.; Zhang, H. Molecular imaging in stem cell therapy for spinal cord injury. Biomed. Res. Int. 2014, 2014, 759514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeRoux, L.G.; Bredow, S.; Grosshans, D.; Schellingerhout, D. Molecular imaging detects impairment in the retrograde axonal transport mechanism after radiation-induced spinal cord injury. Mol. Imaging Biol. 2014, 16, 204–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albadawi, H.; Chen, J.W.; Oklu, R.; Wu, Y.; Wojtkiewicz, G.; Pulli, B.; Milner, J.D.; Cambria, R.P.; Watkins, M.T. Spinal cord inflammation: Molecular Imaging after Thoracic Aortic Ischemia reperfusion injury. Radiology 2017, 282, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Minakov, A.N.; Chernov, A.S.; Asutin, D.S.; Konovalov, N.A.; Telegin, G.B. Experimental models of spinal cord injury in laboratory rats. Acta Nat. 2018, 10, 4–10. [Google Scholar] [CrossRef]
- Baldwin, S.A.; Broderick, R.; Blades, D.A.; Scheff, S.W. Alterations in temporal/spatial distribution of GFAP- and vimentin-positive astrocytes after spinal cord contusion with the New York University Spinal cord injury device. J. Neurotrauma 1998, 15, 1015–1026. [Google Scholar] [CrossRef]
- Nestrasil, I.; Shapiro, E.; Svatkova, A.; Dickson, P.; Chen, A.; Wakumoto, A.; Ahmed, A.; Stehel, E.; McNeil, S.; Gravance, C.; et al. Intrathecal enzyme replacement therapy reverses cognitive decline in mucopolysaccharidosis type I. Am. J. Med. Genet. Part A 2017, 173, 780–783. [Google Scholar] [CrossRef] [Green Version]
- Ineichen, B.V.; Schnell, L.; Gullo, M.; Kaiser, J.; Schneider, M.P.; Mosberger, A.C.; Good, N.; Linnebank, M.; Schwab, M.E. Direct, long-term intrathecal application of therapeutics to the rodent CNS. Nat. Protoc. 2017, 12, 104–131. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.-S.; Correa, D.; Imobersteg, S.; Maurer, M.A.; Kaiser, J.; Augath, M.A.; Schwab, M.E. Targeting Therapeutic antibodies to the CNS: A comparative study of intrathecal, intravenous, and subcutaneous Anti-Nogo a antibody treatment after stroke in rats. Neurotherapeutics 2020, 17, 1153–1159. [Google Scholar] [CrossRef]
- Harkey, H.L., III; White, E.A., IV; Tibbs, R.E., Jr.; Haines, D.E. A clinician’s view of spinal cord injury. Anat. Rec. B New Anat. 2003, 271, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Martín, S.; Renán, S.; Ortiz, S.D. Aspectos epidemiológicos de la lesión medular de la población del Centro Nacional de Rehabilitación. Rev. Mex. Med. Física Rehabil. 2008, 20, 74–82. [Google Scholar]
- De Esclarín Ruz, A. Lesión Medular, Enfoque Multidisciplinario, 1st ed.; Panamericana, E.M., Ed.; Editorial Médica Panamericana S.A.: Madrid, Spain, 2011; ISBN 9788491106326. [Google Scholar]
- Rodríguez Fernández, M. Lesión Medular: Atención Sociosanitaria; Formación Alcalá: Alcalá la Real, Spain, 2020; ISBN 978-84-85539-77-2. [Google Scholar]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, A.; Steinstrasser, A. A novel approach to Tc-99m labeled monoclonal antibodies (Abstract). J. Nucl. Med. 1987, 28, 721. [Google Scholar]
- Zambrano-Rodríguez, P.C.; Bolaños-Puchet, S.; Reyes-Alva, H.J.; García-Orozco, L.E.; Romero-Piña, M.E.; Martinez-Cruz, A.; Guízar-Sahagún, G.; Medina, L.A. Micro-CT myelography using contrast-enhanced digital subtraction: Feasibility and initial results in healthy rats. Neuroradiology 2019, 61, 323–330. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo-Sánchez, V.; Zambrano-Rodríguez, P.C.; Peña-Merino, N.; Bolaños-Puchet, S.; Reyes-Alva, H.J.; Martínez-Cruz, A.; Muñiz-Hernández, S.; Guízar-Sahagún, G.; Medina, L.A. Evaluation of Traumatic Spinal Cord Injury in a Rat Model Using 99mTc-GA-5 as a Potential In Vivo Tracer. Molecules 2021, 26, 7138. https://doi.org/10.3390/molecules26237138
Izquierdo-Sánchez V, Zambrano-Rodríguez PC, Peña-Merino N, Bolaños-Puchet S, Reyes-Alva HJ, Martínez-Cruz A, Muñiz-Hernández S, Guízar-Sahagún G, Medina LA. Evaluation of Traumatic Spinal Cord Injury in a Rat Model Using 99mTc-GA-5 as a Potential In Vivo Tracer. Molecules. 2021; 26(23):7138. https://doi.org/10.3390/molecules26237138
Chicago/Turabian StyleIzquierdo-Sánchez, Vanessa, Pablo C. Zambrano-Rodríguez, Nadia Peña-Merino, Sirio Bolaños-Puchet, Horacio J. Reyes-Alva, Angelina Martínez-Cruz, Saé Muñiz-Hernández, Gabriel Guízar-Sahagún, and Luis Alberto Medina. 2021. "Evaluation of Traumatic Spinal Cord Injury in a Rat Model Using 99mTc-GA-5 as a Potential In Vivo Tracer" Molecules 26, no. 23: 7138. https://doi.org/10.3390/molecules26237138
APA StyleIzquierdo-Sánchez, V., Zambrano-Rodríguez, P. C., Peña-Merino, N., Bolaños-Puchet, S., Reyes-Alva, H. J., Martínez-Cruz, A., Muñiz-Hernández, S., Guízar-Sahagún, G., & Medina, L. A. (2021). Evaluation of Traumatic Spinal Cord Injury in a Rat Model Using 99mTc-GA-5 as a Potential In Vivo Tracer. Molecules, 26(23), 7138. https://doi.org/10.3390/molecules26237138





