Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies
Abstract
:1. Introduction
2. Results
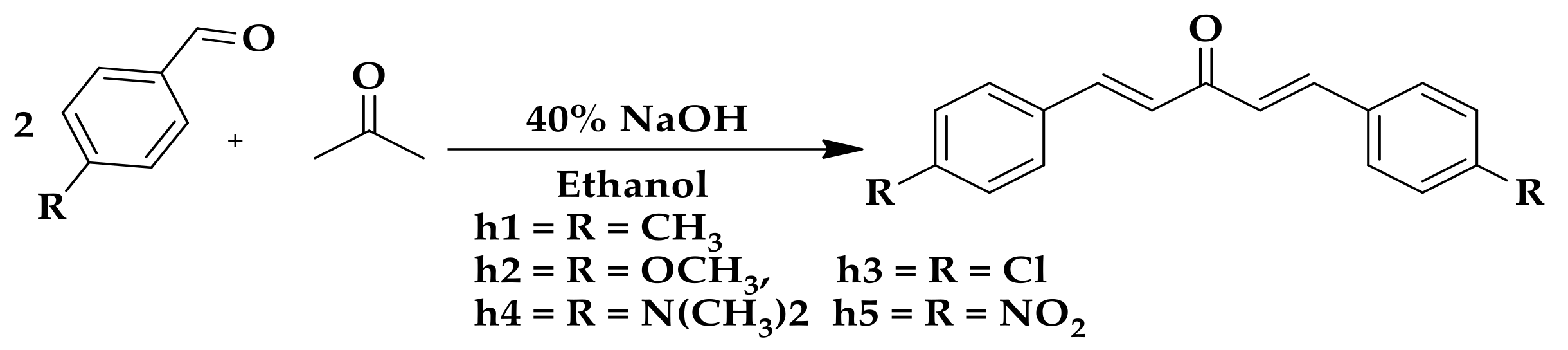
2.1. Chemistry
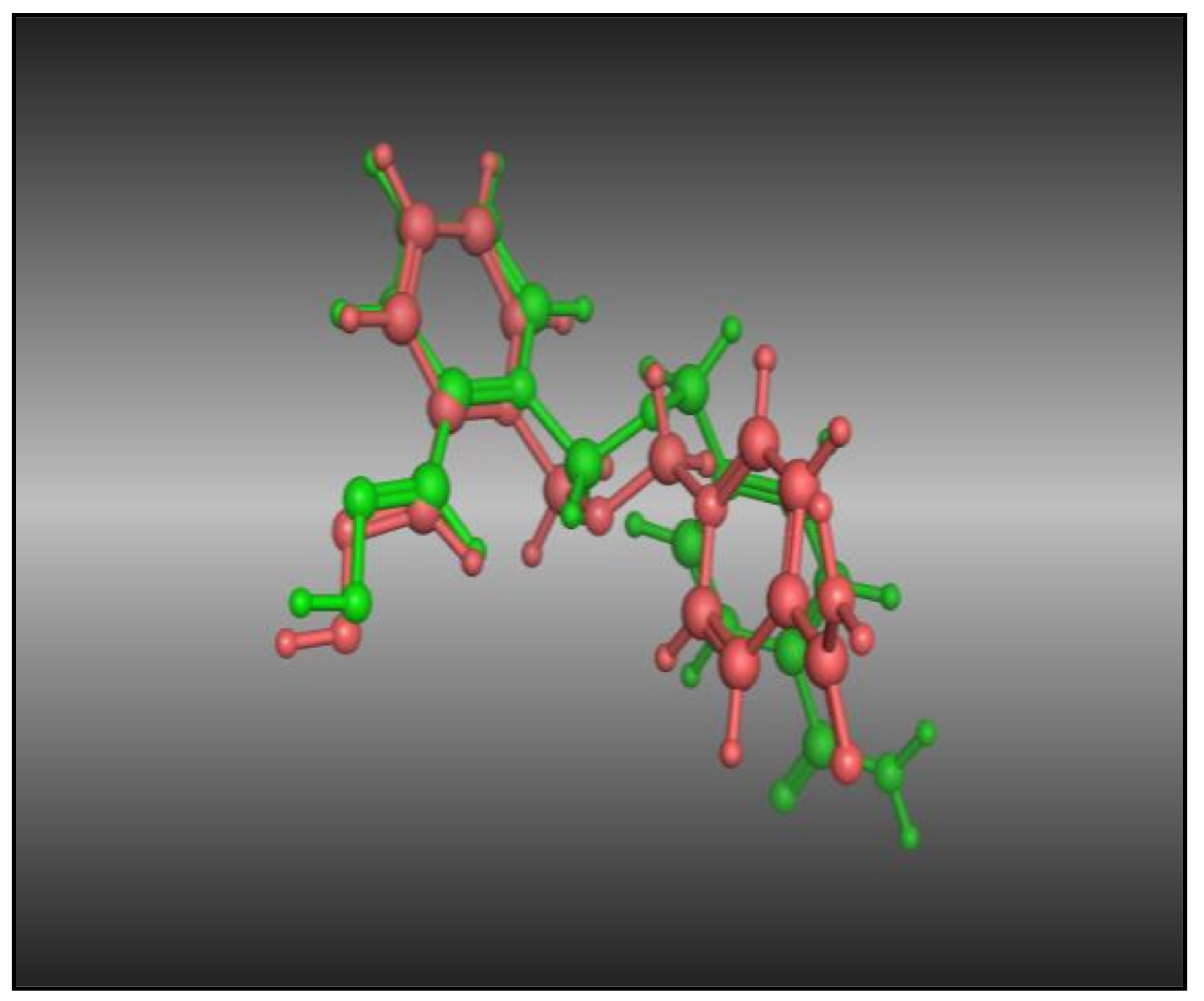
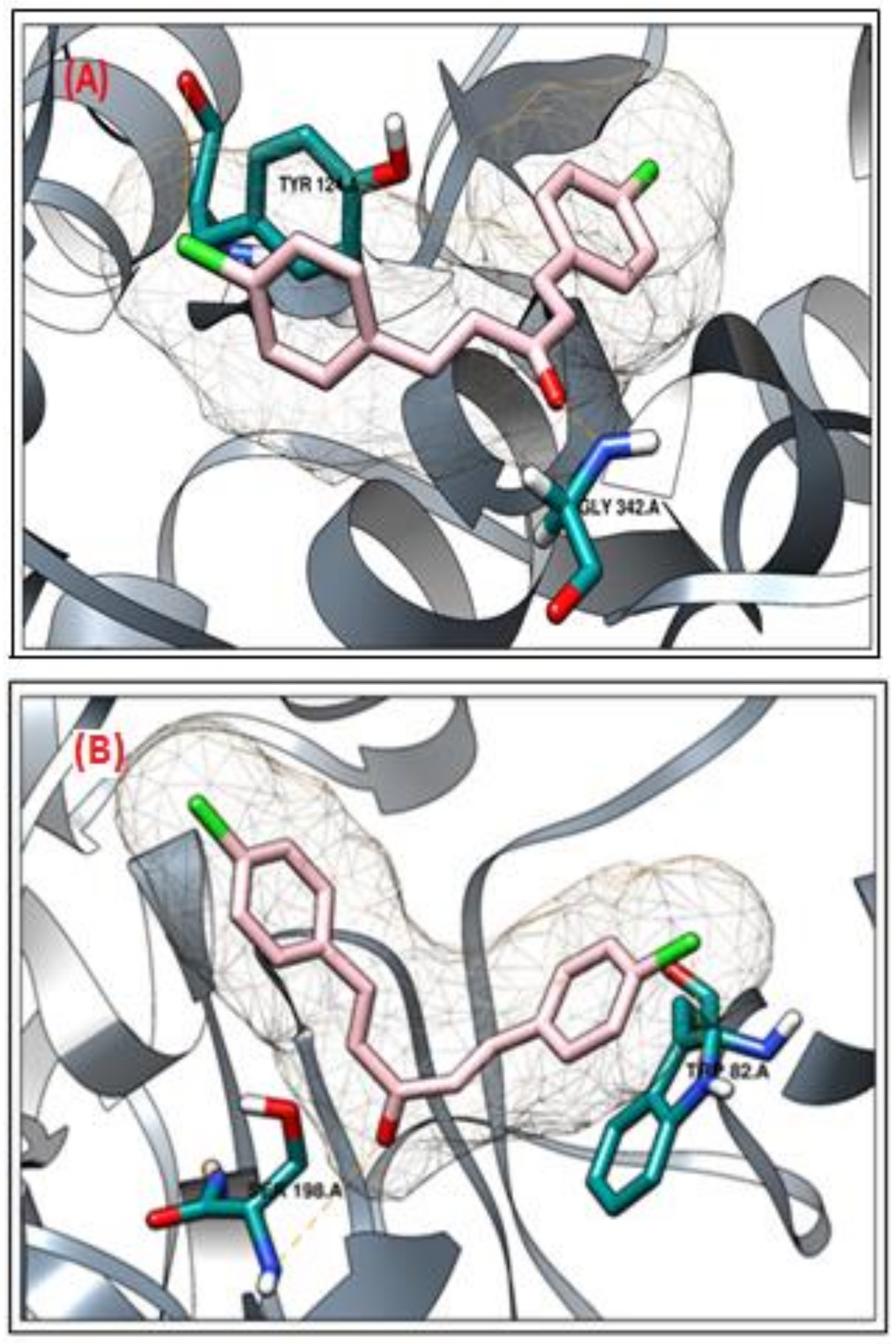
2.2. Molecular Docking Study
Molecular Docking Validation of Synthesized Compounds for Anticholinesterases
2.3. Pharmacological Activities
2.3.1. In Vitro Cholinesterase Inhibition Potential of Curcumin Analogs
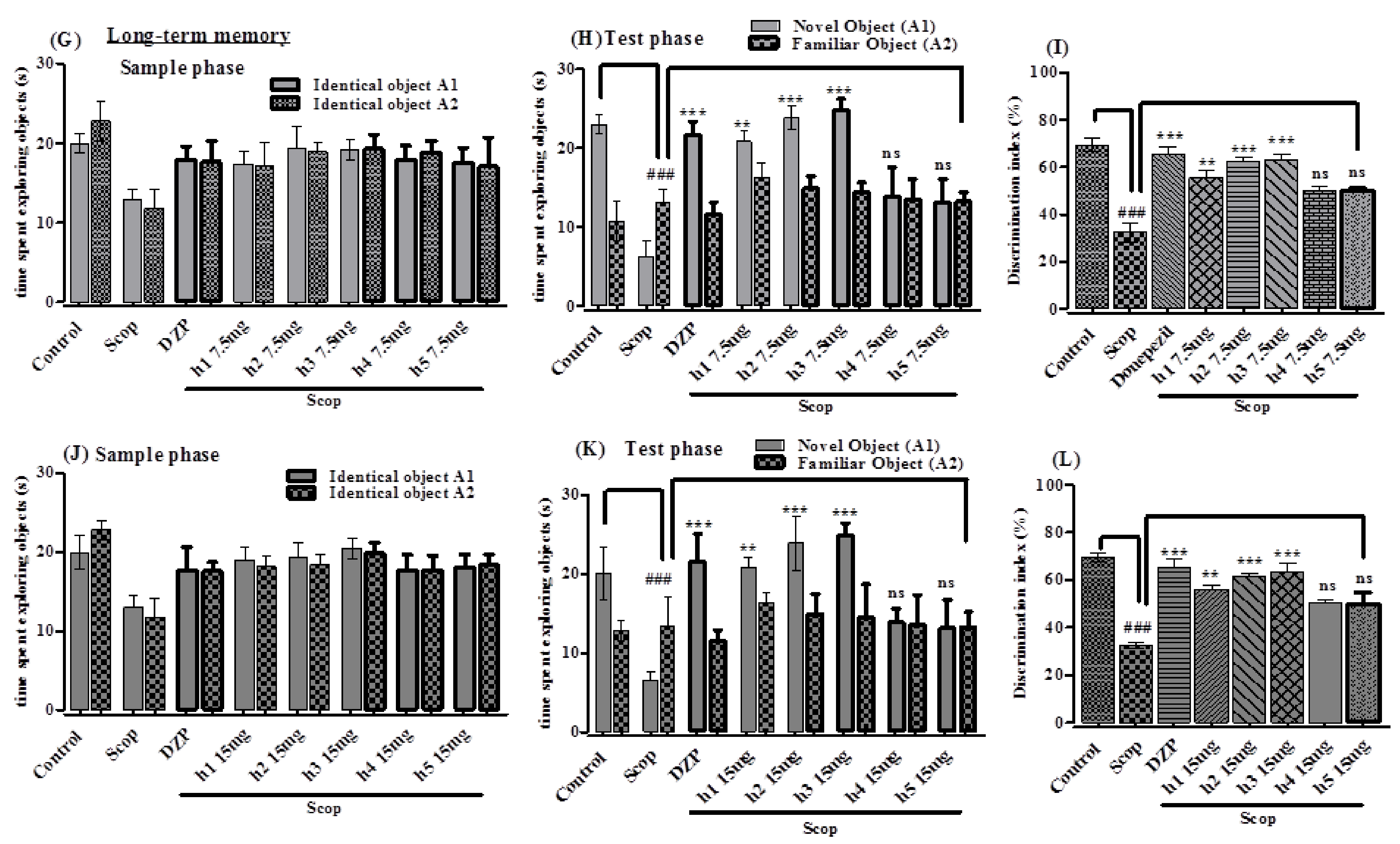
2.3.2. In Vivo Study
Elevated Plus Maze
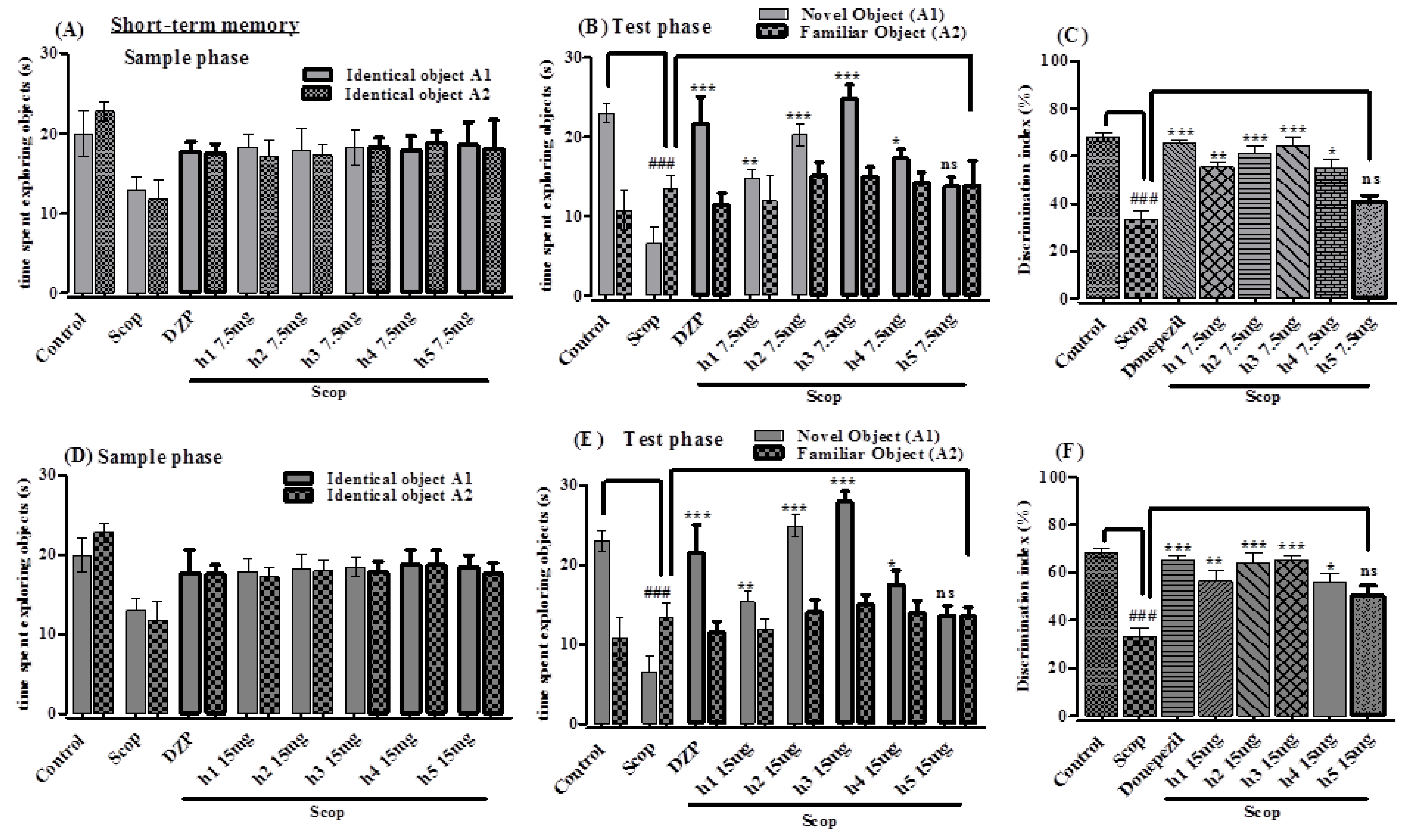
Novel Object Recognition Test
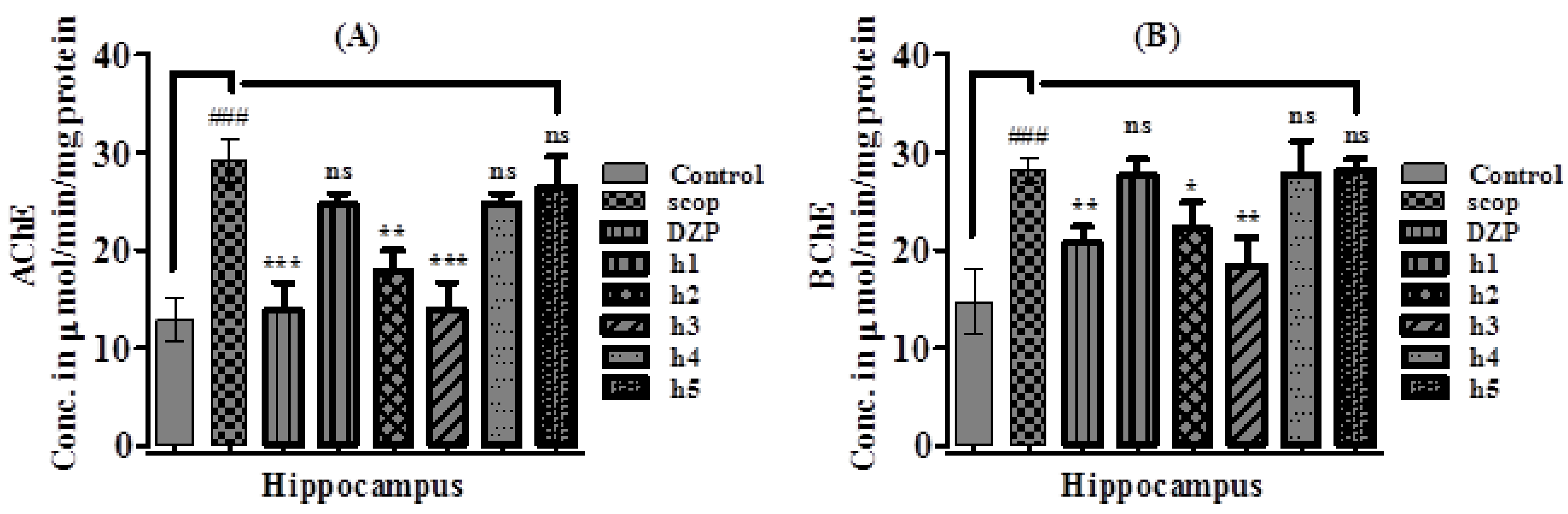
2.3.3. Effect of Synthesized Curcumin Analogs on Brain Hippocampus Cholinesterase (AChE and BChE) Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methodology
4.2.1. General Procedure for the Synthesis Curcumin Analogs
4.2.2. Synthesis of (1E,4E)-1,5-Di-p-tolylpenta-1,4-dien-3-one (h1)
4.2.3. Synthesis of (1E,4E)-1,5-bis(4-Methoxyphenyl)penta-1,4-dien-3-one (h2)
4.2.4. Synthesis of (1E,4E)-1,5-bis(4-Chlorophenyl)penta-1,4-dien-3-one (h3)
4.2.5. Synthesis of (1E,4E)-1,5-bis(4-(Dimethylamino)phenyl)penta-1,4-dien-3-one (h4)
4.2.6. Synthesis of (1E,4E)-1,5-bis(4-Nitrophenyl)penta-1,4-dien-3-one (h5)
4.3. Molecular Docking Study
4.3.1. Protein Preparation
4.3.2. Ligand Preparation
4.3.3. Receptor Preparation
4.3.4. Re-docking Setup
4.4. Pharmacological Activities
4.4.1. Anticholinesterase Assay
4.4.2. Animals, Dosing and Grouping
4.4.3. In Vivo Analysis
Elevated Plus Maze Model (EPM)
Novel Object Recognition Test
4.4.4. Isolation of Brain Hippocampus for Biochemical Assessment
Cholinesterase (AChE and BChE) Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Ioan, S.; Chiriac, B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shal, B.; Khan, A.; Khan, A.U.; Ullah, R.; Ali, G.; Islam, S.U.; Haq, I.U.; Ali, H.; Seo, E.K.; Khan, S. Alleviation of memory deficit by bergenin via the regulation of reelin and Nrf-2/NF-κB pathway in transgenic mouse model. Int. J. Mol. Sci. 2021, 22, 6603. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Nisar, M.; Karim, N.; Latif, A.; Ahmad, S.; Uddin, Z. Evaluation of neuroprotective and antiamnesic effects of elaeagnus umbellate thunb. On scopolamine-induced memory impairment in mice. BMC Complement. Med. Ther. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L) a very potent antioxidant attenuates dementia in scopolamine induced memory deficit mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ghias, M.; Shoaib, M.; Ali Shah, S.W.; Umar, M.N.; Ullah, S.; Ali, N.; Shah, I.; Ullah, S. Nootropic effects of synthetic flavonoid derivatives on scopolamine induced memory impairment in mice via cholinesterase inhibition and antioxidant system. Pak. J. Pharm. Sci. 2019, 32, 2325–2332. [Google Scholar]
- Orhan, I.E.; Kartal, M.; Sener, B. Activity of Essential Oils and Individual Components against Acetyl- and Butyrylcholinesterase. Naturforschung 2008, 547–553. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Piasecka, A.; Kachlicki, P.; Kujawski, R.; Bogacz, A.; Bartkowiak-wieczorek, J.; Szulc, M.; Kaminska, E.; Kujawska, M.; et al. Influence of the Melissa officinalis Leaf Extract on Long-Term Memory in Scopolamine Animal Model with Assessment of Mechanism of Action. Evidence-Based Complement. Altern. Med. 2016, 2016, 9729818. [Google Scholar] [CrossRef] [Green Version]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Eugenio, O.S. Memory Deficits in Alzheimer’s Patients: A Comprehensive Review. Neuropsychol. Rev. 1992, 3, 119–169. [Google Scholar]
- Arciniegas, D.B. The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Curr. Psychiatry Rep. 2003, 5, 391–399. [Google Scholar] [CrossRef]
- Knox, D. The Role Of Basal Forebrain Cholinergic Neurons In Fear and Extinction Memory. Neurobiol Learn. Mem 2016, 133, 9–52. [Google Scholar] [CrossRef] [Green Version]
- Hasselmo, M.E.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.M.R.; Ribeiro, F. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Lian, W.; Fang, J.; Xu, L.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.L.; Du, G.H. DL0410 ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in mice. Molecules 2017, 22, 410. [Google Scholar] [CrossRef] [Green Version]
- Castro, A.; Martinez, A. Targeting Beta-Amyloid Pathogenesis Through Acetylcholinesterase Inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef]
- Orhan, G.; Orhan, I.; Sener, B. Recent Developments in Natural and Synthetic Drug Research for Alzheimers Disease. Lett. Drug Des. Discov. 2006, 3, 268–274. [Google Scholar] [CrossRef]
- Olsen, C.E.; Poulsen, H.D.; Lublin, H.K.F. Drug therapy of dementia in elderly patients. A review. Nord. J. Psychiatry 2005, 59, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Jiang, L.; Zhao, Y.; Shao, L.; Xiao, J.; Ye, F.; Li, Y.; Li, X. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaguchi, T.; Ono, K.; Yamada, M. Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef]
- Lee, W.; Loo, C.; Bebawy, M.; Luk, F.; Mason, R.S. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21 st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, F.; Haider, S.; Naqvi, F.; Saleem, S.; Perveen, T.; Batool, Z. A comparative study showing greater effects of curcumin compared to donepezil on memory function in rats. Pak. J. Pharm. Sci. 2019, 32, 53–60. [Google Scholar] [PubMed]
- Wadood, A.; Riaz, M.; Jamal, S.B.; Shah, M. Interactions of ketoamide inhibitors on HCV NS3/4A protease target: Molecular docking studies. Mol. Biol. Rep. 2014, 41, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Pahaye, D.B.; Bum, E.N.; Taïwé, G.S.; Ngoupaye, G.T.; Sidiki, N.; Clarisse, F.; Moto, O.; Kouemou, N.; Jacqueline, S.; Njapdounke, K.; et al. Neuroprotective and Antiamnesic Effects of Mitragyna inermis Willd (Rubiaceae) on Scopolamine-Induced Memory Impairment in Mice. Behav. Neurol. 2017, 2017, 5952897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Kahol, A.; Singh, I.P.; Saraf, I.; Shri, R. Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in-vitro and in-vivo models. J. Ethnopharmacol. 2016, 193, 490–499. [Google Scholar] [CrossRef]
- Reeta, K.H.; Mehla, J.; Gupta, Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009, 1301, 52–60. [Google Scholar] [CrossRef]
- Noorafshan, A.; Asadi-Golshan, R.; Karbalay-Doust, S.; Abdollahifar, M.A.; Rashidiani-Rashidabadi, A. Curcumin, the Main Part of Turmeric, Prevents Learning and Memory Changes Induced by Sodium Metabisulfite, a Preservative Agent, in Rats. Exp. Neurobiol. 2013, 22, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Bevins, R.A.; Besheer, J.; Palmatier, M.I.; Jensen, H.C.; Pickett, K.S.; Eurek, S. Novel-object place conditioning: Behavioral and dopaminergic processes in expression of novelty reward. Behav. Brain Res. 2002, 129, 41–50. [Google Scholar] [CrossRef]
- Baxter, M.G. “I’ve seen it all before” Explaining age-related impairments in object recognition. Theoretical comment on Burke et al. (2010). Behav. Neurosci. 2010, 124, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Biala, M.A.G. The novel object recognition memory: Neurobiology, test procedure and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, Z.; Setorki, M. Effect of hydroalcoholic echium amoenum extract on scopolamine-induced learning and memory impairment in rats. Pharm. Biol. 2018, 56, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carapina da Silva, C.; Pacheco, B.S.; das Neves, R.N.; Dié Alves, M.S.; Sena-Lopes, Â.; Moura, S.; Borsuk, S.; de Pereira, C.M.P. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- Naikwadi, P.; Takate, S.K.; Malujkar, S.K.; Arun-Wakchaure, N.D.; Phatangare, R.B.; Rutuja, R.; Deshmukh, A.B.; Dhokare, S.V.S.P. Preparation of Various Derivatives of Dibenzalpropanone by using Lithum Hydroxide as Green Approach. Int. J. Sci. Res. Sci. Technol. 2017, 3, 84–86. [Google Scholar]
- Faheem, M.; Jamal, S.B. Identification of Zika Virus NS5 Novel Inhibitors through Virtual Screening and Docking Studies. Life Sci. 2020, 1, 2–6. [Google Scholar] [CrossRef]
- Jamal, S.B.; Hassan, S.S.; Tiwari, S.; Viana, M.V.; Turjanski, G.; Barh, D.; Benevides, D.J.; Ullah, A.; Baumbach, J.; Ghosh, P.; et al. An integrative in-silico approach for therapeutic target identification in the human pathogen Corynebacterium diphtheriae. PLoS ONE 2017, 12, e0186401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadood, A.; Jamal, S.B.; Riaz, M.; Mir, A. Computational analysis of benzofuran-2-carboxlic acids as potent Pim-1 kinase inhibitors. Pharm. Biol. 2014, 52, 1170–1178. [Google Scholar] [CrossRef]
- Shoaib, M.; Shah, I.; Adikhari, A.; Ali, N.; Ayub, T.; Ul-Haq, Z.; Shah, S.W.A. Isolation of flavonoides from Artemisia macrocephala anticholinesterase activity: Isolation, characterization and its in vitro anticholinesterse activity supported by molecular docking. Pak. J. Pharm. Sci. 2018, 31, 1347–1354. [Google Scholar] [PubMed]
- George, L.; Ellman, K. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
| Comp. | AChEI (IC50) | Docking Score | Binding Energy (GBVI/WSA) | BChEI (IC50) | Docking Score | Binding Energy (GBVI/WSA) |
|---|---|---|---|---|---|---|
| h1 | 104.72 | –8.354 | –14.164 | 243.12 | –7.874 | –13.491 |
| h2 | 93.27 | –10.176 | –17.317 | 147.92 | –8.360 | –15.082 |
| h3 | 29.39 | –12.412 | –21.543 | 67.35 | –11.537 | –19.728 |
| h4 | 294.77 | –7.954 | –13.674 | 512.77 | –7.612 | –14.011 |
| h5 | 632.37 | –7.018 | –13.006 | 988.35 | –6.603 | –11.225 |
| Comp. | Conc. µg/mL | Mean ± SEM | AChEI (IC50) | Mean ± SEM | BChEI (IC50) |
|---|---|---|---|---|---|
| h1 | 1000 | 79.67 ± 0.21 ns | 104.72 | 62.16 ± 0.04 *** | 243.12 |
| 500 | 68.07 ± 0.18 *** | 56.42 ± 0.16 *** | |||
| 250 | 58.12 ± 0.16 *** | 51.91 ± 0.14 *** | |||
| 125 | 54.34 ± 0.17 *** | 46.25 ± 0.13 *** | |||
| 62.5 | 45.63 ± 0.13 *** | 37.16 ± 0.12 *** | |||
| h2 | 1000 | 76.23 ± 0.15 ns | 93.27 | 69.77 ± 0.03 *** | 147.92 |
| 500 | 73.79 ± 0.12 ns | 64.38 ± 0.06 *** | |||
| 250 | 61.21±0.18 *** | 57.22 ± 0.01 *** | |||
| 125 | 53.57 ± 0.17 *** | 48.61 ± 0.15 *** | |||
| 62.5 | 46.88 ± 0.15 *** | 41.82 ± 0.18 *** | |||
| h3 | 1000 | 83.31 ± 0.13 ns | 29.39 | 78.72 ± 0.16 * | 67.35 |
| 500 | 77.25 ± 0.11 ns | 69.52 ± 0.12 *** | |||
| 250 | 69.51 ± 0.13 ns | 62.41 ± 0.19 *** | |||
| 125 | 62.44 ± 0.16 ns | 58.33 ± 0.13 *** | |||
| 62.5 | 53.53±0.12 * | 49.25 ± 0.02 *** | |||
| h4 | 1000 | 63.51 ± 0.17 *** | 294.77 | 56.32 ± 0.18 *** | 512.77 |
| 500 | 58.16 ± 0.11 *** | 49.51 ± 0.16 *** | |||
| 250 | 46.32 ± 0.15 *** | 42.62 ± 0.12 *** | |||
| 125 | 39.45 ± 0.04 *** | 35.33 ± 0.19 *** | |||
| 62.5 | 31.41 ± 0.13 *** | 27.16 ± 0.11 *** | |||
| h5 | 1000 | 58.41 ± 0.13 *** | 632.37 | 51.82 ± 0.16 *** | 988.35 |
| 500 | 47.33 ± 0.16 *** | 43.51 ± 0.13 *** | |||
| 250 | 41.27 ± 0.04 *** | 32.73 ± 0.01 *** | |||
| 125 | 35.51 ± 0.06 *** | 20.26 ± 0.31 *** | |||
| 62.5 | 26.45 ± 0.18 *** | 16.22 ± 0.83 *** | |||
| Galanthamine | 1000 | 81.85 ± 0.18 | 26.58 | 83.53 ± 0.20 | 21.30 |
| 500 | 76.59 ± 0.30 | 78.62 ± 0.17 | |||
| 250 | 69.75 ± 0.14 | 73.42 ± 0.11 | |||
| 125 | 64.47 ± 0.49 | 66.20 ± 0.15 | |||
| 62.5 | 59.12 ± 0.34 | 61.35 ± 0.18 |
| Samples | Treatment/Dose | Retention (sec) |
|---|---|---|
| Control | Normal saline, p.o | 36.42 ± 1.81 |
| Scopolamine (Scop) | 1 mg/kg, i.p negative control | 67.31 ± 1.24 *** |
| Donepezil (DZP) | 2 mg/kg p.o | 25.57 ± 1.93 *** |
| h1 | 7.5 mg/kg, p.o | 33.13 ± 2.16 * |
| 15 mg/kg, p.o | 26.18 ± 2.32 *** | |
| h2 | 7.5 mg/kg, p.o | 31.51 ± 2.73 ** |
| 15 mg/kg, p.o | 25.32 ± 2.42 *** | |
| h3 | 7.5 mg/kg, p.o | 25.26 ± 2.51 *** |
| 15 mg/kg, p.o | 21.27 ± 2.92 *** | |
| h4 | 7.5 mg/kg, p.o | 35.71 ± 1.13 * |
| 15 mg/kg, p.o | 30.42 ± 1.24 ** | |
| h5 | 7.5 mg/kg, p.o | 34.71 ± 1.37 * |
| 15 mg/kg, p.o | 31.51 ± 1.54 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ghias, M.; Ullah, A.; Rahman, S.U.; Kamal, Z.; Khan, F.A.; Khan, N.M.; Muhammad, J.; et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules 2021, 26, 7168. https://doi.org/10.3390/molecules26237168
Hussain H, Ahmad S, Shah SWA, Ghias M, Ullah A, Rahman SU, Kamal Z, Khan FA, Khan NM, Muhammad J, et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules. 2021; 26(23):7168. https://doi.org/10.3390/molecules26237168
Chicago/Turabian StyleHussain, Haya, Shujaat Ahmad, Syed Wadood Ali Shah, Mehreen Ghias, Abid Ullah, Shafiq Ur Rahman, Zul Kamal, Farman Ali Khan, Nasir Mehmood Khan, Juma Muhammad, and et al. 2021. "Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies" Molecules 26, no. 23: 7168. https://doi.org/10.3390/molecules26237168
APA StyleHussain, H., Ahmad, S., Shah, S. W. A., Ghias, M., Ullah, A., Rahman, S. U., Kamal, Z., Khan, F. A., Khan, N. M., Muhammad, J., Almehmadi, M., Abdulaziz, O., & Alghamdi, S. (2021). Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules, 26(23), 7168. https://doi.org/10.3390/molecules26237168







