Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves
Abstract
:1. Introduction
2. Ethnobotany and Ethnopharmacology of Allium Species
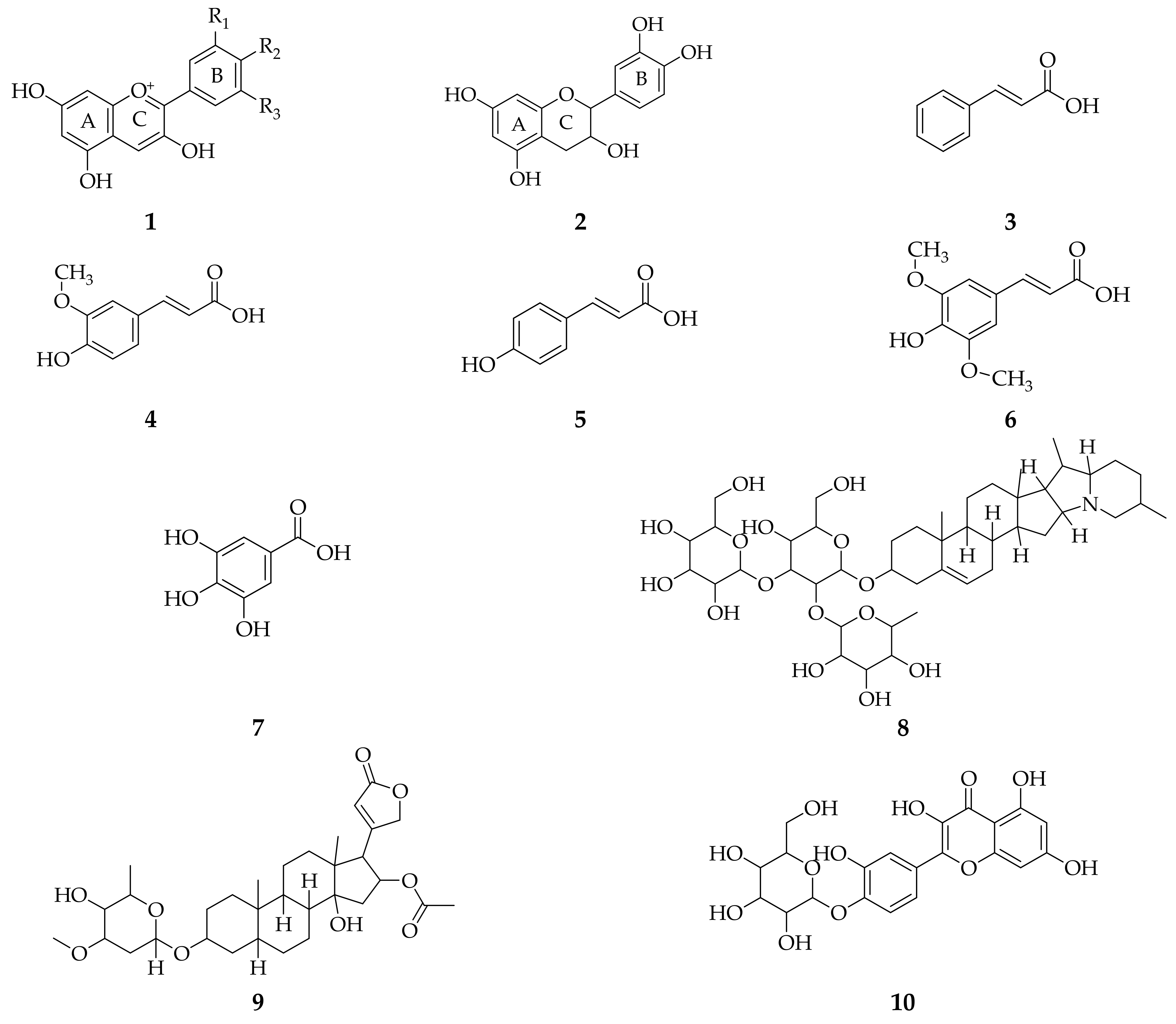
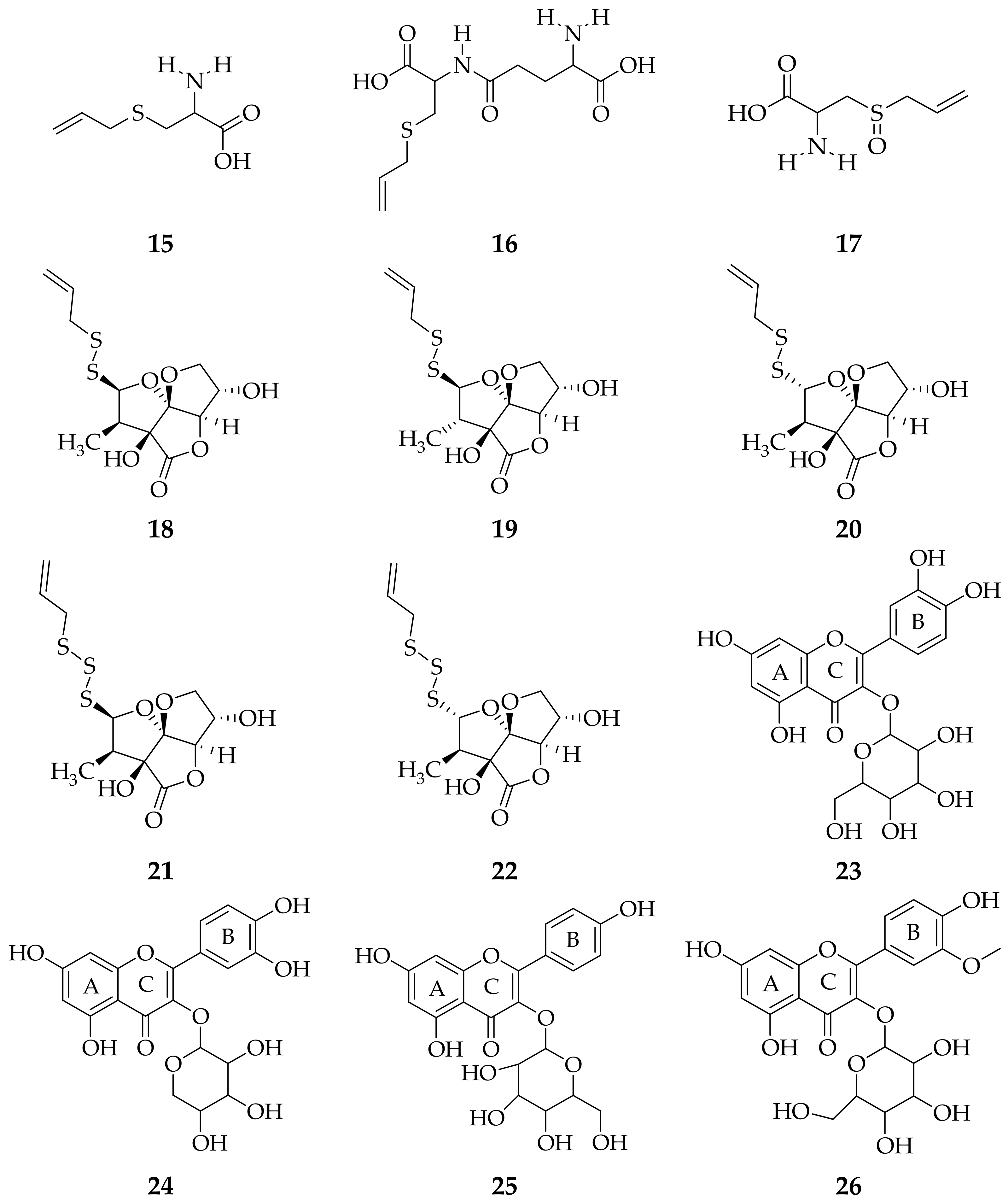
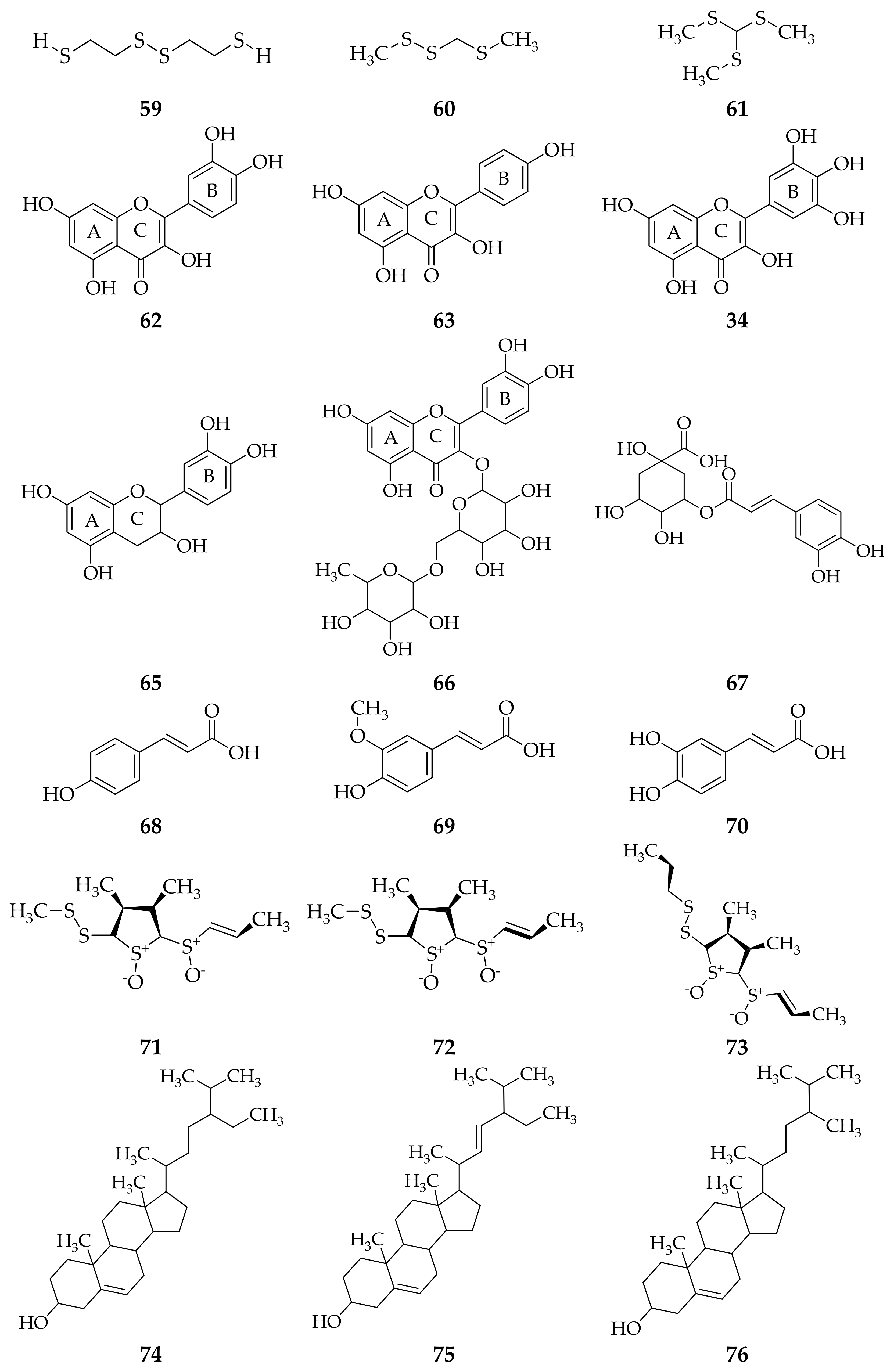
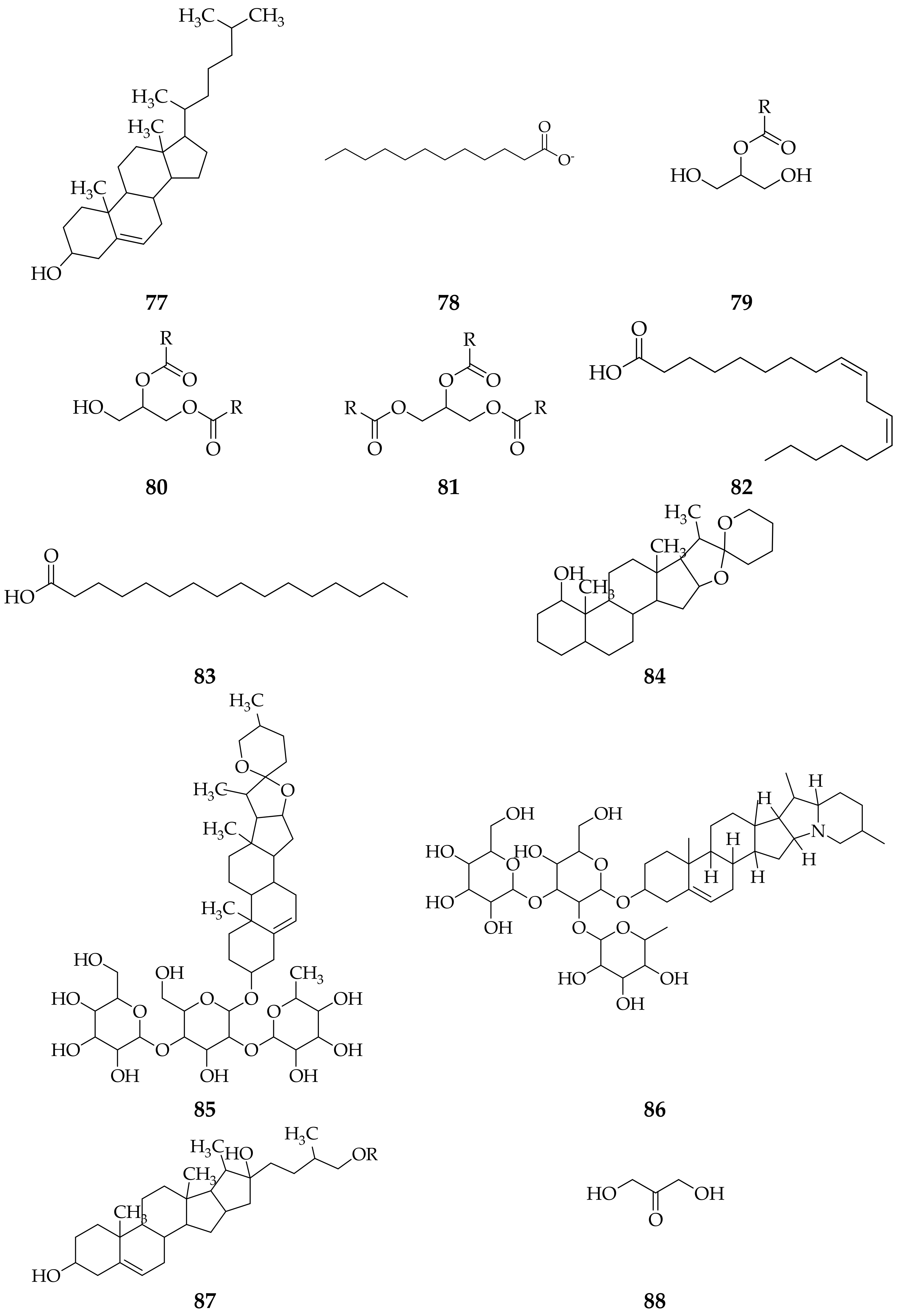
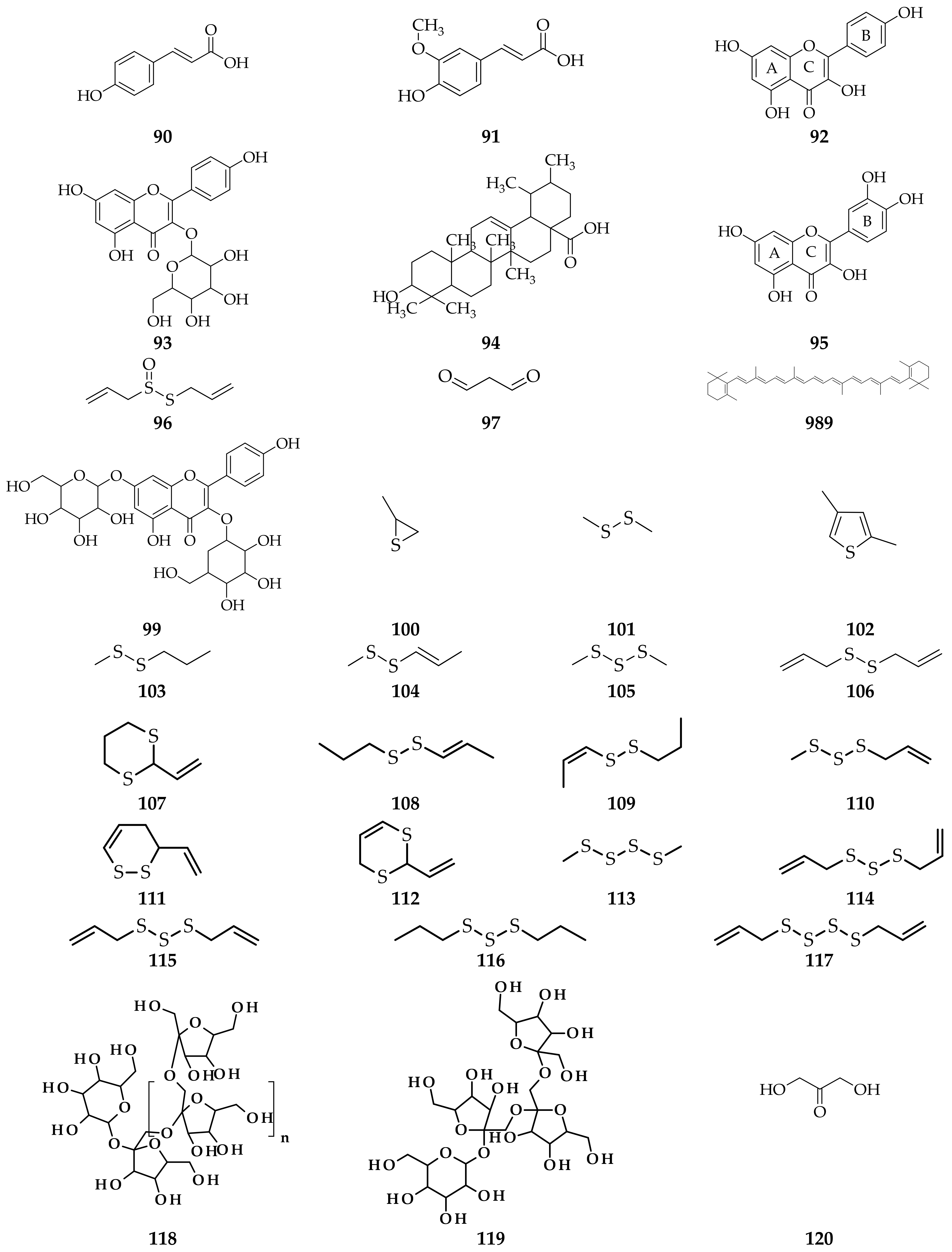
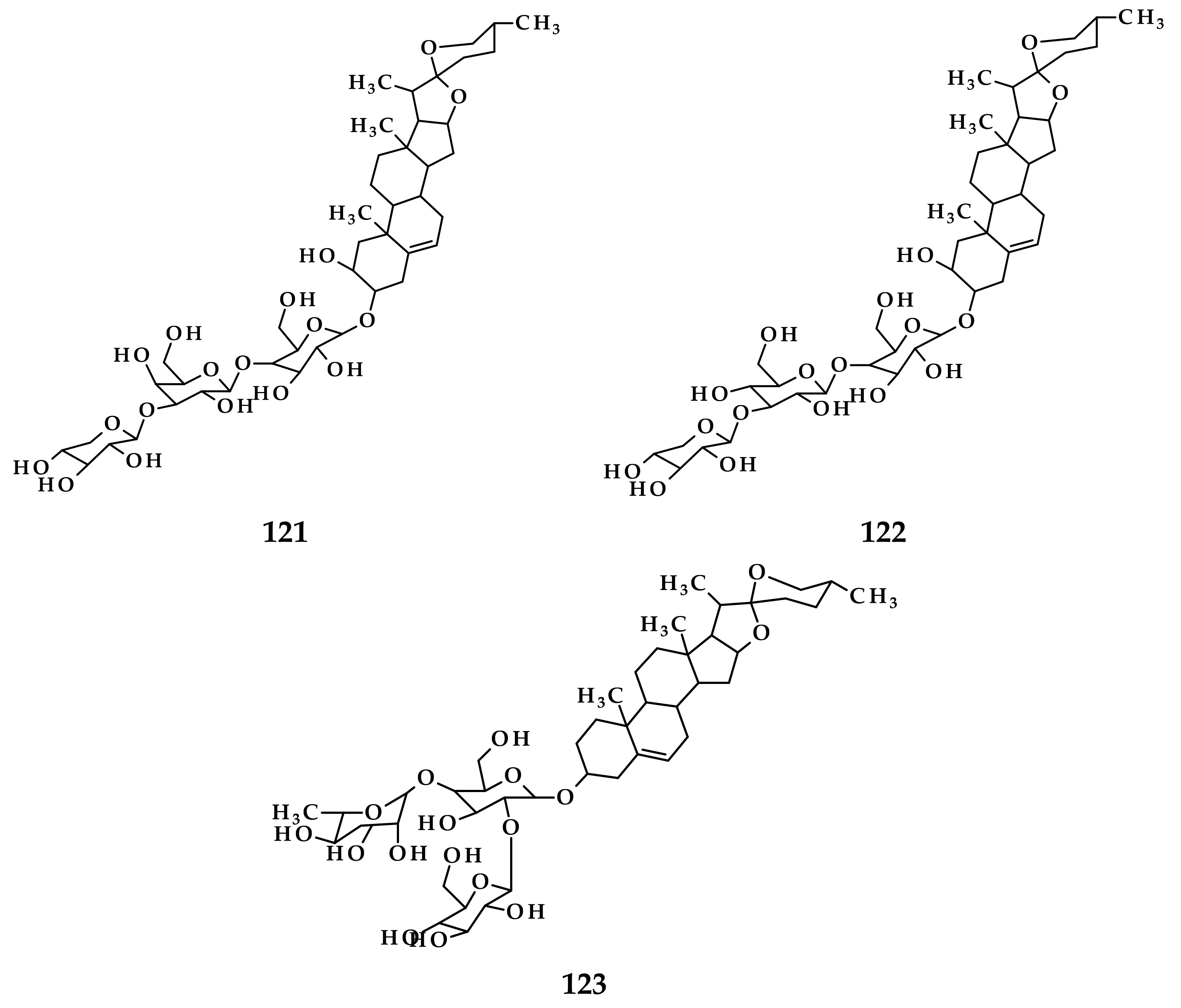
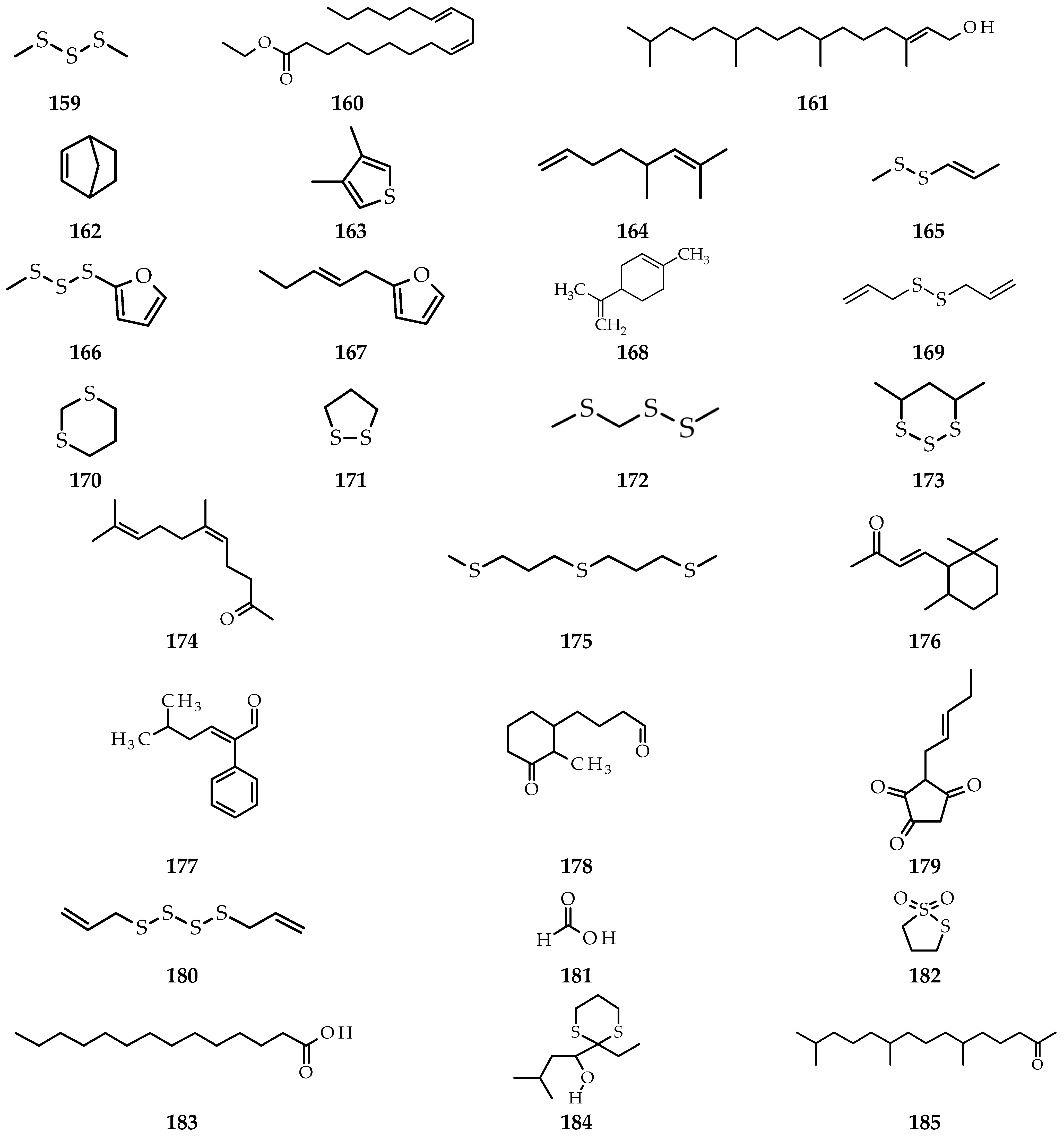
3. Isolation Methods and Compound Content of Allium Species Leaves
3.1. Allium cepa
3.2. Allium sativum
3.3. Allium fistulosum
3.4. Allium schoenoprasum
3.5. Allium ursinum
3.6. Allium flavum
3.7. Allium scorodoprasum
3.8. Allium vineale
3.9. Allium atroviolaceum
4. Allium Species Leave Bioactivity and Test Methods
4.1. Antimicrobial
4.1.1. Antibacterial
4.1.2. Antifungal
4.2. Antioxidant
4.3. Anti-Inflammatory
4.4. Antitumor
4.5. Antiplatelet
4.6. Pancreatic α-Amylase and Glucoamylase Enzyme Inhibitor
| Species | Antioxidant Test Methods | Other Bioactivities |
|---|---|---|
| A. sativum | The phosphomolybdenum reduction assay [102] | Antimicrobial [103,104]; Anti-inflammatory [88]; Inhibitor pancreatic α-amylase and glucoamylase [53] |
| A. ursinum | DPPH radical-scavenging ability [70,71,105]; ferric reducing antioxidant power (FRAP) assay [68]; ABTS radical scavenging assays [23] | Antimicrobial [72,105]; Antifungal [21]; Antiplatelet [17] |
| A. schoenoprasum | DPPH radical-scavenging ability; ferric reducing antioxidant power (FRAP) assay [68]; DPPH bleaching method; the Trolox equivalent antioxidant capacity (TEAC) assay [64]; the total iron reduction’s potential technique [62]; ORAC (oxygen radical absorbance capacity) [65] | Anti-inflammatory [73]; Antitumor [67]; Antiplatelet [65] |
| A. fistulosum | DPPH free radical scavenging assay [61]; ORAC (oxygen radical absorbance capacity) [65] | Anti-inflammatory [87]; Antiplatelet [65] |
| A. scorodoprasum | Ferric reducing/antioxidant power (FRAP); DPPH radical scavenging activity assay [12] | - |
| A. vineale | Ferric thiocyanate method; ferric ions (Fe3+) reducing antioxidant power assay (FRAP); DPPH free radical-scavenging activity [16] | - |
| A. cepa | Antioxidant enzyme method [27] | Antimicrobial [40,79]; Antibacterial [80]; Anticardioprotective [49] |
| A. flavum | - | Anticancer [11] |
| A. atroviolaceum | - | Antimicrobial [46]; Antiplatelet [74] |
5. Antioxidant Properties
6. Structure-Antioxidant Activity Relationship Compounds in Allium
6.1. Apigenin
6.2. Myricetin
6.3. Naringenin
6.4. Kaempferol
6.5. Catechin
6.6. Quercetin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.; Oliveira, R.; Oliveira, A.; Serafini, M.; Araújo, A.; Gelain, D.; Moreira, J.; Almeida, J.; Quintans, J.; Quintans-Junior, L.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, G.; Huang, H. The antioxidant activities of garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2020, 145, 819–826. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Soković Bajić, S.; Jovanović, Ž.; Matić, I.; Tolinački, M.; Popović, D.; Popović, N.; Terzić-Vidojević, A.; Golić, N.; Beškoski, V.; et al. Antioxidant and Antiproliferative Activity of Allium ursinum and Their Associated Microbiota During Simulated in vitro Digestion in the Presence of Food Matrix. Front. Microbiol. 2020, 11, 601616. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- Gitin, L.; Dinica, R.; Parnavel, R. The Influence of Extraction Method on the Apparent Content of Bioactive Compounds in Romanian Allium spp. Leaves. Not. Bot. Horti Agrobot. 2012, 40, 93. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Pradheep, K.; Khar, A.; Negi, K.; Rana, J. Collection and Characterization of Allium Species from Himachal Pradesh. Indian J. Plant Genet. Resour. 2008, 21, 225–228. [Google Scholar]
- Verma, S.; Kaur, S.; Singh, J.; Garg, A. Anti-bacterial Effect of Garlic (Allium sativum L.) Extract on Different Pathogenic and Non-pathogenic Bacteria. RJPBCS 2015, 6, 1103. [Google Scholar]
- Krivokapic, M.Z.; Tomovic, M.T.; Jakovljevic, V.L.; Sovrlic, M.M.; Bradic, J.V.; Petkovic, A.M.; Radojevic, I.D.; Brankovic, S.R.; Comic, L.R.; Andjic, M.M.; et al. Biological activities of different extracts from Allium ursinum leaves. Acta Pol. Pharm. Drug Res. 2020, 77, 121–129. [Google Scholar] [CrossRef]
- Rezgui, A.; Mitaine-Offer, A.-C.; Paululat, T.; Delemasure, S.; Dutartre, P.; Lacaille-Dubois, M.-A. Cytotoxic Steroidal Glycosides from Allium flavum. Fitoterapia 2014, 93, 121–125. [Google Scholar] [CrossRef]
- Taşcı, B.; Kütük, H.; Koca, İ. Antioxidant Activity of Allium scorodoprasum L. subsp. rotundum (L.) Stearn Plant Grown in Turkey. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1561. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Kim, S.-K. Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Aydin, Ç.; Mammadov, R. Phytochemical analysis, phenolic content, antioxidant, antibacterial, insecticidal and cytotoxic activites of Allium reuterianum Boiss. extracts. Indian J. Tradit. Knowl. 2019, 9, 290–298. Available online: http://nopr.niscair.res.in/handle/123456789/47083 (accessed on 15 November 2021).
- Chehregani, A.; Azimishad, F.; Alizade, H.H. Study on Antibacterial Effect of Some Allium Species from Hamedan-Iran. Int. J. Agri. Biol. 2007, 9, 6. [Google Scholar]
- Demirtas, I.; Erenler, R.; Elmastas, M.; Goktasoglu, A. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem. 2013, 136, 34–40. [Google Scholar] [CrossRef]
- Hiyasat, B.; Sabha, D.; Grötzinger, K.; Kempfert, J.; Rauwald, J.-W.; Mohr, F.-W.; Dhein, S. Antiplatelet Activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef]
- Durmaz, H.; Sagun, E.; Tarakci, Z.; Ozgokce, F. Antibacterial activities of Allium vineale, Chaerophyllum macropodum and Prangos ferulacea. Afr. J. Biotechnol. 2006, 5, 1795–1798. [Google Scholar]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [Green Version]
- Kopeć, A.; Skoczylas, J.; Jędrszczyk, E.; Francik, R.; Bystrowska, B.; Zawistowski, J. Chemical Composition and Concentration of Bioactive Compounds in Garlic Cultivated from Air Bulbils. Agriculture 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Pârvu, M.; Pârvu, A.E.; Vlase, L.; Rosca-Casian, O.; Pârvu, O. Antifungal Aroperties of allium ursinum L. Ethanol Extract. J. Med. Plants Res. 2011, 5, 2041–2046. [Google Scholar]
- Pejatović, T.; Samardžić, D.; Krivokapić, S. Antioxidative properities of a traditional tincture and several leaf extracts of Allium ursinum L. (collected in Montenegro and Bosnia and Herzegovina). J. Mater. Environ. Sci. 2017, 8, 1929–1934. [Google Scholar]
- Sapunjieva, T.; Alexieva, I.; Mihaylova, D.; Popova, A. Antimicrobial and antioxidant activity of extracts of Allium ursinum L. J. BioSci. Biotech. 2012, 3, 143–145. [Google Scholar]
- Štajner, D.; Čanadanović-Brunet, J.; Pavlović, A. Allium schoenoprasum L., as a natural antioxidant. Phytother. Res. 2004, 18, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Tóth, T.; Kovarovič, J.; Bystrická, J.; Vollmannová, A.; Musilová, J.; Lenková, M. The content of polyphenols and antioxidant activity in leaves and flowers of wild garlic (Allium ursinum L.). Acta Aliment. 2018, 47, 252–258. [Google Scholar] [CrossRef]
- Upadhyay, R. Nutritional and Therapeutic Potential of Allium Vegetables. J. Nutr. Ther. 2017, 6, 18–37. [Google Scholar] [CrossRef]
- Stajner, D.; Varga, I.S. An evaluation of the antioxidant abilities of Allium species. Acta Biol. Szeged. 2003, 47, 103–106. [Google Scholar]
- Upadhyay, R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. Int. J. Green Pharm. 2016, 10, 19. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-90-481-8660-0. [Google Scholar]
- Koske, T. Allium Crops Onions Shallots and Garlic. LSU AgCenter Reserch & Ext. 1914. Available online: https://www.lsuagcenter.com/NR/rdonlyres/9E30F191-2704-4BB9-A051-7A3FF7D9715E/44481/pub2318AlliumCropsLOWRES.pdf (accessed on 15 November 2021).
- Peterson, J. The Allium Species (Onions, Garlic, Leeks, Chives, and Shallots). Camb. World Hist. Food 2013, 249–271. [Google Scholar] [CrossRef]
- Newenhouse, A. Growing Fresh Market Onions, Garlic, and Leeks; University of Wisconsin: Madison, WI, USA, 2011; pp. 1–39. [Google Scholar]
- Sanjay, S.S.; Kn, D.; Db, R.; Kabbinavar, S.; Zaranappa, D. Phytochemicals and Potential Biological Activities of Allium sativum Linn. J. Pharmacogn. Phytochem. 2018, 7, 662–670. [Google Scholar]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran. J. Basic Med. Sci. 2013, 16, 18. [Google Scholar]
- Immaculate, V.T.M.; Shlini, P.; Mary Clare, H. Isolation and Purification of Apigenin From Allium fistulosum. Int. J. Curr. Pharm. Res. 2020, 12, 67–71. [Google Scholar] [CrossRef]
- Bede, D.; Zaixiang, L. Dietary Polysaccharides from Allium Species: A Critical Review in Dietary Polysaccharides from Allium Species: Extraction, Characterization, Bioactivity, And Potential Utilization. Acta Sci. Agric. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Singh, V.; Chauhan, G.; Krishan, P.; Shri, R. Allium schoenoprasum L.: A review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 2018, 32, 2202–2216. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Comparison of Phenolic Content and Antioxidant Capacity of Bear Garlic (Allium ursinum L.) in Different Maturity Stages: Phenolics and Antioxidants in Bear Garlic. J. Food Process. Preserv. 2017, 41, e12921. [Google Scholar] [CrossRef]
- Gebregiorgis Amabye, T. In Vitro Antimicrobial Efficacy of Fractions from Onion (Allium cepa) Leaves Extract from Wukro, Ethiopia. Am. J. Life Sci. 2015, 3, 365. [Google Scholar] [CrossRef]
- Curcic, M.G.; Stankovic, M.S.; Radojevic, I.D.; Stefanovic, O.D.; Comic, L.R.; Topuzovic, M.D.; Djacic, D.S.; Markovic, S.D. Biological Effects, Total Phenolic Content and Flavonoid Concentrations of Fragrant Yellow Onion (Allium flavum L.). Med. Chem. 2012, 8, 46–51. [Google Scholar] [CrossRef]
- Firat, M. The Ethnobotanical Usage of Some East Anatolian (Turkey) Allium L. Species. Manas J. Agric. Vet. Life Sci. 2015, 5, 80–86. [Google Scholar]
- Tasci, B.; Koca, I. Use of Allium scorodoprasum L. subsp. rotundum as food. Acta Hortic. 2016, 153–158. [Google Scholar] [CrossRef]
- Defelice, M.S. Wild Garlic, Allium vineale L.—Little to Crow About. Weed Technol. 2003, 17, 890–895. [Google Scholar] [CrossRef]
- Satyal, P.; Craft, J.; Dosoky, N.; Setzer, W. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Hafeznia, B.; Anvar, S.A.; Kakoolaki, S.; Choobkar, N. Antimicrobial efficiency of Allium atroviolaceum extract on Rainbow trout in different temperature and storage time. Iran. J. Aquat. Anim. Health 2018, 4, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, S.; Abdul Hamid, R.; Ramachandran, V.; Mohd Esa, N.; Pandurangan, A.K.; Danazadeh, F.; Ismail, P. Cytotoxicity and Proapoptotic Effects of Allium atroviolaceum Flower Extract by Modulating Cell Cycle Arrest and Caspase-Dependent and p53 -Independent Pathway in Breast Cancer Cell Lines. Evid.-Based Complement. Alternat. Med. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Albert, N.W.; McCallum, J.A.; Pither-Joyce, M.; Crowhurst, R.N.; Eady, C.; Davies, K.M. The Onion (Allium cepa L.) R2R3-MYB Gene MYB1 Regulates Anthocyanin Biosynthesis. Front. Plant Sci. 2016, 7, 1865. [Google Scholar] [CrossRef] [Green Version]
- Jalaiah, M.; Sarvanan, R.; Gowtham, C.H.; Vinay, Y.; Akhila, Y. Evaluation of Cardioprotective Activity of Allium cepa Aerial Leaves. Int. J. Curr. Pharm. Res. 2017, 9, 27. [Google Scholar] [CrossRef]
- Štajner, D.; Milić, N.; Čanadanović-Brunet, J.; Kapor, A.; Štajner, M.; Popović, B.M. Exploring Allium species as a source of potential medicinal agents. Phytother. Res. 2006, 20, 581–584. [Google Scholar] [CrossRef]
- Wilson, A.; Pandya, D.; Solanki, D.H. Comparative Phytochemical Screening of Leaves and Bulb of Allium sativum L. Int. J. Inf. Comput. Sci. 2019, 6, 5. [Google Scholar]
- Fukaya, M.; Nakamura, S.; Hayashida, H.; Noguchi, D.; Nakashima, S.; Yoneda, T.; Matsuda, H. Structures of Cyclic Organosulfur Compounds From Garlic (Allium sativum L.) Leaves. Front. Chem. 2020, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Meshram, G.A.; Khamkar, S.S. Effect Of Oleanolic Acid Isolated From Garlic Leaves On Carbohydrate Metabolizing Enzymes, In Vitro. Int. J. Pharm. Sci. Res. 2014, 5, 988–991. [Google Scholar]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Yabe, A.; Sugino, Y.; Murakami, S.; Sai-ngam, N.; Sumi, S.; Tsuneyoshi, T.; Saito, K. Garlic Î3-glutamyl transpeptidases that catalyze deglutamylation of biosynthetic intermediate of alliin. Front. Plant Sci. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, N.; Onuma, M.; Mizuno, S.; Sugino, Y.; Nakabayashi, R.; Imai, S.; Tsuneyoshi, T.; Sumi, S.; Saito, K. Identification of a flavin-containing S -oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J. 2015, 83, 941–951. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kim, Y.-C.; Chung, S.-K. Identification andin vitro biological activities of flavonols in garlic leaf and shoot: Inhibition of soybean lipoxygenase and hyaluronidase activities and scavenging of free radicals. J. Sci. Food Agric. 2005, 85, 633–640. [Google Scholar] [CrossRef]
- Fukaya, M.; Nakamura, S.; Nakagawa, R.; Kinka, M.; Nakashima, S.; Matsuda, H. Cyclic sulfur-containing compounds from Allium fistulosum ‘Kujou’. J. Nat. Med. 2019, 73, 397–403. [Google Scholar] [CrossRef]
- Ajayi, G.O.; Akinsanya, M.A.; Agbabiaka, A.T.; Oyebanjo, K.S.; Hungbo, T.D.; Olagunju, J.A. D-Limonene: A major bioactive constituent in Allium fistulosum identified by GC-MS analysis. J. Phytopharm. 2019, 8, 257–259. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y.; Kudo, R.; Yamaguchi, K.; Ikeda, T.; Murakami, K.; Ono, M.; Kajimoto, T.; Takeya, M. Isolation and Characterization of New Onionins A2 and A3 from Allium cepa, and of Onionins A1, A2, and A3 from Allium fistulosum. Chem. Pharm. Bull. 2014, 62, 1141–1145. [Google Scholar] [CrossRef] [Green Version]
- El-Hadidy, E.M.; Mossa, M.E.A.; Habashy, H.N. Effect of freezing on the pungency and antioxidants activity in leaves and bulbs of green onion in Giza 6 and Photon varieties. Ann. Agric. Sci. 2014, 59, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, O.G.P.; Pesantes, K.E.B.; Martínez, M.M.; Gaitén, Y.G. Estudio químico y evaluación biológica del extracto etanólico de Allium schoenoprasum L. Regel & Tiling (Cebollín). Rev. Cuba. Farm. 2019, 52, 13. [Google Scholar]
- Díaz, A.B.; Vera, J.R.; Fermín, L.R.; Méndez, A.M.; Zambrano, R.A.; Contreras, L.R. Composition of the essential oil of leaves and roots of Allium schoenoprasum L. (Alliaceae). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2011, 10, 218–221. [Google Scholar]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory Effects of Allium schoenoprasum L. Leaves. J. Physiol. Pharmacol. 2014, 65, 309–315. [Google Scholar]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships between bioactive compound content and the antiplatelet and antioxidant activities of six Allium vegetable species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef]
- Fukaya, M.; Nakamura, S.; Kyoku, Y.; Nakashima, S.; Yoneda, T.; Matsuda, H. Cyclic sulfur metabolites from Allium schoenoprasum var. foliosum. Phytochem. Lett. 2019, 29, 125–128. [Google Scholar] [CrossRef]
- Shirshova, T.I.; Beshlei, I.V.; Deryagina, V.P.; Ryzhova, N.I.; Matistov, N.V. Chemical composition of Allium schoenoprasum leaves and inhibitory effect of their extract on tumor growth in mice. Pharm. Chem. J. 2013, 46, 672–675. [Google Scholar] [CrossRef]
- Petkova, N.T.; Ivanov, I.G.; Raeva, M.; Topuzova, M.G.; Todorova, M.M.; Denev, P.P. Fructans and antioxidants in leaves of culinary herbs from Asteraceae and Amaryllidaceae families. Food Res. 2019, 3, 407–415. [Google Scholar] [CrossRef]
- Barla, G.-F.; Seritan, M.P.; Sanduleac, E.; Ciornei, S.E. Antioxidant Activity and Total Phenolic Content in Allium ursinum and Ranunculus ficaria. Food Environ. Saf. 2014, 8, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Sahnoun, D.; Ksouri, W.M.; Younes, I.; Hammami, M.; Bettaieb, I.; Saada, M.; Mkadmini, K.; Ksouri, R.; Serairi, R.B. Antioxidant activity and biochemical composition of fresh bulbs and leaves of wild garlic Allium ursinum. J. New Sci. 2017, 44, 8. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Characterization and Content of Flavonol Derivatives of Allium ursinum L. Plant. J. Agric. Food Chem. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical Composition and Antimicrobial Activity of Wild Garlic Allium ursinum of Bulgarian Origin. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef] [Green Version]
- Sebtosheikh, P.; Qomi, M.; Ghadami, S.; Mojab, F. Analysis of essential oil from leaves and Bulb of Allium atroviolaceum. Int. Pharm. Acta 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Ayatollahi, S.A.; Amidi, S.; Kobarfard, F. Evaluation of Anti-Platelet Aggregation Effect of Some Allium Species. Iran. J. Pharm. Res. 2015, 14, 1225. [Google Scholar]
- Ye, C.-L.; Dai, D.-H.; Hu, W.-L. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Parmar, P.; Salman, A.; Kalavathy, C.M.; Jesudasan, C.N.; Thomas, P.A. Pneumococcal keratitis: A clinical profile. Clin. Experiment. Ophthalmol. 2003, 31, 44–47. [Google Scholar] [CrossRef]
- Shouval, D.S.; Greenberg, D.; Givon-Lavi, N.; Porat, N.; Dagan, R. Site-Specific Disease Potential of Individual Streptococcus pneumoniae Serotypes in Pediatric Invasive Disease, Acute Otitis Media and Acute Conjunctivitis. Pediatric Infect. Dis. J. 2006, 25, 602–607. [Google Scholar] [CrossRef]
- Ying, Q. Antimicrobial resistance, serotypes and genotypes of Streptococcus pneumoniae isolates associated with ocular infection in China. Afr. J. Microbiol. Res. 2012, 6, 160–164. [Google Scholar] [CrossRef]
- Roopa, V.M.; Suvarna, V.C.; Natesh, N. Original Research Article Antimicrobial activity of plant extracts against post harvest spoilage of onions. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 388–394. [Google Scholar]
- Sulaiman, A.F.; Alwan, W.M.; Salman, S.A.; Al-Abodi, E.E. A Comparative Study of Chemical Compounds and Anti-Bacterial Efficacy of Different Allium cepa Plant Extracts. Syst. Rev. Pharm. 2021, 12, 4. [Google Scholar]
- Stankov, S.V. Definition of Inflammation, Causes of Inflammation and Possible Anti-inflammatory Strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 287–302. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Aslam, Z.; Akhtar, S.; Imran, M.; Nadeem, M.; Gilani, S.A.; Elnashar, M.; El-ghorab, A. Antioxidant Activity, Anti-Inflammatory Activities, Anti-Cancer and Chemical Composition of Spring Onion (Allium fistolisum) Extracts. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1880–1890. [Google Scholar]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-S.; Huang, G.-J.; Lu, Y.-H.; Chang, L.-W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013, 138, 751–756. [Google Scholar] [CrossRef]
- Pan, S.; Aparna, L.I.; Priyanka, P. Anti-inflammatory activity of aqueous extract of Allium satium Leaves. Asian J. Pharm. Clin. Res. 2015, 8, 78–80. [Google Scholar]
- Sinha, T. Tumors: Benign and Malignant. Cancer Ther. Oncol. Int. J. 2018, 10, 1–3. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y. Antitumor Sulfur Compounds from Allium Species (Onion, Welsh Onion, and Garlic). Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef] [Green Version]
- Twomey, L.; Wallace, R.G.; Cummins, P.M.; Degryse, B.; Sheridan, S.; Harrison, M.; Moyna, N.; Meade-Murphy, G.; Navasiolava, N.; Custaud, M.-A.; et al. Platelets: From Formation to Function. In Homeostasis—An Integrated Vision; Lasakosvitsch, F., Dos Anjos Garnes, S., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-077-2. [Google Scholar]
- Clemetson, K.J. Platelets and Primary Haemostasis. Thromb. Res. 2012, 129, 220–224. [Google Scholar] [CrossRef]
- Younis, L.S.; Mohammed, I.M.; Najah, H.T.; Haider, A.M. Antiplatelet drugs overview. GSC Biol. Pharm. Sci. 2020, 10, 81–89. [Google Scholar] [CrossRef]
- Saplonţai-Pop, A.; Moţ, A.; Moldovan, M.; Oprean, R.; Silaghi-Dumitrescu, R.; Orășan, O.; Pârvu, M.; Gal, E.; Ionescu, C. Testing antiplatelet and antioxidant activity of the extract of seven varieties of Allium cepa L. Open Life Sci. 2015, 10, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.Y.; Nile, S.H.; Jung, Y.-S.; Keum, Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech 2018, 8, 155. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.C.P.; Bettoni, M.M.; Bona, C.; Foerster, L.A. Morphological and chemical characteristics of onion plants (Allium cepa L.) associated with resistance to onion thrips. Acta Sci. Agron. 2014, 37, 85. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Srivastava, R.; Singh, C.; Shukla, K.; Singh, R.; Singh, P.; Singh, R.; Singh, N.; Sharma, R. Amylases: An Overview with Special Reference to Alpha Amylase. J. Glob. Biosci. 2015, 4, 1886–1901. [Google Scholar]
- Williams, J.A. Amylase. Pancreapedia 2017, 1–8. [Google Scholar] [CrossRef]
- Adefisoye, S.A.; Sakariyau, A.O. Production of Glucoamylase by Aspergillus niger in Solid State Fermentation. Adv. Biol. Res. 2018, 2, 7–11. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, R. Study of Phytochemical Analysis and Antioxidant Activity of Allium sativum of Bundelkhand Region. Int. J. Life Sci. Sci. Res. 2017, 3, 1451–1458. [Google Scholar] [CrossRef]
- Shakurfow, F.A.A.; Buazzi, M.M.; Gamal, M.A.B. Assessment of antimicrobial activity of onion (Allium cepa) and garlic (Allium sativum) extracts on Listeria monocytogenes; in vitro study. Lebda Med. J. 2015, 1, 1–5. [Google Scholar]
- Khalid, N.; Ahmed, I.; Latif, M.S.Z.; Rafique, T.; Fawad, S.A. Comparison of antimicrobial activity, phytochemical profile and minerals composition of garlic Allium sativum and Allium tuberosum. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 311–317. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D.; Alexieva, I. GC-MS chemical composition of volatile oil and mineral element content of Allium ursinum and Nectaroscordum siculum. Pak. J. Bot. 2018, 50, 2351–2354. [Google Scholar]
- Elsayed Azab, A.; Adwas, A.A.; Ibrahim Elsayed, A.S.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [Green Version]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Sunitha, D. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Azat Aziz, M.; Shehab Diab, A.; Abdulrazak Mohammed, A. Antioxidant Categories and Mode of Action. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-919-5. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Nencini, C.; Cavallo, F.; Capasso, A.; Franchi, G.G.; Giorgio, G.; Micheli, L. Evaluation of antioxidative properties of Allium species growing wild in Italy. Phytother. Res. 2007, 21, 874–878. [Google Scholar] [CrossRef]
- Kruzlicova, D.; Danihelova, M.; Veverka, M. Quantitative Structure-Antioxidant Activity Relationship of Quercetin and its New Synthetised Derivatives. Nova Biotechnol. Chim. 2012, 11, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.; Welsh, W.J.; Shi, L.; Fang, H.; Perkins, R. Structure–activity relationship approaches and applications. Environ. Toxicol. Chem. 2003, 22, 1680. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar] [CrossRef]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Bilyk, A.; Cooper, P.L.; Sapers, G.M. Varietal differences in distribution of quercetin and kaempferol in onion (Allium cepa L.) tissue. J. Agric. Food Chem. 1984, 32, 274–276. [Google Scholar] [CrossRef]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and Characterization of Flavonoids from the Leaves of Zanthoxylum bungeanum and Correlation between Their Structure and Antioxidant Activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Tu, B.; Liu, Z.J.; Chen, Z.F.; Ouyang, Y.; Hu, Y.J. Understanding the structure-activity relationship between quercetin and naringenin: In vitro. RSC Adv. 2015, 5, 106171–106181. [Google Scholar] [CrossRef]
- Aoyama, S.; Yukiko, Y. Antioxidant Activity and Flavonoid Content of Welsh Onion (Allium fistulosum) and the Effect of Thermal Treatment. Food Sci. Technol. Res. 2007, 13, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Farkas, O.; Jakus, J.; Héberger, K. Quantitative Structure—Antioxidant Activity Relationships of Flavonoid Compounds. Molecules 2004, 9, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Biswas, S.; Dayan, N.A.; Sarwat, M. Antioxidant Role of Kaempferol in the Management of Hepatocellular Carcinoma. Med. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Nguyen Ngoc, H.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol Within Inflammatory Pathways—A Hint Towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–Antioxidant–Antiproliferative Activity Relationships of Natural C7 and C7–C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Z.; Deng, G.; Guo, R.; Chen, D.-F.; Fu, Z.-M. DFT Studies on the Antioxidant Activity of Naringenin and Its Derivatives: Effects of the Substituents at C3. Int. J. Mol. Sci. 2019, 20, 1450. [Google Scholar] [CrossRef] [Green Version]
- Silva dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant Activity Relationships of Flavonoids: A Re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Bajerska, J.; Mildner-Szkudlarz, S.; Jeszka, J.; Szwengiel, A. Catechin stability, antioxidant properties and sensory profiles of rye breads fortified with green tea extracts. J. Food Nutr. Res. 2010, 49, 104–111. [Google Scholar]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, S.A.B.E.; de Groot, M.J.; van den Berg, D.-J.; Tromp, M.N.J.L.; Donné-Op den Kelder, G.; van der Vijgh, W.J.F.; Bast, A. A Quantum Chemical Explanation of the Antioxidant Activity of Flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Update Ejournal 2007, 2, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Vásquez-Espinal, A.; Yañez, O.; Osorio, E.; Areche, C.; García-Beltrán, O.; Ruiz, L.M.; Cassels, B.K.; Tiznado, W. Theoretical Study of the Antioxidant Activity of Quercetin Oxidation Products. Front. Chem. 2019, 7, 818. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves. Molecules 2021, 26, 7175. https://doi.org/10.3390/molecules26237175
Kurnia D, Ajiati D, Heliawati L, Sumiarsa D. Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves. Molecules. 2021; 26(23):7175. https://doi.org/10.3390/molecules26237175
Chicago/Turabian StyleKurnia, Dikdik, Dwipa Ajiati, Leny Heliawati, and Dadan Sumiarsa. 2021. "Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves" Molecules 26, no. 23: 7175. https://doi.org/10.3390/molecules26237175
APA StyleKurnia, D., Ajiati, D., Heliawati, L., & Sumiarsa, D. (2021). Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves. Molecules, 26(23), 7175. https://doi.org/10.3390/molecules26237175








