Pesticides Burden in Neotropical Rivers: Costa Rica as a Case Study
Abstract
:1. Introduction
2. Results and Discussion
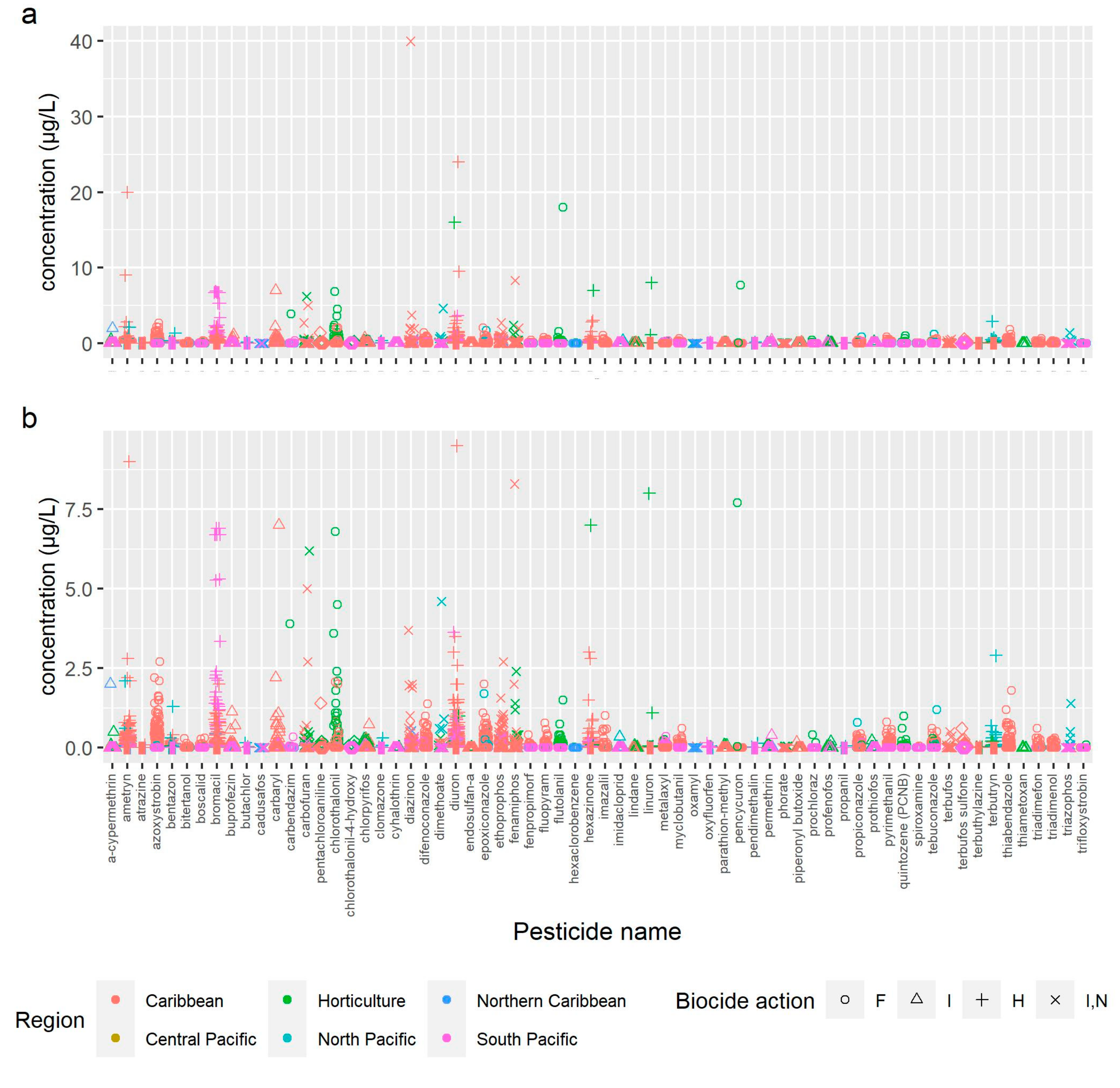
2.1. Pesticide Detection and Frequency
2.2. Comparison with International Regulations
2.3. Ecological Risk Multi-Substance Potentially Affected Fraction (msPAF) Model
3. Materials and Methods
3.1. Area of Study
3.2. Database
3.3. Pesticide Analysis
3.4. Comparison with International Regulations
3.5. Ecological Risk Multi-Substance Potentially Affected Fraction (msPAF) Model
4. Conclusions
- -
- Pesticides are ubiquitous contaminants of fresh waters in Costa Rica and other Neotropical countries;
- -
- Several of the highly toxic active ingredients are detected in high frequencies (>20%) throughout Costa Rica, increasing the risks for aquatic biota;
- -
- Concentrations at which individual analyzed pesticides are found in the country exceed criteria for biodiversity protection (HC5) and international standards, therefore representing a risk for the integrity and ecological functioning of aquatic ecosystems;
- -
- msPAF reveals moderate and high risk derived from pesticide mixtures in water samples across Costa Rica;
- -
- Pesticides consistently representing risk in Costa Rica (high frequency of detection, exceeding environmental standards, and identified as risk contributors within the msPAF model and literature) are a-cypermethrin, ametryn, azoxystrobin, bromacil. carbofuran, chlorothalonil, chlorpyrifos, diazinon, diuron, epoxiconazole, ethoprophos, fenamiphos, hexazinone, terbufos, and terbutryn;
- -
- We believe these pesticides (except bromacil, which has already been forbidden) should be re-evaluated if their registration did not take into account current risk assessment tools;
- -
- Several high-risk pesticides in Costa Rica are detected in other Neotropical countries;
- -
- Deeper analysis of the responses of biota to the detected pesticides might be used to complement the development of numerical water-quality criteria and also for retrospective environmental risk evaluations for Neotropical countries;
- -
- There is an urgent need for systematic pesticide residue monitoring of fresh waters in the Neotropical region.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Antonelli, A.; Sanmartín, I. Why are there so many plant species in the Neotropics? TAXON 2011, 60, 403–414. [Google Scholar] [CrossRef]
- MINAE. Actividades y eventos que generan presión e impacto en el ambiente costarricense. In Informe Estado del Ambiente Costa Rica 2017; MINAE: San José, Costa Rica, 2018; 713p. [Google Scholar]
- Alpízar, F.; Madrigal, R.; Salas, A. Retos ambientales de Costa Rica; Banco Interamericano de Desarrollo: Washington, DC, USA, 2018; 66p. [Google Scholar] [CrossRef]
- Bravo Durán, V.; de la Cruz, E.; Herrera Ledezma, G.; Ramírez, F. Agriculture pesticide use as a tool for monitoring health. Uniciencia 2013, 27, 351–376. [Google Scholar]
- De La Cruz, E.; Bravo-Durán, V.; Ramírez, F.; Castillo, L.E. Environmental hazards associated with pesticide import into Costa Rica, 1977–2009. J. Environ. Biol. 2014, 35, 43–55. [Google Scholar]
- FAOSTAT. Available online: http//www.fao.org/faostat/en/#data/RP (accessed on 26 September 2019).
- Arias-Andrés, M.; Rämö, R.; Mena Torres, F.; Ugalde, R.; Grandas, L.; Ruepert, C.; Gunnarsson, J.S. Lower tier toxicity risk assessment of agriculture pesticides detected on the Río Madre de Dios watershed, Costa Rica. Environ. Sci. Pollut. Res. 2018, 25, 13312–13321. [Google Scholar] [CrossRef]
- Castillo, L.E.; Ruepert, C.; Solis, E. Pesticide residues in the aquatic environment of banana plantation areas in the north Atlantic zone of Costa Rica. Environ. Toxicol. Chem. 2000, 19, 1942–1950. [Google Scholar] [CrossRef]
- Castillo, L.E.; Ruepert, C.; Savage, C.; Gilek, M.; Pinnock, M.; Solis, E. Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica. Sci. Total Environ. 2006, 367, 418–432. [Google Scholar] [CrossRef]
- Echeverría-Sáenz, S.; Mena, F.; Pinnock, M.; Ruepert, C.; Solano, K.; de la Cruz, E.; Campos, B.; Sánchez-Avila, J.; Lacorte, S.; Barata, C. Environmental hazards of pesticides from pineapple crop production in the Río Jiménez watershed (Caribbean Coast, Costa Rica). Sci. Total Environ. 2012, 440, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Echeverría-Sáenz, S.; Mena, F.; Arias-Andrés, M.; Vargas, S.; Ruepert, C.; Van den Brink, P.J.; Castillo, L.E.; Gunnarsson, J.S. In situ toxicity and ecological risk assessment of agro-pesticide runoff in the Madre de Dios River in Costa Rica. Environ. Sci. Pollut. Res. 2018, 25, 13270–13282. [Google Scholar] [CrossRef]
- Fournier, M.L.; Echeverría-Sáenz, S.; Mena, F.; Arias-Andrés, M.; de la Cruz, E.; Ruepert, C. Risk assessment of agriculture impact on the Frío River watershed and Caño Negro Ramsar wetland, Costa Rica. Environ. Sci. Pollut. Res. 2018, 25, 13347–13359. [Google Scholar] [CrossRef]
- Carazo-Rojas, E.; Pérez-Rojas, G.; Pérez-Villanueva, M.; Chinchilla-Soto, C.; Chin-Pampillo, J.S.; Aguilar-Mora, P.; Alpízar-Marín, M.; Masís-Mora, M.; Rodríguez-Rodríguez, C.E.; Vryzas, Z. Pesticide monitoring and ecotoxicological risk assessment in surface water bodies and sediments of a tropical agro-ecosystem. Environ. Pollut. 2018, 241, 800–809. [Google Scholar] [CrossRef]
- Mena, F.; San Juan, M.F.; Campos, B.; Sánchez-Ávila, J.; Faria, M.; Pinnock, M.; Barata, C. Pesticide residue analyses and biomarker responses of native Costa Rican fish of the Poeciliidae and Cichlidae families to assess environmental impacts of pesticides in Palo Verde National Park. J. Environ. Biol. 2014, 35, 19–27. [Google Scholar]
- Rizo-Patrón, V.; Kumar, F.; McCoy, A.; Colton, M.B.; Springer, M.; Trama, F.A. Macroinvertebrate communities as bioindicators of water quality in conventional and organic irrigated rice fields in Guanacaste, Costa Rica. Ecol. Indic. 2013, 29, 68–78. [Google Scholar] [CrossRef]
- Echeverría-Sáenz, S.; Ugalde-Salazar, R.; Vargas-Villalobos, S. Efectos de múltiples factores ambientales sobre la comunidad macrobentónica de una quebrada con influencia agrícola. Presented at V Congreso Latinoamericano de Macroinvertebrados y Ecosistemas Acuáticos, Online, 18–22 October 2021. [Google Scholar]
- Fournier, M.L.; F. Ramírez, C.; Ruepert, S.; Vargas, S.; Echeverría-Sáenz, S. Echeverría-Sáenz, S. Diagnóstico Sobre Contaminación de Aguas, Suelos y Productos Hortícolas por el Uso de Agroquímicos en la Microcuenca de las Quebradas Plantón y Pacayas en Cartago, Costa Rica; Final Project Report; National University: Heredia, Costa Rica, 2010. [Google Scholar]
- Ramírez-Morales, D.; Pérez-Villanueva, M.E.; Chin-Pampillo, J.S.; Aguilar-Mora, P.; Arias-Mora, V.; Masís-Mora, M. Pesticide occurrence and water quality assessment from an agriculturally influenced Latin-American tropical region. Chemosphere 2021, 262, 127851. [Google Scholar] [CrossRef]
- Cornejo, A.; Tonin, A.M.; Checa, B.; Tuñon, A.R.; Pérez, D.; Coronado, E.; González, S.; Ríos, T.; Macchi, P.; Correa-Araneda, F.; et al. Effects of multiple stressors associated with agriculture on stream macroinvertebrate communities in a tropical catchment. PLoS ONE 2019, 14, e0220528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornejo, A.; Pérez, J.; López-Rojo, N.; Tonin, A.M.; Rovira, D.; Checa, B.; Jaramillo, N.; Correa, K.; Villarreal, A.; Villarreal, V.; et al. Agriculture impairs stream ecosystem functioning in a tropical catchment. Sci. Total Environ. 2020, 745, 140950. [Google Scholar] [CrossRef] [PubMed]
- Barizon, R.; Figueiredo, R.; Dutra, D.; Regitano, J.; Ferracini, V. Pesticides in the surface waters of the Camanducaia River watershed, Brazil. J. Environ. Sci. Health Part B 2019, 55, 283–292. [Google Scholar] [CrossRef]
- Hernández, F.; Portolés, T.; Ibáñez, M.; Bustos-López, M.C.; Díaz, R.; Botero-Coy, A.M.; Peñuela, G. Use of time-of-flight mass spectrometry for large screening of organic pollutants in surface waters and soils from a rice production area in Colombia. Sci. Total Environ. 2012, 439, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deknock, A.; De Troyer, N.; Houbraken, M.; Dominguez-Granda, L.; Nolivos, I.; Van Echelpoel, W.; Eurie Forio, M.A.; Spanoghe, P.; Goethals, P. Distribution of agricultural pesticides in the freshwater environment of the Guayas River basin (Ecuador). Sci. Total Environ. 2019, 646, 996–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva Morales, J.B.; Valdez Torres, J.B.; Bastidas Bastidas, P.J.; Angulo Escalante, M.A.; Sarmiento Sánchez, J.I.; Barraza Lobo, A.L.; Olmeda Rubio, C.; Chaidez Quiroz, C. Monitoring of pesticides residues in northwestern Mexico rivers. Acta Univ. 2017, 27, 45–54. [Google Scholar] [CrossRef]
- Cárdenas, S.; Marquez, A.; Guevara, E.; Rey, D. Characterization of organochlorated pesticides in water and sediments, Tucutunemo River, Venezuela. Tecnol. Cienc. Agua 2018, 9, 131–169. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernandez-Alba, R.; Barcelo, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Ramírez Muñoz, F.; Orozco Aceves, M.; Fournier Leiva, M.L.; Berrocal Montero, S.E.; Echeverría Sáenz, S.; de la Cruz, E.; Chaverri Fonseca, F.; Moraga López, G.; Solano Díaz, K.; Alfaro Alfaro, A.; et al. Las Buenas Prácticas Agrícolas en el Uso y Manejo de Agroquímicos en la Zona Hortícola de Zarcero, Alajuela. Final Project Report; National University: Heredia, Costa Rica, 2017. [Google Scholar]
- Vryzas, Z.; Ramwell, C.; Sans, C. Pesticide prioritization approaches and limitations in environmental monitoring studies, From Europe to Latin America and the Caribbean. Environ. Int. 2020, 143, 105917. [Google Scholar] [CrossRef]
- Bravo-Durán, V.; Berrocal-Montero, S.E.; Ramírez-Muñoz, F.; de la Cruz, E.; Canto-Mai, N.; Tatis-Ramírez, A.; Mejía-Merino, W.; Rodríguez-Altamirano, T. Pesticides import and health hazards. Central America, 2005–2009. Uniciencia 2015, 29, 84–106. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, R.B.; van den Brink, P.J.; Liess, M. Impacts of Pesticides on Freshwater Ecosystems. In Ecological Impacts of Toxic Chemicals; Sánchez-Bayo, F., van den Brink, P.J., Mann, R.M., Eds.; Open Access; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011; pp. 111–137. [Google Scholar] [CrossRef] [Green Version]
- Liess, M.; Beketov, M. Traits and stress, Keys to identify community effects of low levels of toxicants in test systems. Ecotoxicology 2011, 20, 1328–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Brink, P.J.; Crum, S.J.H.; Gylstra, R.; Bransen, F.; Cuppen, J.G.M.; Brock, T.C.M. Effects of a herbicide-insecticide mixture in freshwater microcosms, Risk assessment and ecological effect chain. Environ. Pollut. 2009, 157, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Beketov, M.A.; Liess, M. An indicator for effects of organic toxicants on lotic invertebrate communities, Independence of confounding environmental factors over an extensive river continuum. Environ. Pollut. 2008, 156, 980–987. [Google Scholar] [CrossRef]
- Jergentz, S.; Pessacq, P.; Mugni, H.; Bonetto, C.; Schulz, R. Linking in situ bioassays and population dynamics of macroinvertebrates to assess agricultural contamination in streams of the Argentine pampa. Ecotoxicol. Environ. Saf. 2004, 59, 133–141. [Google Scholar] [CrossRef]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [Green Version]
- Stehle, S.; Bub, S.; Schulz, R. Compilation and analysis of global surface water concentrations for individual insecticide compounds. Sci. Total Environ. 2018, 639, 516–525. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [Green Version]
- Ruepert, C.; de la Cruz, E.; Solano, K.; Arguello, S. DDT y sus metabolitos en muestras ambientales y sangre de niños en viviendas de Estradas y Olivia-Paraíso, Costa Rica. Resumen de actividades Ejecutadas 2004–2008. In Programa Regional de Acción y Demostración de Alternativas Sostenibles para el Control de la Malaria sin Uso del DDT en Mexico y América Central, El Caso de Costa Rica; Serie de Informes Técnicos; Herrero-Acosta, M.V., Ed.; IRET: Heredia, Costa Rica, 2008; Volume 5, pp. 31–44. [Google Scholar]
- Pérez-Maldonado, I.N.; Trejo, A.; Ruepert, C.; Jovel, R.D.C.; Méndez, M.P.; Ferrari, M.; Saballos-Sobalvarro, E.; Alexander, C.; Yáñez-Estrada, L.; López, D.; et al. Assessment of DDT levels in selected environmental media and biological samples from Mexico and Central America. Chemosphere 2010, 78, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- De Paz, J.M.; Rubio, J.L. Application of a GIS–AF/RF model to assess the risk of herbicide leaching in a citrus-growing area of the Valencia Community, Spain. Sci. Total Environ. 2006, 371, 44–54. [Google Scholar] [CrossRef] [PubMed]
- ENSR International. Bromacil, Ecological Risk Assessment, Final Report 2005. All U.S. Government Documents (Utah Regional Depository). Paper 78. Available online: https://digitalcommons.usu.edu/govdocs/78 (accessed on 25 August 2021).
- Rodríguez-Rodríguez, C.E.; Matarrita, J.; Herrero-Nogareda, L.; Pérez-Rojas, G.; Alpízar-Marín, M.; Chinchilla-Soto, C.; Pérez-Villanueva, M.; Vega-Méndez, D.; Masís-Mora, M.; Cedergreen, N.; et al. Environmental monitoring and risk assessment in a tropical Costa Rican catchment under the influence of melon and watermelon crop pesticides. Environ. Pollut. 2021, 284, 117498. [Google Scholar] [CrossRef] [PubMed]
- PAN (Pesticide Action Network), highly Hazardous Pesticides. Available online: http://pan-international.org/wp-content/uploads/PAN_HHP_List.pdf (accessed on 21 May 2021).
- RIVM—National Institute for Health and Environment (The Netherlands). Zoeksysteem Risico’s van Stoffen (Risks of Substances Sytem Search). Available online: https://rvszoeksysteem.rivm.nl/Stoffen (accessed on 21 May 2021).
- EPA—Environmental Protection Agency National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table and https://www.atsdr.cdc.gov/spl/#2019spl; (accessed on 21 May 2021).
- Australian New Zealand Guidelines for Fresh Marine Water Quality. Available online: https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/water-quality-toxicants/search (accessed on 21 May 2021).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/dir/2013/39/oj (accessed on 21 May 2021).
- Rämö, R.A.; van den Brink, P.J.; Ruepert, C.; Castillo, L.E.; Gunnarsson, J.S. Environmental risk assessment of pesticides in the River Madre de Dios, Costa Rica using PERPEST, SSD, and msPAF models. Environ. Sci. Pollut. Res. 2018, 25, 13254–13269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burden, R.S.; Cooke, D.T.; Carter, G.A. Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry 1989, 28, 1791–1804. [Google Scholar] [CrossRef]
- Fungicide Resistance Action Committee. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2021--final.pdf?sfvrsn=f7ec499a_2 (accessed on 10 September 2021).
- Sandoval-Herrera, N.; Mena, F.; Espinoza, M.; Romero, A. Neurotoxicity of organophosphate pesticides could reduce the ability of fish to escape predation under low doses of exposure. Sci. Rep. 2019, 9, 10530. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, K.; Solano, K.; Scholz, C.; Redondo-López, S.; Mena, F. Early Toxic Effects in a Central American Native Fish (Parachromis dovii) Exposed to Chlorpyrifos and Difenoconazole. Environ. Toxicol. Chem. 2021, 40, 1940–1949. [Google Scholar] [CrossRef]
- Hunt, L.; Marrochi, N.; Bonetto, C.; Liess, M.; Buss, D.F.; Vieira da Silva, C.; Chiu, M.-C.; Resh, V.H. Do riparian buffers protect stream invertebrate communities in South American Atlantic Forest agricultural areas? Environ. Manag. 2017, 60, 1155–1170. [Google Scholar] [CrossRef]
- Knillmann, S.; Orlinskiy, P.; Kaske, O.; Foit, K.; Liess, M. Indication of pesticide effects and recolonization in streams. Sci. Total Environ. 2018, 630, 1619–1627. [Google Scholar] [CrossRef]
- Herrera, W. Climate of Costa Rica. In Costa Rican Ecosystems; Kappelle, M., Ed.; The University of Chicago Press: Chicago, IL, USA, 2016; pp. 19–29. [Google Scholar]
- Holdridge, L.R.; Grenke, W.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environments in Tropical Life Zones, A Pilot Study; Pergamon Press: Oxford, UK, 1971; 747p. [Google Scholar]
- De Zwart, D.; Posthuma, L.; Gevrey, M.; von der Ohe, P.C.; de Deckere, E. Diagnosis of ecosystem impairment in a multiple-stress context—how to formulate effective river basin management plans. Integr. Environ. Assess. Manag. 2009, 5, 38. [Google Scholar] [CrossRef]
- Traas, T.P.; Van de Meent, D.; Posthuma, L.; Hamers, T.; Kater, B.J.; De Zwart, D.; Aldenberg, T. The potentially affected fraction as a measure of ecological risk. In Species Sensitivity Distributions in Ecotoxicology; Posthuma, L., Suter, G.W., II, Traas, T.P., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2002; pp. 315–344. [Google Scholar]
- U.S. Environmental Protection Agency, Washington, DC, USA. ECOTOX User Guide, ECOTOX Knowledgebase Reference Guide EPA/600/F-19/089. Available online: Epa.gov/ecotox/ (accessed on 5 September 2021).
- Insecticide Resistance Action Committee. Available online: http://www.irac-online.org/ (accessed on 10 September 2021).
- Herbicide Resistance Action Committee. Available online: http://www.hracglobal.com/ (accessed on 10 September 2021).
- De la Cruz, E.; Pinnock-Branford, M.; Echeverría-Sáenz, S.; Mena, F.; Ugalde Salazar, R. Impacto de los Plaguicidas en el Recurso Hídrico de la Zona Baja de la Cuenca del Río Tempisque (Palo Verde) Costa Rica. Bases Científicas para la Gestión Ambiental Sostenible. 2012. Available online: http://www.iret.una.ac.cr/index.php/component/joomd/joomdtypepublicaciones/items/view/proyecto11 (accessed on 22 January 2020).
- Ruepert, C.; National University, Heredia, Costa Rica). Personal communication, 2020.
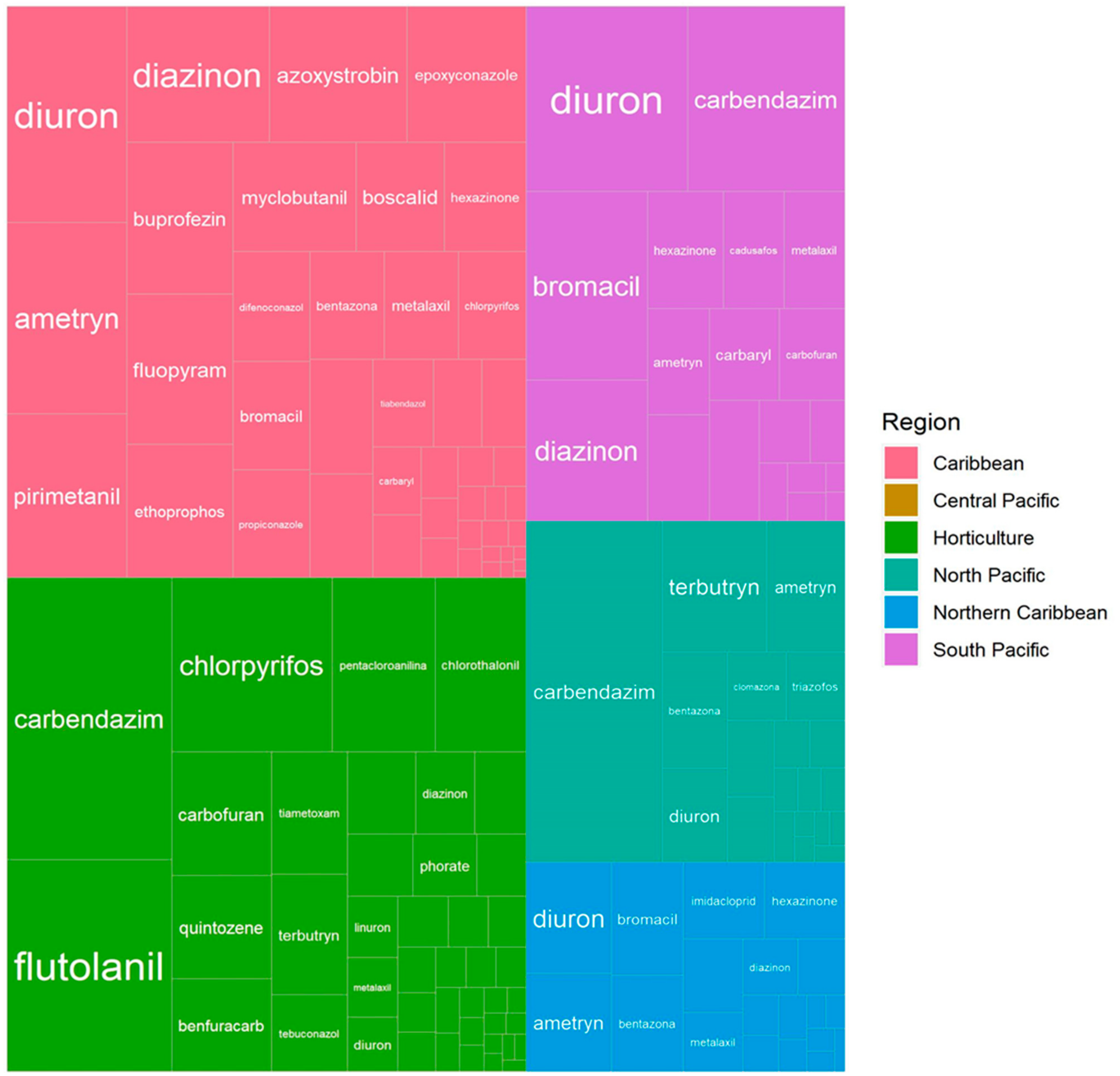


| Active Ingredient | Num. of Analyzed Samples | Num. of Detections | Detection Frequency | Observations | Year of Prohibition/Restriction |
|---|---|---|---|---|---|
| diuron | 917 | 339 | 36.97 | A | |
| ametryn | 991 | 315 | 31.79 | ||
| pyrimethanil | 549 | 170 | 30.97 | A | |
| flutolanil | 432 | 130 | 30.09 | A | |
| pentachloroaniline (M) | 216 | 62 | 28.70 | ||
| diazinon | 1000 | 279 | 27.90 | A | |
| azoxystrobin | 602 | 158 | 26.25 | A | |
| buprofezin | 431 | 99 | 22.97 | ||
| epoxiconazole | 822 | 180 | 21.90 | A | |
| chlorpyrifos | 1029 | 204 | 19.83 | R | 2007 |
| myclobutanil | 456 | 90 | 19.74 | ||
| ethoprophos | 914 | 180 | 19.69 | R | 2007 |
| fluopyram | 296 | 53 | 17.91 | ||
| bromacil | 967 | 149 | 15.41 | F | 2017 |
| chlorothalonil | 914 | 136 | 14.88 | A | |
| hexazinone | 979 | 135 | 13.79 | ||
| bentazone | 293 | 39 | 13.31 | ||
| difenoconazole | 725 | 91 | 12.55 | A | |
| metalaxyl | 919 | 114 | 12.40 | ||
| propiconazole | 846 | 99 | 11.70 | A | |
| boscalid | 291 | 32 | 11.00 | A | |
| fenpropimorph | 401 | 40 | 9.98 | A | |
| thiabendazole | 637 | 56 | 8.79 | A | |
| carbendazim | 126 | 11 | 8.73 | A | |
| terbutryn | 930 | 77 | 8.28 | ||
| tebuconazole | 779 | 54 | 6.93 | A | |
| carbofuran | 846 | 58 | 6.86 | F | 2014 |
| quintozene (PCNB) | 783 | 41 | 5.24 | ||
| terbufos sulfone (M) | 746 | 38 | 5.09 | ||
| fenamiphos | 999 | 50 | 5.01 | ||
| imidacloprid | 173 | 8 | 4.62 | A | |
| carbaryl | 837 | 36 | 4.30 | ||
| clorotalonil 4-hidroxi (M) | 125 | 4 | 3.20 | ||
| profenophos | 179 | 5 | 2.79 | ||
| hexachlorobenzene | 545 | 15 | 2.75 | F | 2005 |
| imazalil | 449 | 12 | 2.67 | A | |
| lindane | 151 | 4 | 2.65 | F | 1999 |
| triadimenol | 827 | 20 | 2.42 | A | |
| oxifluorfen | 688 | 15 | 2.18 | A | |
| dimethoate | 750 | 16 | 2.13 | A | |
| terbufos | 992 | 18 | 1.81 | R | 2007 |
| triadimefon | 803 | 14 | 1.74 | ||
| linuron | 787 | 13 | 1.65 | ||
| clomazone | 290 | 4 | 1.38 | A | |
| triazophos | 531 | 7 | 1.32 | A | |
| oxamyl | 166 | 2 | 1.20 | ||
| phorate | 917 | 11 | 1.20 | ||
| permethrin | 685 | 7 | 1.02 | A | |
| carbofuran phenol (M) | 846 | 8 | 0.95 | ||
| bitertanol | 768 | 7 | 0.91 | A | |
| prothiofos | 660 | 6 | 0.91 | ||
| tecnazene | 146 | 1 | 0.68 | ||
| a-cypermethrin | 794 | 5 | 0.63 | A | |
| piperonyl butoxide | 164 | 1 | 0.61 | ||
| cadusafos | 346 | 2 | 0.58 | ||
| terbuthylazine | 834 | 4 | 0.48 | ||
| butachlor | 633 | 3 | 0.47 | A | |
| spiroxamine | 460 | 2 | 0.43 | ||
| prochloraz | 550 | 2 | 0.36 | A | |
| parathion-methyl | 842 | 3 | 0.36 | R | 2007 |
| pendimethalin | 654 | 2 | 0.31 | A | |
| tolclofos-methyl | 657 | 2 | 0.30 | ||
| trifloxystrobin | 393 | 1 | 0.25 | A | |
| pencycuron | 801 | 2 | 0.25 | ||
| atrazine | 953 | 2 | 0.21 | ||
| propanil | 543 | 1 | 0.18 | A | |
| cyhalothrin | 685 | 1 | 0.15 | A | |
| endosulfan-a | 1003 | 1 | 0.10 | F * | 2015 |
| metribuzin | 1 | 1 | 100 | ||
| dimetomorph | 5 | 4 | 80 | ||
| benfuracarb | 5 | 1 | 20 | ||
| thiametoxan | 5 | 1 | 20 | A | |
| endosulfan-b | 992 | 0 | 0 | ||
| deltametryn | 727 | 0 | 0 | A | |
| malathion | 670 | 0 | 0 | ||
| bifenthrin | 626 | 0 | 0 | ||
| fenthion | 620 | 0 | 0 | ||
| cyproconazole | 582 | 0 | 0 | A | |
| fenbuconazole | 439 | 0 | 0 | A | |
| endosulfan sulfate | 418 | 0 | 0 | ||
| pentachlorobenzene (M) | 154 | 0 | 0 | ||
| pentachloroanisol (M) | 147 | 0 | 0 | ||
| DDE-pp (M) | 142 | 0 | 0 | ||
| DDD-pp (M) | 134 | 0 | 0 | ||
| pp-DDE (M) | 42 | 0 | 0 |
| Active Ingredient. | Biocide Action | Mean Detected Conc. (µg/L) | Max. Detected Conc. (µg/L) | HC5 (µg/L) | AA-EQS (EU) (µg/L) | MAC-EQS (EU) (µg/L) | MTR eco (µg/L) | EPA (Chronic) (µg/L) | EPA (Acute) (µg/L) | Aust (µg/L) | HHP (PAN) | Priority (EU) | Priority (EPA) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a-cypermethrin | insecticide | 0.06 | 2 | 1.77 | 0.00008 | 0.0006 | YES | YES | |||||
| ametryn | herbicide | 0.27 | 20 | 0.23 | 0.01 | ||||||||
| atrazine | herbicide | 0.09 | 0.09 | nc | 0.6 | 2 | 13 | YES | |||||
| azoxystrobin | fungicide | 0.39 | 2.7 | 43.7 | 0.02 | 4.1 | |||||||
| bentazone | herbicide | 0.16 | 1.3 | 828 | 73 | 450 | |||||||
| bitertanol | fungicide | 0.10 | 0.29 | nc | 0.31 | ||||||||
| boscalid | fungicide | 0.07 | 0.3 | nc | 0.55 | ||||||||
| bromacil | herbicide | 0.66 | 6.9 | 3.8 | 0.0068 | ||||||||
| buprofezin | insecticide | 0.06 | 1.13 | nc | 0.56 | ||||||||
| butachlor | herbicide | 0.001 | 0.04 | nc | 0.00023 | YES | |||||||
| cadusafos | insecticide | 0.03 | 0.03 | nc | 0.023 | 0.023 | YES | ||||||
| carbaryl | insecticide | 0.60 | 7 | 1.02 | 0.23 | 2.1 | 2.1 | YES | |||||
| carbendazim | fungicide | 0.13 | 0.34 | 11.7 | 0.6 | 0.6 | YES | ||||||
| carbofuran | insecticide | 0.41 | 6.2 | 0.4 | 0.91 | 1.2 | YES | ||||||
| chlorothalonil | fungicide | 0.28 | 6.8 | 6.2 | 0.06 | YES | |||||||
| chlorpyrifos | insecticide | 0.06 | 0.73 | 0.108 | 0.03 | 0.1 | 0.041 | 0.083 | 0.01 | YES | YES | YES | |
| clomazone | herbicide | 0.19 | 0.3 | nc | 0.56 | ||||||||
| cyhalothrin | insecticide | 0.03 | 0.025 | nc | 0.0003 | YES | |||||||
| diazinon | insecticide | 0.28 | 40 | 0.2 | 0.037 | 0.17 | 0.17 | 0.01 | YES | YES | |||
| difenoconazole | fungicide | 0.15 | 1.38 | 100.9 | 0.76 | 7.8 | |||||||
| dimethoate | insecticide | 0.08 | 0.9 | 1.25 | 0.07 | 0.7 | 0.15 | YES | YES | ||||
| diuron | herbicide | 0.43 | 24 | 2.6 | 0.2 | 1.8 | 0.2 | YES | YES | YES | |||
| endosulfan-a | insecticide | 0.03 | 0.03 | nc | 0.005 | 0.01 | 0.056 | 0.22 | 0.2 | YES | YES * | YES | |
| epoxiconazole | fungicide | 0.19 | 2 | nc | 0.19 | 1.8 | YES | ||||||
| ethoprophos | insecticide | 0.15 | 2.7 | 3.1 | 0.063 | YES | |||||||
| fenamiphos | insecticide | 0.29 | 8.3 | 0.8 | 0.012 | 0.027 | YES | ||||||
| fenpropimorf | fungicide | 0.06 | 0.4 | nc | 0.22 | ||||||||
| fluopyram | fungicide | 0.16 | 0.78 | nc | 2.7 | 32 | |||||||
| flutolanil | fungicide | 0.24 | 18 | nc | 22 | ||||||||
| hexachlorobenzene | fungicide | 0.01 | 0.02 | nc | - | 0.05 | 0.1 | YES | YES * | YES | |||
| hexazinone | herbicide | 0.22 | 7 | 6.1 | 0.56 | ||||||||
| imazalil | fungicide | 0.38 | 1.01 | nc | 0.87 | YES | |||||||
| imidacloprid | insecticide | 0.35 | 0.35 | 0.52 | 0.0083 | 0.2 | YES | ||||||
| lindane | insecticide | 0.04 | 0.08 | nc | 0.02 | 0.04 | - | 0.95 | 0.2 | YES | |||
| linuron | herbicide | 0.025 | 0.025 | nc | 0.17 | 0.29 | YES | ||||||
| metalaxyl | fungicide | 0.08 | 0.36 | 5530 | 46 | ||||||||
| myclobutanil | fungicide | 0.09 | 0.6 | nc | 55 | ||||||||
| oxamyl | insecticide | 0.06 | 0.06 | nc | 1.8 | YES | |||||||
| oxyfluorfen | herbicide | 0.05 | 0.15 | 0.5 | YES | ||||||||
| parathion-methyl | insecticide | 0.08 | 0.09 | nc | 11 | YES | |||||||
| pencycuron | fungicide | 1.97 | 3.9 | nc | 2.7 | ||||||||
| pendimethalin | herbicide | 0.14 | 0.14 | 3.26 | 0.018 | 0.024 | YES | ||||||
| permethrin | insecticide | 0.18 | 0.4 | nc | 0.0003 | YES | |||||||
| phorate | insecticide | 0.03 | 0.05 | nc | 0.00017 | YES | YES | ||||||
| piperonyl butoxide | insecticide | 0.17 | 0.17 | nc | |||||||||
| prochloraz | fungicide | 0.28 | 0.4 | nc | 1.3 | ||||||||
| profenofos | insecticide | 0.13 | 0.2 | nc | 0.00003 | 0.02 | YES | ||||||
| propanil | herbicide | 0.025 | 0.025 | 12 | 0.07 | ||||||||
| propiconazole | fungicide | 0.10 | 1 | 386.8 | 10 | YES | |||||||
| prothiofos | insecticide | 0.06 | 0.22 | nc | YES | ||||||||
| pyrimethanil | fungicide | 0.10 | 0.81 | 1740 | 7 | 33 | |||||||
| quintozene (PCNB) | fungicide | 0.09 | 1 | nc | 3.1 | ||||||||
| spiroxamine | fungicide | 0.05 | 0.05 | nc | 0.002 | ||||||||
| tebuconazole | fungicide | 0.10 | 1.2 | 848.1 | 0.63 | 14 | YES | ||||||
| terbufos | insecticide | 0.03 | 0.5 | 0.1 | 0.00003 | YES | |||||||
| terbuthylazine | herbicide | 0.03 | 0.04 | 5.74 | 0.2 | 1.3 | |||||||
| terbutryn | herbicide | 0.10 | 2.9 | 5.4 | 0.065 | 0.34 | YES | ||||||
| thiabendazole | fungicide | 0.28 | 1.2 | nc | 3.3 | YES | |||||||
| thiametoxan | insecticide | 0.025 | 0.025 | nc | 0.14 | YES | |||||||
| triadimefon | fungicide | 0.28 | 0.6 | 754.3 | 0.91 | ||||||||
| triadimenol | fungicide | 0.17 | 0.31 | 2160 | 3.2 | YES | |||||||
| triazophos | insecticide | 0.03 | 0.5 | nc | 0.001 | 0.02 | YES | ||||||
| trifloxystrobin | fungicide | 0.08 | 0.08 | nc | 0.27 | 0.81 |
| Active Ingredient | Biocide Action | MoA * | Algae | Aquatic Plants | Primary Producers | Insects | Crustaceans | Arthropods | Fish | Fish and Arthropods |
|---|---|---|---|---|---|---|---|---|---|---|
| metalaxyl | fungicide | FA1 | 1 | 1 | 1 | |||||
| carbendazim | fungicide | FB1 | 2 | |||||||
| thiabendazole | fungicide | FB1 | 2a | |||||||
| flutolanil | fungicide | FC2 | 3 | 3 | ||||||
| azoxystrobin | fungicide | FC3 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| trifloxystrobin | fungicide | FC3 | 4 | |||||||
| pyrimethanil | fungicide | FD1 | 5 | 5 | 5 | |||||
| quintozene (PCNB) | fungicide | FF3 | 6 | |||||||
| difenoconazole | fungicide | FG1 | 7 | 7b | 7 | 7 | ||||
| imazalil | fungicide | FG1 | 7a | |||||||
| myclobutanil | fungicide | FG1 | 7a | 7b | ||||||
| propiconazole | fungicide | FG1 | 7a | 7a | 7b | 7a | ||||
| tebuconazole | fungicide | FG1 | 7a | 7a | 7b | 7a | ||||
| triadimefon | fungicide | FG1 | 7b | 7b | ||||||
| triadimenol | fungicide | FG1 | 7b | 7a | 7c | |||||
| spiroxamine | fungicide | FG2 | 8 | 8 | ||||||
| chlorothalonil | fungicide | FM | 9 | 9 | 9 | 9 | 9 | 9 | 9 | |
| clomazone | herbicide | H13 | 10 | 10 | 10 | |||||
| oxyfluorfen | herbicide | H14 | 11 | 11 | ||||||
| butachlor | herbicide | H15 | 12 | 12 | 12 | 12 | 12 | 12 | ||
| pendimethalin | herbicide | H3 | 13 | 13 | 13 | 13 | 13 | |||
| ametryn | herbicide | H5 | 14 | 14 | 14a | 14a | 14 | 14b | ||
| atrazine | herbicide | H5 | 14a | 14 | 14a | 14c | 14 | |||
| bromacil | herbicide | H5 | 14 | 14a | 14 | 14 | 14 | |||
| diuron | herbicide | H5 | 14a | 14b | 14 | 14a | 14a | 14a | 14b | |
| hexazinone | herbicide | H5 | 14 | 14 | 14 | 14 | 14b | 14a | ||
| linuron | herbicide | H5 | 14 | 14b | 14 | 14a | ||||
| propanil | herbicide | H5 | 14a | 14 | 14a | 14a | 14 | 14 | ||
| terbuthylazine | herbicide | H5 | 14 | 14 | 14a | 14d | ||||
| terbutryn | herbicide | H5 | 14a | 14 | 14b | 14b | 14c | |||
| bentazon | herbicide | H6 | 15 | 15 | ||||||
| buprofezin | insecticide | I16 | 16 | |||||||
| carbaryl | insecticide | I1A | 17 | 17 | 17a | |||||
| carbofuran | insecticide | I1A | 17a | 17 | 17 | 17a | 17a | 17 | ||
| oxamyl | insecticide | I1A | 17 | 17a | 17 | 17 | 17 | 17 | ||
| cadusafos | insecticide | I1B | 18 | 18 | ||||||
| chlorpyrifos | insecticide | I1B | 18b | 18 | 18a | 18b | ||||
| diazinon | insecticide | I1B | 18 | 18b | 18 | 18a | 18a | 18 | ||
| dimethoate | insecticide | I1B | 18a | 18a | 18a | 18b | 18b | 18b | ||
| ethoprophos | insecticide | I1B | 18 | 18 | ||||||
| fenamiphos | insecticide | I1B | 18 | 18 | 18 | |||||
| parathion-methyl | insecticide | I1B | 18b | 18 | 18 | 18a | ||||
| phorate | insecticide | I1B | 18 | 18b | 18b | 18b | 18 | |||
| profenophos | insecticide | I1B | 18a | 18a | 18b | 18 | 18 | |||
| terbufos | insecticide | I1B | 18a | 18a | 18 | 18 | ||||
| triazophos | insecticide | I1B | 18b | 18 | ||||||
| endosulfan-a | insecticide | I2A | 19 | 19 | 19 | 19a | ||||
| lindane | insecticide | I2A | 19 | 19 | 19 | 19a | 19 | 19a | 19 | |
| a-cypermethrin | insecticide | I3A | 20 | 20 | 20 | 20 | 20 | 20a | 20 | |
| cyhalothrin | insecticide | I3A | 20a | 20 | 20 | 20b | ||||
| permethrin | insecticide | I3A | 20 | 20a | 20 | 20 | 20a | |||
| imidacloprid | insecticide | I4A | 21 | |||||||
| thiametoxam | insecticide | I4A | 21 | 21 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría-Sáenz, S.; Spínola-Parallada, M.; Soto, A.C. Pesticides Burden in Neotropical Rivers: Costa Rica as a Case Study. Molecules 2021, 26, 7235. https://doi.org/10.3390/molecules26237235
Echeverría-Sáenz S, Spínola-Parallada M, Soto AC. Pesticides Burden in Neotropical Rivers: Costa Rica as a Case Study. Molecules. 2021; 26(23):7235. https://doi.org/10.3390/molecules26237235
Chicago/Turabian StyleEcheverría-Sáenz, Silvia, Manuel Spínola-Parallada, and Ana Cristina Soto. 2021. "Pesticides Burden in Neotropical Rivers: Costa Rica as a Case Study" Molecules 26, no. 23: 7235. https://doi.org/10.3390/molecules26237235
APA StyleEcheverría-Sáenz, S., Spínola-Parallada, M., & Soto, A. C. (2021). Pesticides Burden in Neotropical Rivers: Costa Rica as a Case Study. Molecules, 26(23), 7235. https://doi.org/10.3390/molecules26237235






