Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae
Abstract
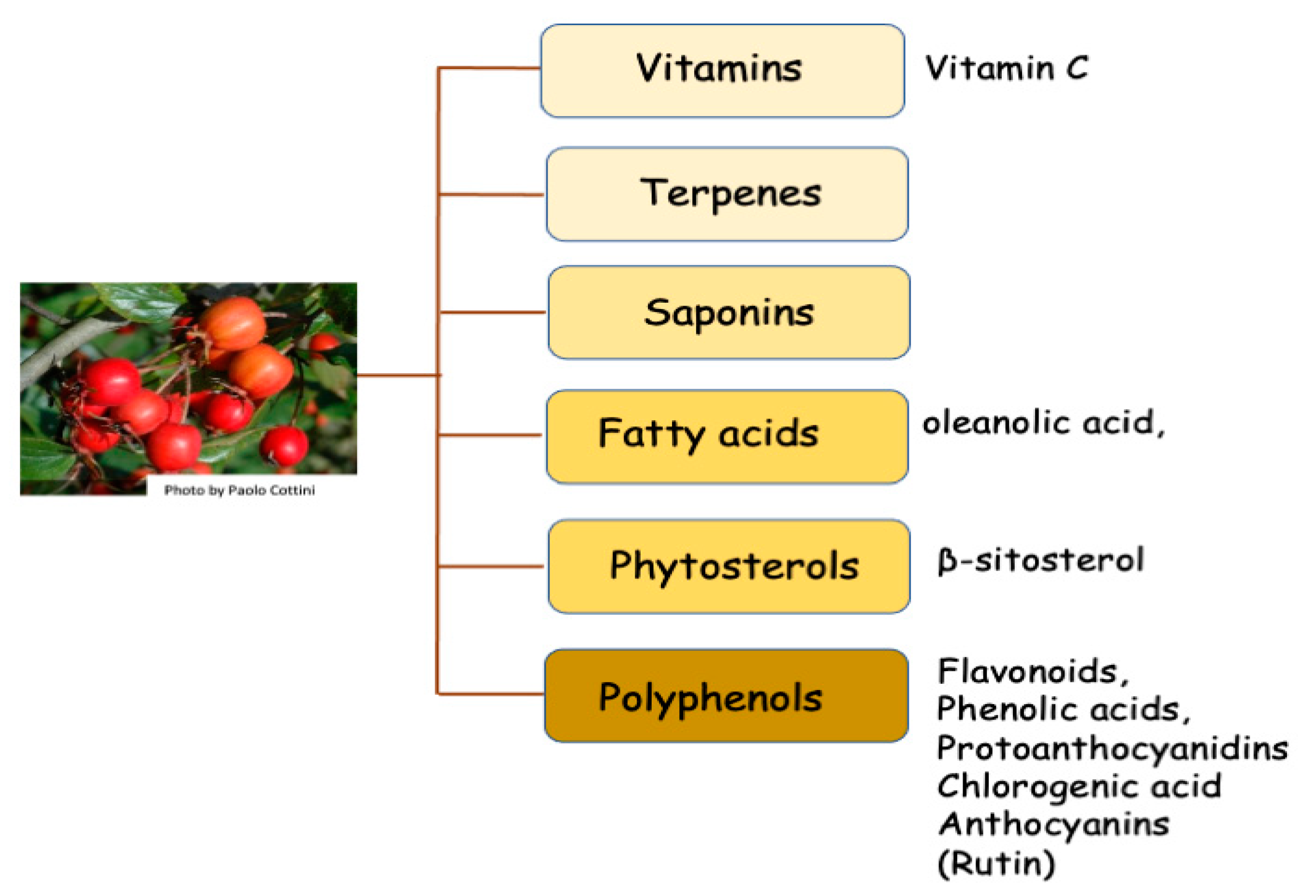
:1. Botanical Aspects
2. Genetic Characterization
3. Phytochemical Components
4. Antioxidant Properties of Hawthorn Extracts
5. Pharmaceutical Properties
5.1. Protective against Cardiac Diseases
5.2. Protection against Atherosclerosis and Cholesterolemia
5.3. Anti-Microbial Activity
5.4. Hepatoprotective Effects
5.5. Protection against Neurological Disorders
5.6. Anti-Anxiety
5.7. Against Dermatitis
5.8. Anti-Cancer
5.9. Gastroprotective Effects
5.10. Anti-Diabetes
5.11. Protective against Renal Diseases
5.12. Genotoxic Effects
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gundogdu, M.; Ozrenk, K.; Ercisli, S.; Kan, T.; Kodad, O.; Hegedus, A. Organic acids, sugars, vitamin C content and some pomological characteristics of eleven hawthorn species (Crataegus spp.) from Turkey. Biol. Res. 2014, 47, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Phipps, J.B. Crataegus—A nomenclator for sectional and serial names. Taxon 1983, 32, 598–604. [Google Scholar] [CrossRef]
- Phipps, J.B.; Robertson, K.R.; Rohrer, J.R.; Smith, P.G. Origins and Evolution of Subfam. Maloideae (Rosaceae). Syst. Botany 1991, 16, 303. [Google Scholar] [CrossRef]
- Campbell, C.S.; Dickinson, T.A. Apomixis, Patterns of Morphological Variation, and Species Concepts in subfam. Maloideae (Rosaceae). Syst. Botany 1990, 15, 124. [Google Scholar] [CrossRef]
- Christensen, K.I. Revision of Crataegus Sect. Crataegus and Nothosect. Crataeguineae (Rosaceae-Maloideae) in the Old World. Syst. Botany Monogr. 1992, 35, 1. [Google Scholar] [CrossRef]
- Attard, E.; Attard, H. Hawthorn: Crataegus oxyacantha, Crataegus monogyna and related species. Nonvit. Nonminer. Nutr. Suppl. 2019, 2019, 289–293. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. In Plant Systematics and Evolution; Springer: Berlin/Heidelberg, Germany, 2007; Volume 266, pp. 5–43. [Google Scholar]
- Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.F.; Cantino, P.D. Plant systematics. A phylogenetic approach. Syst. Biol. 1999, 48, 826–828. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.M.; Han, S.; Stefanović, S.; Dickinson, T.A. Widespread generalist clones are associated with range and niche expansion in allopolyploids of Pacific Northwest Hawthorns (Crataegus L.). Mol. Ecol. 2017, 26, 5484–5499. [Google Scholar] [CrossRef] [PubMed]
- Phipps, J.B.; O’Kennon, R.; Lance, R.W. Hawthorns and Medlars; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Benabderrahmane, W.; Lores, M.; Benaissa, O.; Lamas, J.P.; de Miguel, T.; Amrani, A.; Benayache, F.; Benayache, S. Polyphenolic content and bioactivities of Crataegus oxyacantha L. (Rosaceae). Nat. Prod. Res. 2021, 35, 627–632. [Google Scholar] [CrossRef]
- Orhan, I.E. Phytochemical and Pharmacological Activity Profile of Crataegus oxyacantha L. (Hawthorn)—A Cardiotonic Herb. Curr. Med. Chem. 2016, 25, 4854–4865. [Google Scholar] [CrossRef]
- Ahmad, S.D.; Sabir, S.M.; Lodhi, N.A. Morphological and biochemical comparison of Hippophae rhamnoides, Elaeagnus umbellata and Crataegus oxyacantha intra- and interspecifically. S. Afr. J. Botany 2005, 71, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Cui, Y.; Wang, Q.; Xu, Z.; Wang, Y.; Lin, Y.; Song, J.; Yao, H. Identification and phylogenetic analysis of five Crataegus species (Rosaceae) based on complete chloroplast genomes. Planta 2021, 254, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sydora, N. Morphological and taxonomic study of oxyacanthae Zbl. section of crataegus L. genus by vegetative characteristics. ScienceRise Pharm. Sci. 2018, 1, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Dönmez, A.A.; Özderin, S. Additional contributions to taxonomy, nomenclature and biogeography of the Turkish Crataegus (Rosaceae) taxa. PhytoKeys 2019, 122, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönmez, A.A. Taxonomic notes on the genus Crataegus (Rosaceae) in Turkey. Botan. J. Linn. Soc. 2007, 155, 231–240. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Brasil. Farmacog. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Ferrazzini, D.; Monteleone, I.; Belletti, P. Small-scale genetic diversity in oneseed hawthorn (Crataegus monogyna Jacq.). Eur. J. Forest Res. 2008, 127, 407–414. [Google Scholar] [CrossRef]
- Rajeb, C.; Messaoud, C.; Chograni, H.; Bejaoui, A.; Boulila, A.; Rejeb, M.N.; Boussaid, M. Genetic diversity in Tunisian Crataegus azarolus L. var. aronia L. populations assessed using RAPD markers. Ann. Forest Sci. 2010, 67, 512. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, K.U.; Yanar, M.; Ercisli, S.; Sahiner, H.; Taskin, T.; Zengin, Y. Genetic relationships among some hawthorn (Crataegus spp.) species and genotypes. Biochem. Genet. 2010, 48, 873–878. [Google Scholar] [CrossRef]
- Serçe, S.; Şimşek, Ö.; Toplu, C.; Kamiloǧlu, Ö.; Çalişkan, O.; Gündüz, K.; Özgen, M.; Kaçar, Y.A. Relationships among Crataegus accessions sampled from Hatay, Turkey, as assessed by fruit characteristics and RAPD. Genet. Resour. Crop Evol. 2011, 58, 933–942. [Google Scholar] [CrossRef]
- Khiari, S.; Boussaid, M.; Messaoud, C. Genetic diversity and population structure in natural populations of Tunisian Azarole (Crataegus azarolus L. var. aronia) assessed by microsatellite markers. Biochem. Syst. Ecol. 2015, 59, 264–270. [Google Scholar] [CrossRef]
- Güney, M.; Kafkas, S.; Keles, H.; Aras, S.; Ercişli, S. Characterization of hawthorn (Crataegus spp.) genotypes by SSR markers. Physiol. Mol. Biol. Plants 2018, 24, 1221–1230. [Google Scholar] [CrossRef]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.M.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.V.; Santos-Buelga, C.; Ferreira, I.C.F.R. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Portnaya, I.; Cogan, U.; Azaizeh, H.; Bomzon, A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J. Ethnopharmacol. 2005, 101, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 2572-0406. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef]
- Cui, T.; Li, J.Z.; Kayahara, H.; Ma, L.; Wu, L.X.; Nakamura, K. Quantification of the polyphenols and triterpene acids in Chinese hawthorn fruit by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef]
- Mehdadi, Z.; Hamdaoui, M.; Latreche, A. Evaluation of Parietal Polysaccharides and Polyphenols of Crataegus monogyna Jacq. and Valorization Prospects. In Proceedings of the International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2015), Phuket, Thailand, 7–8 April 2015. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Glew, R.H.; Huang, H.S.; Chuang, L.T.; VanderJagt, D.J.; Strnad, M. Evolution of fatty acids in medlar (Mespilus germanica L.) mesocarp at different stages of ripening. Grasas y Aceites 2002, 53, 352–356. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Huang, H.S.; Chuang, L.T.; Vanderjagt, D.J.; Glew, R.H. Fatty acid composition of medlar (Mespilus germanica L.) fruit at different stages of development. Italian J. Food Sci. 2002, 14, 439–446. [Google Scholar]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; VanderJagt, D.J.; Huang, H.S.; Chuang, L.T.; Strnad, M. Effect of postharvest period on sugars, organic acids and fatty acids composition in commercially sold medlar [Mespilus germanica ’Dutch’) fruit. Eur. Food Res. Technol. 2003, 216, 390–394. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus monogyna subsp. monogyna jacq.). J. Int. Ethnopharmacol. 2014, 3, 51. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Rad. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yen, G.C. Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J. Agric. Food Chem. 2003, 51, 1474–1479. [Google Scholar] [CrossRef]
- Issaadi, O.; Fibiani, M.; Picchi, V.; Scalzo, R.L.; Madani, K. Phenolic composition and antioxidant capacity of hawthorn (Crataegus oxyacantha L.) flowers and fruits grown in Algeria. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venskutonis, P.R. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): Review of recent research advances. J. Food Bioact. 2018, 4, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Maleki, H.H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef] [Green Version]
- Velickovic, J.M.; Ilic, S.; Mitic, S.S.; Mitic, M.N.; Kostic, D.A. Comparative Analysis of Phenolic and Mineral Composition of Hawthorn and Blackthorn from Southeast Serbia. Oxidat. Commun. 2016, 39, 2280–2290. [Google Scholar]
- García-Mateos, R.; Aguilar-Santelises, L.; Soto-Hernández, M.; Nieto-Angel, R. Flavonoids and antioxidant activity of flowers of Mexican Crataegus spp. Nat. Prod. Res. 2013, 27, 834–836. [Google Scholar] [CrossRef]
- Khokhlova, K.; Zdoryk, O.; Vyshnevska, L. Chromatographic characterization on flavonoids and triterpenes of leaves and flowers of 15 crataegus L. species. Nat. Prod. Res. 2020, 34, 317–322. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Kartal, M.; Özdeveci, B.; Duman, H. HPLC Quantification of Vitexine-2″-O-rhamnoside and Hyperoside in Three Crataegus Species and Their Antimicrobial and Antiviral Activities. Chromatographia 2007, 66, 153–157. [Google Scholar] [CrossRef]
- Bilia, A.R.; Eterno, F.; Bergonzi, M.C.; Mazzi, G.; Vincieri, F.F. Evaluation of the content and stability of the constituents of mother tinctures and tinctures: The case of Crataegus oxyacantha L. and Hieracium pilosella L. J. Pharm. Biomed. Anal. 2007, 44, 70–78. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.; Mokdad-Bzéouich, I.; Maatouk, M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Antitumoral, antioxidant, and antimelanogenesis potencies of Hawthorn, a potential natural agent in the treatment of melanoma. Melanoma Res. 2016, 26, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Rad. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keser, S.; Celik, S.; Turkoglu, S.; Yılmaz, Ö.; Turkoglu, I. Hydrogen Peroxide Radical Scavenging and Total Antioxidant Activity of Hawthorn. Chem. J. 2012, 2, 9–12. [Google Scholar]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and oxidative stress in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, A.; Pintaudi, A.M.; Traina, A.; Carruba, G.; Attanzio, A.; Gentile, C.; Tesoriere, L.; Livrea, M.A. Raman Spectroscopic Measurements of Dermal Carotenoids in Breast Cancer Operated Patients Provide Evidence for the Positive Impact of a Dietary Regimen Rich in Fruit and Vegetables on Body Oxidative Stress and BC Prognostic Anthropometric Parameters: A Five-Year Study. Oxidat. Med. Cell. Long. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Peluso, I.; Raguzzini, A.; Catasta, G.; Cammisotto, V.; Perrone, A.; Tomino, C.; Toti, E.; Serafini, M. Effects of high consumption of vegetables on clinical, immunological, and antioxidant markers in subjects at risk of cardiovascular diseases. Oxid. Med. Cell. Long. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.S.; Hasanzadeh, S. Crataegus monogyna fruit aqueous extract as a protective agent against doxorubicin-induced reproductive toxicity in male rats. Avicen. J. Phytomed. 2013, 3, 159. [Google Scholar]
- Lucconi, G.; Chlapanidas, T.; Martino, E.; Gaggeri, R.; Perteghella, S.; Rossi, D.; Faragò, S.; Vigo, D.; Faustini, M.; Collina, S.; et al. Formulation of microspheres containing Crataegus monogyna Jacq. extract with free radical scavenging activity. Pharm. Dev. Technol. 2014, 19, 65–72. [Google Scholar] [CrossRef]
- Deveci, E.; Cayan, G.T.; Karakurt, S.; Duru, M.E. Antioxidant, Cytotoxic, and Enzyme Inhibitory Activities of Agropyron repens and Crataegus monogyna Species. Eur. J. Biol. 2020, 79, 98–105. [Google Scholar] [CrossRef]
- Kostić, D.A.; Velicković, J.M.; Mitić, S.S.; Mitic, M.N.; Randelović, S.S. Phenolic content, and antioxidant and antimicrobial activities of Crataegus oxyacantha L (Rosaceae) fruit extract from Southeast Serbia. Trop. J. Pharm. Res. 2012, 11, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Barreira, J.C.M.; Rodrigues, S.; Carvalho, A.M.; Ferreira, I.C.F.R. Development of hydrosoluble gels with Crataegus monogyna extracts for topical application: Evaluation of antioxidant activity of the final formulations. Ind. Crops Prod. 2013, 42, 175–180. [Google Scholar] [CrossRef]
- Stelmakiene, A.; Ramanauskiene, K.; Petrikaite, V.; Jakstas, V.; Briedis, V. Application of dry hawthorn (Crataegus oxyacantha L.) extract in natural topical formulations. Acta Poloniae Pharm.—Drug Res. 2016, 73, 955–965. [Google Scholar]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Sokół-Łetowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef]
- Magnus, S.; Gazdik, F.; Anjum, N.A.; Kadlecova, E.; Lackova, Z.; Cernei, N.; Brtnicky, M.; Kynicky, J.; Klejdus, B.; Necas, T.; et al. Assessment of antioxidants in selected plant rootstocks. Antioxidants 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.Y.; Lee, M.J.; Liao, C.L.; Lin, W.L.; Yin, Y.F.; Tseng, T.H. Inhibitory Effect of Hot-Water Extract from Dried Fruit of Crataegus pinnatifida on Low-Density Lipoprotein (LDL) Oxidation in Cell and Cell-Free Systems. J. Agric. Food Chem. 2003, 51, 7583–7588. [Google Scholar] [CrossRef]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Yin, Y.F.; Wang, C.P.; Tseng, T.H. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) procyanidins microcapsules with inulin and maltodextrin. J. Sci. Food Agric. 2017, 97, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, P.; Ficarra, R.; De Pasquale, A.; Monforte, M.T.; Calabro, M.L. High-performance liquid chromatography of flavonoids in Crataegus oxyacantha L. IV-reversed-phase high-pressure liquid chromatography in flower, leaf and bud extractives of Crataegus oxyacantha L. Farmaco 1990, 45, 247–255. [Google Scholar]
- Ruiz-Rodríguez, B.M.; de Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; Sánchez-Mata, M.d.C.; Cámara, M.; Tardío, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.L. Anti-microbial and anti-oxidant activities of illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengineered 2013, 4, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.; Jahan, N.; Rahman, K.U.; Zafar, F.; Ashraf, M.Y. Synergistic interactions of polyphenols & their effect on antiradical Potential. Pakistan J. Pharm. Sci. 2017, 30, 1297–1304. [Google Scholar]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Djeddi, S.; Boutaleb, H. Evaluation of antioxidative and antibacterial potentials of Crataegus monogyna Jacq. from Mahouna mountain (Algeria). J. Adv. Sci. Appl. Eng. 2015, 1, 60–63. [Google Scholar]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Szczypka, M.; Suszko-Pawłowska, A.; Sokół-ŁĘtowska, A.; Kucharska, A.; Obmińska-Mrukowicz, B. Hawthorn (Crataegus monogyna) Phenolic Extract Modulates Lymphocyte Subsets and Humoral Immune Response in Mice. Planta Med. 2020, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Predescu, C.N.; Tudoreanu, L.; Nicorescu, V.; Gâjâilă, I. Comparative study of the influence of hawthorn (Crataegus monogyna) berry ethanolic extract and butylated hydroxylanisole (BHA) on lipid peroxidation, myoglobin oxidation, consistency and firmness of minced pork during refrigeration. J. Sci. Food Agric. 2018, 98, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Chopdat, F.I. The Efficacy of Crataegus Oxyacantha ⊖ on Refractory Hypertension in Males. Ph.D. Thesis, University of Witwatersrand, Johannesburg, South Africa, 2007. [Google Scholar]
- Li, F.; Yuan, Q.; Rashid, F. Isolation, purification and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifida Bge. Carbohydrate Polym. 2009, 78, 80–88. [Google Scholar] [CrossRef]
- Kanyonga, M.P.; Faouzi, M.Y.A.; Zellou, A.; Essassi, M.; Cherrah, Y. Effects of methanolic extract of Crataegus oxyacantha on blood homeostasis in rat. J. Chem. Pharm. Res. 2011, 3, 713–717. [Google Scholar]
- Akila, M.; Devaraj, H. Synergistic effect of tincture of Crataegus and Mangifera indica L. extract on hyperlipidemic and antioxidant status in atherogenic rats. Vasc. Pharmacol. 2008, 49, 173–177. [Google Scholar] [CrossRef]
- Shanthi, S.; Parasakthy, K.; Deepalakshmi, P.D.; Devaraj, S.N. Hypolipidemic activity of tincture of Crataegus in rats. Indian J. Biochem. Biophys. 1994, 31, 143–146. [Google Scholar]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef]
- Zick, S.M.; Gillespie, B.; Aaronson, K.D. The effect of Crataegus oxycantha special extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. Eur. J. Heart Failure 2008, 10, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenring, F.H.; Suter, A.; Weber, M.; Saller, R. A randomised double blind placebo controlled clinical trial of a standardised extract of fresh Crataegus berries (Crataegisan®) in the treatment of patients with congestive heart failure NYHA II. Phytomedicine 2003, 10, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr. Med. Res. Opin. 2004, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, R.; Thirupurasundari, C.J.; Devaraj, S.N. Pretreatment with alcoholic extract of shape Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol—Induced myocardial infarction in rats. Mol. Cell. Biochem. 2006, 292, 59–67. [Google Scholar] [CrossRef]
- Long, S.R.; Carey, R.A.; Crofoot, K.M.; Proteau, P.J.; Filtz, T.M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 2006, 13, 643–650. [Google Scholar] [CrossRef]
- Jayachandran, K.S.; Khan, M.; Selvendiran, K.; Devaraj, S.N.; Kuppusamy, P. Crataegus oxycantha extract attenuates apoptotic incidence in myocardial ischemia-reperfusion injury by regulating akt and Hif-1 signaling pathways. J. Cardiovasc. Pharmacol. 2010, 56, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Elango, C.; Devaraj, S.N. Immunomodulatory effect of Hawthorn extract in an experimental stroke model. J. Neuroinflam. 2010, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xiong, X.; Feng, B. Effect of crataegus usage in cardiovascular disease prevention: An evidence-based approach. Evid.-based Complement. Alternat. Med. 2013, 2013, 149363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.T.; Dao, J.; Shao, Z.H. Hawthorn: Potential roles in cardiovascular disease. Am. J. Chinese Med. 2005, 33, 1–10. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Trumbeckaite, S.; Majiene, D.; Baniene, R.; Baliutyte, G.; Savickas, A.; Toleikis, A. The effect of crataegus fruit extract and some of its flavonoids on mitochondrial oxidative phosphorylation in the heart. Phytother. Res. 2009, 23, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- Jayalakshmi, R.; Devaraj, S.N. Cardioprotective effect of tincture of Crataegus on isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2010, 56, 921–926. [Google Scholar] [CrossRef]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn extract for treating chronic heart failure: Meta-analysis of randomized trials. Am. J. Med. 2003, 114, 665–674. [Google Scholar] [CrossRef]
- Veveris, M.; Koch, E.; Chatterjee, S.S. Crataegus special extract WS® 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004, 74, 1945–1955. [Google Scholar] [CrossRef]
- Zhang, Z.; Ho, W.K.K.; Huang, Y.; Anthony, E.J.; Lam, L.W.; Chen, Z.Y. Hawthorn fruit is hypolipidemic in rabbits fed a high cholesterol diet. J. Nutr. 2002, 132, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef]
- Mecheri, A.; Benabderrahmane, W.; Amrani, A.; Boubekri, N.; Benayache, F.; Benayache, S.; Zama, D. Hepatoprotective Effects of Algerian Crataegus oxyacantha Leaves. Recent Patents Food Nutr. Agric. 2018, 10, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Lashin, F.M.; Gamel, M.; Hassanin, S.O.; Abdalla, Y.; Amin, A. Hawthorn herbal preparation from crataegus oxyacantha attenuates in vivo carbon tetrachloride-induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Muhammad, S.; Shah, M.R.; Khan, A.; Rashid, U.; Farooq, U.; Ullah, F.; Sadiq, A.; Ayaz, M.; Ali, M.; et al. Neurologically potent molecules from Crataegus oxyacantha; isolation, anticholinesterase inhibition, and molecular docking. Front. Pharmacol. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, M.; Slama-Ben Salem, R.; Ben Salem, M.; Brahmi, N.; Badraoui, R.; Nasri, M.; El Feki, A. Beneficial effects of crataegus oxyacantha extract on neurobehavioral deficits and brain tissue damages induced by an insecticide mixture of deltamethrin and chlorpyrifos in adult wistar rats. Biomed. Pharmacother. 2019, 114, 108795. [Google Scholar] [CrossRef] [PubMed]
- Zeouk, I.; Balouiri, M.; Bekhti, K. Antistaphylococcal Activity and Phytochemical Analysis of Crude Extracts of Five Medicinal Plants Used in the Center of Morocco against Dermatitis. Int. J. Microbiol. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Belšcak-Cvitanovic, A.; Durgo, K.; Bušić, A.; Franekić, J.; Komes, D. Phytochemical attributes of four conventionally extracted medicinal plants and cytotoxic evaluation of their extracts on human laryngeal carcinoma (HEp2) Cells. J. Med. Food 2014, 17, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Jouad, H.; Lemhadri, A.; Maghrani, M.; Burcelin, R.; Eddouks, M. Hawthorn Evokes a Potent Anti-Hyperglycemic Capacity in Streptozotocin-Induced Diabetic Rats. J. Herbal Pharmacother. 2003, 3, 19–29. [Google Scholar] [CrossRef]
- Ranjbar, K.; Zarrinkalam, E.; Salehi, I.; Komaki, A.; Fayazi, B. Cardioprotective effect of resistance training and Crataegus oxyacantha extract on ischemia reperfusion–induced oxidative stress in diabetic rats. Biomed. Pharmacother. 2018, 100, 455–460. [Google Scholar] [CrossRef]
- Masteiková, R.; Klimas, R.; Samura, B.B.; Savickas, A.; Samura, B.A.; Belaij, S.I.; Samura, I.B.; Rabišková, M.; Chalupová, Z.; Bernatoniene, J. An orientational examination of the effects of extracts from mixtures of herbal drugs on selected renal functions. Ceska Slovenska Farm. Casopis Ceske Farmaceut. Spolecn. Sloven. Farmaceut. Spol. 2007, 56, 85–89. [Google Scholar]
- de Quadros, A.P.O.; Mazzeo, D.E.C.; Marin-Morales, M.A.; Perazzo, F.F.; Rosa, P.C.P.; Maistro, E.L. Fruit extract of the medicinal plant Crataegus oxyacantha exerts genotoxic and mutagenic effects in cultured cells. J. Toxicol. Environ. Health—Part A Curr. Issues 2017, 80, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Massoni, G. L’impiego di un estratto di biancospino (Crataegus) nel trattamento di alcune forme di miocardiopatia ischemica dell’età avanzata. Giornale Gerontol. 1968, 16, 979–984. [Google Scholar]
- Hobbs, C.; Foster, S. Hawthorn: A literature review. HerbalGram 1990, 19, 119. [Google Scholar]
- Alp, H.; Soner, B.C.; Baysal, T.; Şahin, A.S. Protective effects of Hawthorn (Crataegus oxyacantha) extract against digoxin-induced arrhythmias in rats. Anatol. J. Cardiol. 2015, 15, 970–975. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Poindexter, B.J.; Bick, R.J.; Dasgupta, A. A comparison of the effects of commercially available hawthorn preparations on calcium transients of isolated cardiomyocytes. J. Med. Food 2008, 11, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Brixius, K.; Willms, S.; Napp, A.; Tossios, P.; Ladage, D.; Bloch, W.; Mehlhorn, U.; Schwinger, R.H.G. Crataegus special extract WS® 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 2006, 20, 177–184. [Google Scholar] [CrossRef]
- Anselm, E.; Socorro, V.F.M.; Dal-Ros, S.; Schott, C.; Bronner, C.; Schini-Kerth, V.B. Crataegus special extract WS 1442 causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of endothelial NO synthase but not via activation of estrogen receptors. J. Cardiovasc. Pharmacol. 2009, 53, 253–260. [Google Scholar] [CrossRef]
- Rieckeheer, E.; Schwinger, R.H.G.; Bloch, W.; Brixius, K. Hawthorn special extract WS® 1442 increases red blood cell NO-formation without altering red blood cell deformability. Phytomedicine 2011, 19, 20–24. [Google Scholar] [CrossRef]
- Bubik, M.F.; Willer, E.A.; Bihari, P.; Jürgenliemk, G.; Ammer, H.; Krombach, F.; Zahler, S.; Vollmar, A.M.; Fürst, R. A novel approach to prevent endothelial hyperpermeability: The Crataegus extract WS® 1442 targets the cAMP/Rap1 pathway. J. Mol. Cell. Cardiol. 2012, 52, 196–205. [Google Scholar] [CrossRef]
- Willer, E.A.; Malli, R.; Bondarenko, A.I.; Zahler, S.; Vollmar, A.M.; Graier, W.F.; Fürst, R. The vascular barrier-protecting hawthorn extract WS 1442 raises endothelial calcium levels by inhibition of SERCA and activation of the IP3 pathway. J. Mol. Cell. Cardiol. 2012, 53, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Idris-Khodja, N.; Auger, C.; Koch, E.; Schini-Kerth, V.B. Crataegus special extract WS® 1442 prevents aging-related endothelial dysfunction. Phytomedicine 2012, 19, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, H.E.; Ryan, D. A study of the comparative effects of Hawthorn fruit compound and Simvastatin on lowering blood lipid levels. Am. J. Chinese Med. 2009, 37, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Littleton, R.M.; Miller, M.; Hove, J.R. Whole plant based treatment of hypercholesterolemia with Crataegus laevigata in a zebrafish model. BMC Complement. Alternat. Med. 2012, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koçyildiz, Z.Ç.; Birman, H.; Olgaç, V.; Akgün-Dar, K.; Melikoǧlu, G.; Meriçli, A.H. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: A morphological study. Phytother. Res. 2006, 20, 66–70. [Google Scholar] [CrossRef]
- Zapfe Jun, G. Clinical efficacy of crataegus extract WS® 1442 in congestive heart failure NYHA class II. Phytomedicine 2001, 8, 262–266. [Google Scholar] [CrossRef]
- Halver, J.; Wenzel, K.; Sendker, J.; García, C.C.; Erdelmeier, C.A.J.; Willems, E.; Mercola, M.; Symma, N.; Könemann, S.; Koch, E.; et al. Crataegus extract WS® 1442 stimulates cardiomyogenesis and angiogenesis from stem cells: A possible new pharmacology for hawthorn? Front. Pharmacol. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M.; et al. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneim.-Forschung/Drug Res. 1996, 46, 1086–1089. [Google Scholar]
- Liperoti, R.; Vetrano, D.L.; Bernabei, R.; Onder, G. Herbal Medications in Cardiovascular Medicine. J. Am. College Cardiol. 2017, 69, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Durán, R.E.; Medrano-Rodríguez, J.C.; Sánchez-Aguilar, M.; Soria-Castro, E.; Rubio-Ruíz, M.E.; Del Valle-Mondragón, L.; Sánchez-Mendoza, A.; Torres-Narvaéz, J.C.; Pastelín-Hernández, G.; Ibarra-Lara, L. Extracts of crataegus oxyacantha and rosmarinus offcinalis attenuate ischemic myocardial damage by decreasing oxidative stress and regulating the production of cardiac vasoactive agents. Int. J. Mol. Sci. 2017, 18, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkov, V. Plants with hypotensive, antiatheromatous and coronarodilatating action. Am. J. Chinese Med. 1979, 7, 197–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid.-Based Complement. Alternat. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef]
- Rajendran, S.; Deepalakshmi, P.D.; Parasakthy, K.; Devaraj, H.; Devaraj, S.N. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis 1996, 123, 235–241. [Google Scholar] [CrossRef]
- Arslan, R.; Bektas, N.; Bor, Z.; Sener, E. Evaluation of the antithrombotic effects of Crataegus monogyna and Crataegus davisii in the carrageenan-induced tail thrombosis model. Pharm. Biol. 2015, 53, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohana, T.; Navin, A.V.; Jamuna, S.; Sakeena Sadullah, M.S.; Niranjali Devaraj, S. Inhibition of differentiation of monocyte to macrophages in atherosclerosis by oligomeric proanthocyanidins -In-vivo and in-vitro study. Food Chem. Toxicol. 2015, 82, 96–105. [Google Scholar] [CrossRef]
- Jalaly, L.; Sharifi, G.; Faramarzi, M.; Nematollahi, A.; Rafieian-Kopaei, M.; Amiri, M.; Moattar, F. Comparison of the effects of Crataegus oxyacantha extract, aerobic exercise and their combination on the serum levels of ICAM-1 and E-Selectin in patients with stable angina pectoris. DARU J. Pharm. Sci. 2015, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Pawlaczyk-Graja, I. Polyphenolic-polysaccharide conjugates from flowers and fruits of single-seeded hawthorn (Crataegus monogyna Jacq.): Chemical profiles and mechanisms of anticoagulant activity. Int. J. Biol. Macromol. 2018, 116, 869–879. [Google Scholar] [CrossRef]
- Remita, F.; Abdennour, C.; Talbi, A.; Khelili, K. The protective effect of Crataegus monogyna Jacq. aqueous extract (fruits and leaves) on blood cells and lipid profile of rats after copper induced-toxicity. Int. J. Minor Fruits Med. Arom. Plants 2021, 7, 19–25. [Google Scholar] [CrossRef]
- Bylka, W.; Matlawska, I.; Pilewski, N. Natural flavonoids as antimicrobial agents. JANA 2004, 7, 24–31. [Google Scholar]
- Saeedi, G.; Jeivad, F.; Goharbari, M.; Gheshlaghi, G.H.; Sabzevari, O. Ethanol Extract of Crataegus Oxyacantha LAmeliorate Dietary Non-Alcoholic Fatty Liver Disease in Rat. Drug Res. 2018, 68, 553–559. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Chahardahcharic, S.V.; Setorki, M. The effect of hydroalcoholic extract of Crataegus monogyna on hyperglycemia, oxidative stress and pancreatic tissue damage in streptozotocin-induced diabetic rats. J. HerbMed Pharmacol. 2018, 7, 294–299. [Google Scholar] [CrossRef]
- Yonekubo, B.T.; Alves, H.d.M.C.; de Souza Marques, E.; Perazzo, F.F.; Rosa, P.C.P.; Gaivão, I.O.d.M.; Maistro, E.L. The genotoxic effects of fruit extract of Crataegus oxyacantha (hawthorn) in mice. J. Toxicol. Environ. Health—Part A Curr. Issues 2018, 81, 974–982. [Google Scholar] [CrossRef]



| Metabolic Compounds or Mixture | Type of Action | Tissue | Type of Evidence (In Vitro, In Vivo, Traditional Medicine) | References |
|---|---|---|---|---|
| Proanthocyanidins | Cardiovascular activity | Leaves, fruits, flowers | In vitro, traditional medicine, in vivo | [94,95,96] |
| Proanthocyanidins | Apoptosis inhibition | Leaves, fruits, flowers | In vitro | [97] |
| Different plant extracts | Anti-inflammatory effects | Berries, leaves | In vivo | [96] |
| Different plant extracts | Myocardial mitochondrial activity | Flowers, leaves | In vitro, in vivo | [90] |
| Different plant extracts | Mitochondrial antioxidant activity | Flowers, leaves | In vitro | [90] |
| Different plant extracts | Protective against Class II and III heart failure | Flower and leaf extracts | In vitro, in vivo | [98] |
| Different plant extracts | Protective against hypertension, heart and digestive disorders | Berries, flowers and leaves | In vitro, in vivo | [67] |
| Different plant extracts | Dilatation of peripheral blood vessels and coronary vessels | Leaves and berries | In vivo | [99] |
| Different plant extracts | Hypo-lipidaemic, anti-inflammatory, antianxiety | Leaves and berries | In vivo | [87] |
| Fruits Fruits extracts | Anti-ipercholesterolemia Lipid-lowering and hypoglycemic effects (rats) | Fruits Fruits | In vivo In vivo | [100] [101] |
| Flavonoids, polyphenols, phenolic acids Flavonoids, polyphenols, phenolic acids (different solvents) | Anti-microbial action Anti-microbial action | Leaves and berries, Leaves and berries | In vivo In vitro In vitro | [74] [13] [13] |
| Fruits | Anti-microbial action | Fruits | In vivo | [61] |
| Leaf extracts | Hepatoprotective effects | Leaves | In vivo | [102] |
| Chlorogenic acid, rutin, epicatechin, vitexin- and quercetin | Protective against hepatic fibrosis | Leaves | In vivo | [103] |
| β-Sitosterol-3-O-β-D-Glucopyranoside, lupeol, β-sitosterol, betulin, betulinic acid, oleanolic acid and chrysin Different extracts | Protective against neurological disorders Protective effects again brain damage by pesticide (wistar rats) | Leaves Different parts of plant | In vivo In vivo Ex vivo In vitro | [104] [105] |
| Different extracts | Anti-anxiety | Leaves, fruits | In vivo | [89] |
| Different extracts | Against dermatitis | Leaves, fruits | In vivo | [106] |
| Different extracts | Anti-cancer | Leaves, fruits | In vitro | [107] |
| Different extracts | Gastroprotective effects | Leaves, fruits | In vivo | [67] |
| Leaf extracts Different extracts | Anti-diabetes Anti-diabetes effects (diabetic rats) | Leaves | In vivo In vivo | [108] [109] |
| Fruit extracts Fruit extracts | Protective against renal diseases Cytotoxic and genotoxic effects | Fruits Fruits | In vivo In vitro | [110] [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. https://doi.org/10.3390/molecules26237266
Martinelli F, Perrone A, Yousefi S, Papini A, Castiglione S, Guarino F, Cicatelli A, Aelaei M, Arad N, Gholami M, et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules. 2021; 26(23):7266. https://doi.org/10.3390/molecules26237266
Chicago/Turabian StyleMartinelli, Federico, Anna Perrone, Sanaz Yousefi, Alessio Papini, Stefano Castiglione, Francesco Guarino, Angela Cicatelli, Mitra Aelaei, Neda Arad, Mansour Gholami, and et al. 2021. "Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae" Molecules 26, no. 23: 7266. https://doi.org/10.3390/molecules26237266
APA StyleMartinelli, F., Perrone, A., Yousefi, S., Papini, A., Castiglione, S., Guarino, F., Cicatelli, A., Aelaei, M., Arad, N., Gholami, M., & Salami, S. A. (2021). Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules, 26(23), 7266. https://doi.org/10.3390/molecules26237266












