1. Introduction
Dysfunction of neuronal α4β2* nicotinic cholinergic receptors (nAChRs) has been implicated in numerous pathologies, including Parkinson’s disease (PD). Due to this clinical importance of α4β2* nAChRs, evaluating these receptors can potentially assist in the treatment of these human diseases. In order to image these receptors using positron emission tomography (PET) and single photon emission computed tomography (SPECT), we developed several α4β2* nAChRs fluorine-18 and iodine-123 labeled radiotracers (
Figure 1). These include 5-fluoropropyl derivatives, [
18F]nifrolidine [
1], [
18F]nifzetidine [
2], [
18F]nifrolene [
3]. The fluoroalkyl group at the 5-position renders antagonist properties at the α4β2* nAChRs. The difference in the three radiotracers was the secondary amine ring, which dictated the in vivo binding kinetics. [
18F]Nifrolene was found most suitable with PET scan times of 90 min to equilibrate and good target to nontarget ratios in brain regions. [
18F]Nifene 4 which lacks the fluoropropyl group, is more nicotine-like and has more agonist like properties [
4,
5]. Imaging of α4β2* nAChRs by SPECT is made possible by inclusion of iodine-123 at the 5-position in [
123I]niodene [
6]. Both PET and SPECT studies with the identical molecule was made possible by using [
18F]niofene or [
123I]niofene, which contains both a fluorine and iodine substituent [
7].
Of the structures shown in
Figure 1, we have taken [
18F]nifene to human PET studies. Nifene binds selectively to the β2 subtype nAChRs, with binding affinities, Ki = 0.83 nM, 0.80 nM and 0.34 nM for α4β2, α3β2 and α2β2 nAChRs, respectively [
5]. [
18F]Nifene yielded the fastest in vivo equilibration in human PET studies amongst the various radiotracers currently being used for imaging α4β2* nAChRs [
8]. A shorter PET scan time is important for patient comfort and improve subject compliance, minimize motion artifacts which can be detrimental in higher resolution scanners, and use less PET scanner time. [
18F]Nifene has been shown to be superior in terms of imaging times in all species, providing reasonable target-to-nontarget ratios and providing the ability to visualize extrathalamic receptors [
5]. Biological properties of [
18F]nifene reported in rodents [
9,
10], monkeys [
4,
11] and humans [
8] suggest some differences in binding of [
18F]nifene across the different species. Additional constraints in the different species occur due to resolution of scanners for a mouse (~25 g), rat (~250 g), monkey (~10,000 g) and human (~70,000 g) as previously discussed for [
18F]nifene [
5].
Cholinergic impairments may occur in PD, a neurodegenerative disease with motor and non-motor symptoms [
12,
13]. The sporadic nature of PD development can make studying specific pathologies challenging [
14]. The endogenous protein α-synuclein, is generally structured as a random coil but in PD, the misfolded α-synuclein aggregates in neurons [
15]. Aggregation of misfolded α-synuclein in intracellular inclusions and Lewy bodies (LB) are some of the hallmarks of human PD [
16]. Glucose metabolism studies using [
18F]FDG-PET have been successfully carried out in human PD [
17,
18]. With [
18F]FDG-PET, specific patterns of deficit in the PD brain can be identified [
19,
20,
21].
Several transgenic mice models of α-synucleinopathy allow the study of PD progression [
22]. Using PET/CT studies in Hualpha-Syn(A53T) α-synuclein mutant mice, we recently reported [
18F]FDG brain deficits, limb muscle deficits and reduction of metabolic activity in regions of the spinal cord [
23]. These findings are consistent with the increased α-synuclein expression in the Hualpha-Syn(A53T) transgenic mice and spontaneously develop neurodegenerative disease between nine to 16 months of age [
24,
25]. Similar to human PD, neuronal abnormalities include pathological accumulations of α-synuclein and ubiquitin and Lewy body inclusions in neurons, with profound deficits in their motor neurons resulting in paralysis [
22]. Our preliminary findings suggested hypometabolism in the lower spinal cord suggesting degeneration as a probable cause for the paralysis [
23]. The Hualpha-Syn(A53T) mice also develop fine, sensorimotor, and synaptic deficits before developing age-related gross motor and cognitive impairment [
25]. Higher levels of acetylcholine have been reported in α-synuclein transgenic mice [
26].
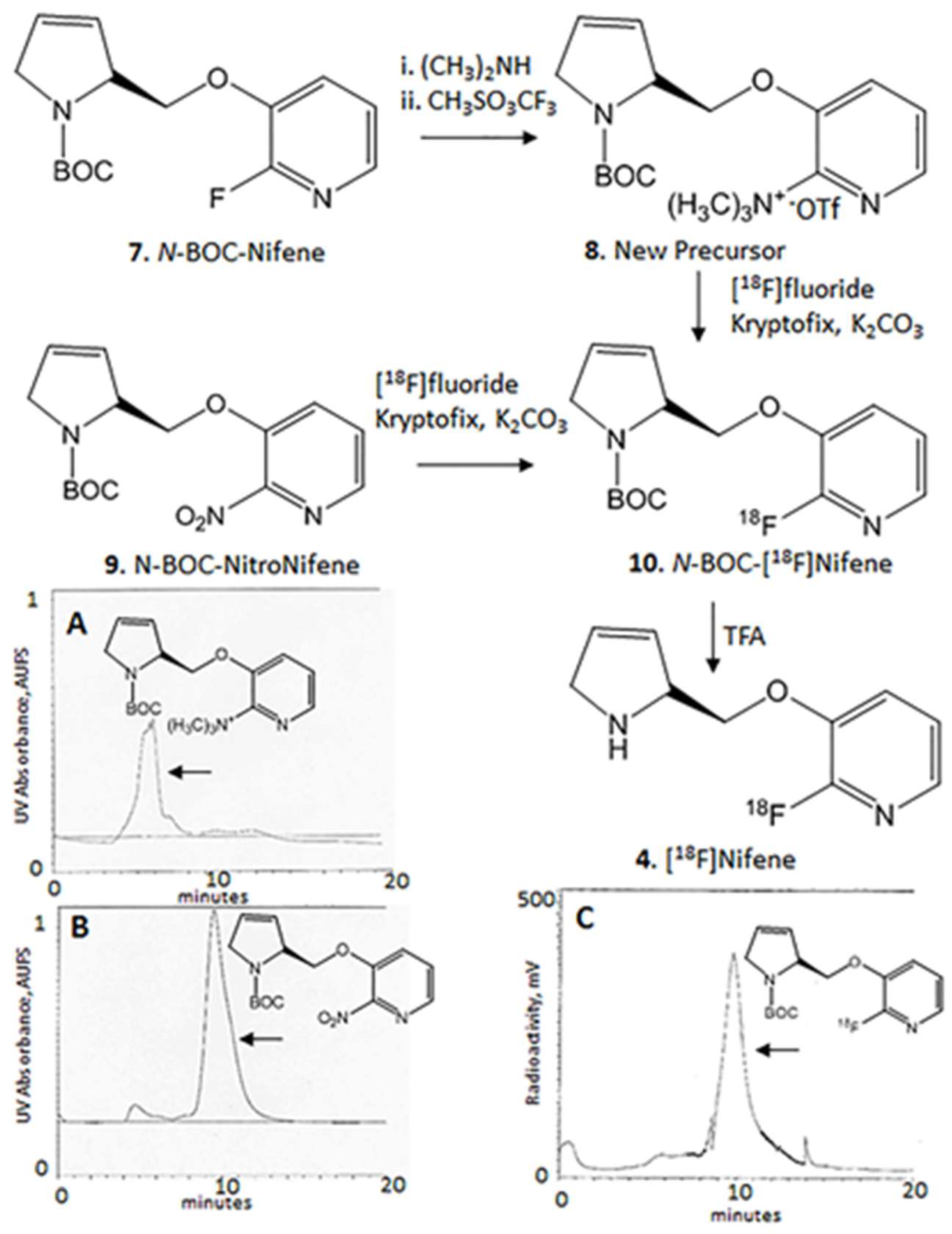
Because of the cholinergic role in PD, we have now investigated binding of [18F]nifene in Hualpha-Syn(A53T) α-synuclein mutant mice and postmortem human PD. Both in vitro and in vivo studies were caried out in Hualpha-Syn(A53T). Preliminary [18F]nifene in postmortem human PD brains comprising of anterior cingulate were carried out to assess potential alterations in α4β2*nAChRs. In order to establish the use of [18F]nifene imaging in mice, we report here the following: (1) Improved radiosynthesis using a new trimethylammonium salt precursor; (2) PET/CT studies in normal mice and evaluation of quantitation methods using mouse brain MRI template; (3) evaluation of [18F]nifene binding in α-synucleinopathy mice model of PD; and (4) binding in post-mortem human anterior cingulate brain sections of control and PD subjects.
2. Materials and Methods
2.1. General Methods
All chemicals and solvents were purchased from Aldrich Chemical and Fisher Scientific. Deionized water was acquired from Millipore Milli-Q Water Purification System (Burlington, MA, USA). Fluorine-18 fluoride in oxygen-18 enriched water was purchased from PETNET Inc. (Riverside, CA, USA), Fluorine-18 radioactivity was counted in a Capintec CRC-15R dose calibrator (Florham Park, NJ, USA) while low level counting was carried out in a Capintec Caprac-R well-counter (Florham Park, NJ, USA). All solvents used were provided by Fisher Scientific (Waltham, MA, USA). Gilson high performance liquid chromatography (HPLC) was used for the semi-preparative reverse-phase column chromatography with UV detector set at dual wavelengths of 254 and 280 nm as well as a radioactivity detector (Middleton, WI, USA). A Semi-preparative HPLC column 100 × 250 mm 10 micron Econosil C18 reverse-phase was used. Analytical thin-layer chromatography (TLC) was used to monitor reactions (Baker-flex, Phillipsburg, NJ, USA). RadioTLC were scanned on an AR-2000 imaging scanner (Eckart and Ziegler, Berlin, Germany). Electrospray mass spectra were obtained from a Model 7250 mass spectrometer (Micromass LCT, Waters Corp., Milford, MA, USA). Proton NMR spectra were recorded on a Bruker OM EGA 500-MHz spectrometer (Billerica, MA, USA). Mice brain slices were prepared at 10 to 40 µm thick using the Lieca 1850 cryotome. In vitro- or ex vivo-labeled brain sections were exposed to phosphor films (Perkin Elmer Multisensitive, Medium MS) and read using the Cyclone Phosphor Imaging System (Waltham, MA, USA). Analysis of autoradiographs was done using Optiquant acquisition and analysis software (Perkin Elmer, Waltham, MA, USA).
2.2. Animals
All animal studies were approved by the Institutional Animal Health Care and Use Committee of University of California-Irvine.
2.2.1. BALB/c Mice
Female mice (n = 4) age 14–18 weeks were used for time–activity of [18F]nifene in this study (24 g). Mice were purchased from Jackson Laboratory and housed under controlled temperatures of 22 ± 1 °C, in a 12 h light–dark cycle, on at 6:00 a.m., with water and food chow ad libitum.
2.2.2. C57BL/6 Mice
Adult male mice (n = 4) were used in this study (28 g; 18–24 weeks). Mice were purchased from Jackson Laboratory and housed under controlled temperatures of 22 ± 1 °C, in a 12 h light–dark cycle, on at 6:00 a.m., with water and food chow ad libitum.
2.2.3. Hualpha-Syn (A53T) Transgenic Mice
The Hualpha-Syn (A53T) transgenic line of mice (Tg(Prnp-SNCA*A53T) 83 Vle/J; stock no. 004479; 4 male and 4 female) and non-carrier mice (4 male and 4 female) were purchased from Jackson Laboratory. Female mice were 20–28 g and male mice weighed 26–38 g and were 11 months old. All mice were housed in sterilized cages. Non-carrier animals did not exhibit any abnormal motor activity whereas Hualpha-Syn (A53T) mice over time had hind limb paralysis and were euthanized when necessary. All animals recovered from the anesthesia required for the PET/CT imaging procedures.
2.3. Human Tissue
Human postmortem brain tissue samples were obtained from Banner Sun Health Research Institute (BHRI), Sun City, AZ brain tissue repository for in vitro experiments. Age and gender matched PD brain and cognitively normal (CN) brain tissue samples were used for the study. Human postmortem brain slices were obtained from chunks of frozen tissue on a Leica 1850 cryotome cooled to −20 °C. Fluorine-18 autoradiographic studies were carried out by exposing tissue samples on storage phosphor screens (Perkin Elmer Multisensitive, Medium MS and tritium sensitive phosphor screens). The apposed phosphor screens were read and analyzed by OptiQuant acquisition and analysis program of the Cyclone Storage Phosphor System (Packard Instruments Co., Boston, MA, USA). Adjacent slices were used for immunostaining with anti-ubiquitin and anti-α-synuclein. All postmortem human brain studies were approved by the Institutional Biosafety Committee of University of California, Irvine.
2.4. Synthesis
2-(Trimethylamino)-3-[2-((
S)-
N-tert-butoxycarbonyl-3-pyrrolinyl)methoxy]pyridine Triflate (TMAT) 8: Synthesis of
N-BOC-nifene was carried out using our procedures reported previously in >95% purity, [
4]).
N-BOC-nifene (0.3 g) was dissolved in 10 mL of dimethylamine in a Wheaton Kimble V-vial. This vial was heated at 100 °C for 23 h in a Lab-Line Multi-Blok heater. After it was cooled to ambient temperature, the solvent was removed in vacuo. The oily residue was then extracted with dichloromethane and purified using preparative TLC in 9:1 dichloromethane-methanol solvent resulting in 2-(dimethylamino)-3-[2-((
S)-
N-tert-butoxycarbonyl-3-pyrrolinyl)methoxy]pyridine (0.2 g). MS,
m/
z, 320 (40%, [M + H]
+), 342 (58%, [M + Na]
+), 661 (100%, [2M + Na]
+). Product 1: 1H NMR (500 MHz, CD
3OD) δ ppm: 7.73 (dd, 1H,
J = 5.04 and 4.98 Hz), 7.21 (d, 1H,
J = 4.98 Hz), 6.80 (m, 1H), 5.93(s, 1H, olefinic), 5.94 (m, 1H), 4.25 (m, 2H), 3.32 (m, 2H), 2.93 (d, 6H, N(CH
3)
2) 1.46 (d, 9H,
J = 5.64 Hz, BOC).
To a solution of 160 mg of 2-(dimethylamino)-3-[2-((S)-N-tert-butoxycarbonyl-3-pyrrolinyl)methoxy]pyridine in 2 mL of toluene, methyl trifluoromethanesulfonate was added (0.08 mL). The solution was stirred at room temperature for 1 h and then diluted with 10 mL of water and dichloromethane (0.5:9.5). The dichloromethane was extracted, dried over magnesium sulfate and filtered. The filtrate was concentrated to an oily residue and purified using preparative TLC in 9:1 dichloromethane-methanol solvent. The purified oily product, 2-(trimethylamino)-3-[2-((S)-N-tert-butoxycarbonyl-3-pyrrolinyl)methoxy]pyridine triflate (TMAT), 18.2 mg was obtained. MS, m/z, 278 (15%, [M − C4H8]+), 275 (23%, [M − NMe3]+), 234 (100%, [M − C4H8CO2]+). 1H NMR (500 MHz, CD3OD) δ ppm: 8.20 (d, 1H, J = 4.43 Hz), 7.94 (d, 1H, J = 8.27 Hz), 7.72 (dd, 1H, J = 4.51 and 4.54 Hz), 6.18 (m, 1H, olefinic), 6.02 (m, 1H, olefinic), 4.62 (d, 1H, J = 10.37), 4.43 (1H, d, J = 10.29 Hz), 4.13 (s, 1H), 3.52 (m, 2H), 2.26 (s, 9H, N(CH3)3) 1.29 (9H, s, BOC).
2.5. Radiosynthesis
The radiosynthesis of [
18F]nifene was performed using nucleophilic displacement of the nitro group in
N-BOC-nitronifene precursor by [
18F]fluoride in an automated synthesizer followed by deprotection using previously described procedures for the various species [
4,
11]. The automated radiosynthesis of [
18F]nifene was carried out in the chemistry-processing control unit (CPCU) box or the GE TRACERlab F X 2 N. High specific activity [
18F]fluoride in H
218O from PETNET was passed through QMA-light Sep-Pak (Waters Corp, Milford, MA, USA), which was previously preconditioned with 2 mL of K
2CO
3 (140 mg/mL), followed by 2 mL of anhydrous acetonitrile. The trapped [
18F]fluoride in QMA was eluted with 2.5 mL of Kryptofix-K
2CO
3 solution (36 mg/7.5 mg in 0.1 mL water and 2.4 mL of acetonitrile) and transferred to the reaction vessel. Initial step in the radiosynthesis involved drying the [
18F]fluoride solution at 125 °C for 10 min. Subsequently, 2 × 1 mL of anhydrous acetonitrile was added to the reaction vessel for azeotropic removal of last traces of moisture by heating at 125 °C for 5 min each time.
The TMAT precursor 2-(trimethylamino)-3-[2-((
S)-
N-tert-butoxycarbonyl-3-pyrrolinyl)methoxy]pyridine triflate, 8 (2 mg dissolved in 0.2 mL anhydrous dimethylsulfoxide and 0.3 mL of anhydrous acetonitrile) or the nitro precursor, 2-nitro-3-[2-((
S)-
N-
tert-butoxycarbonyl-3-pyrroline)methoxy]pyridine 9 (2 mg dissolved in 0.2 mL anhydrous dimethylsulfoxide and 0.3 mL of anhydrous acetonitrile) was transferred to the reaction vessel and the mixture was heated at 126 °C for 30 min for the nitro precursor and 15 min for the TMAT precursor. After the reaction, the contents of the reaction vessel were extracted by the addition of methanol (5 mL). This methanol extract was passed through neutral alumina to remove any unreacted [
18F]fluoride in order to provide the intermediate
N-BOC-[
18F]nifene 10 (
Figure 2). Reverse-phase HPLC purification of the methanol extract using an Alltech C
18 column (10 μm, 250 × 10 mm), mobile phase: 60% acetonitrile-40% with 0.1% triethylamine, flow rate 2.5 mL/min, provided the product
N-BOC-[
18F]nifene 10 (
Figure 2C). The collected fraction was taken to dryness in vacuo and the residue was taken in dichloromethane (1 mL) and TFA (0.2 mL). The mixture was heated at 80 °C (external temperature of heating block) for 30 min and subsequently evaporated to dryness. The residue was neutralized with 10% NaHCO
3 to pH 7.0. The final formulation was carried out using 1-3 mL of saline (0.9% NaCl INJ) followed by sterile filtration through a membrane filter (0.22 μm), and filtered solution was collected in a dose vial. The radiolabeled product [
18F]nifene (370–740 MBq) was obtained in >99% purity. Radiochemical yields of [
18F]nifene with both the precursors were in the 40–50% range decay corrected, and specific activities ranged from 37–185 GBq/μmol and above.
2.6. BALB\c Mice PET and CT Scanning
Subjects had free access to food and water during housing. All animals were fasted for 18–24 h prior to PET imaging. In preparation for the scans, the mice were induced into anesthesia with 4% isoflurane. Inveon preclinical Dedicated PET (Siemen’s Inc., Erlangen, Germany) was used for the MicroPET studies which has a resolution of 1.45 mm [
27]. The Inveon PET and MM CT scanners were placed in the “docked mode” for combined PET/CT experiments (Siemens Medical Solutions, Knoxville, TN, USA). A Sigma Delta anesthetic vaporizer (DRE, Louisville, KY, USA) was used to induce and maintain anesthesia during injections and PET/CT acquisitions.
Mice received intravenous (IV) using tail vein or intraperitoneal (IP) injections (approx. 7 MBq) of [18F]nifene off the scanner bed and then were immediately placed in the mouse imaging chamber for PET acquisition and scanned for 120 min in an Inveon dedicated PET scanner. The average delay between the time of injection and the start of the scan was 4 min. The animals were maintained under 2% isoflurane anesthesia throughout the scanning period. CT images were reconstructed with a cone beam algorithm (bilinear interpolation, Shepp-Logan filter) into 480 × 480 × 632 image arrays with a 206 μm pixel size. Following the reconstruction the CT images were spatially transformed to match the PET images. In addition to being reconstructed into an image, the CT data were used for attenuation correction of PET images. Quantitative calibration of PET images was performed by scanning 6.2 MBq of well mixed [18F]nifene and Millipore water solution inside a 56.5 mL plastic container. The [18F]nifene activity used in calibration was measured in the same dose calibrator used to measure the activities administered to the subjects.
2.7. C57BL\6 Mice PET and CT Scanning
Mice received intravenous (IV) using tail vein or intraperitoneal (IP) injections (approx. 7 MBq) of [18F]nifene off the scanner bed and then were immediately placed in the mouse imaging chamber for PET acquisition and scanned for 120 min in an Inveon dedicated PET scanner. The average delay between the time of injection and the start of the scan was 4 min. The animals were maintained under 2% isoflurane anesthesia throughout the scanning period.
2.7.1. C57BL\6 Mice Ex Vivo Autoradiography
The brain after the ex vivo MicroPET acquisition in
Section 2.5 was removed from the dry ice and rapidly prepared for sectioning. Horizontal sections (20 µm thick) containing [
18F]nifene labeled brain regions of the thalamus, subiculum, cortex, striatum, hippocampus, and cerebellum were cut using the Leica CM1850 cryotome. The sections were air dried and exposed to phosphor films overnight. Films were read using the Cyclone Phosphor Imaging System. Region-of-interest (ROI) of same size were drawn and analyzed on brain regions rich in α4β2* nicotinic receptors using OptiQuant software and binding of [
18F]nifene measured in Digital Light Units/mm
2 (DLU/mm
2).
2.7.2. In Vitro Mice Brain Autoradiography
Mice were decapitated, the brain was rapidly removed and frozen in isopentane at −20 °C. Sagittal sections (10 μm thick) containing the cortex, striatum, thalamus, hippocampus and cerebellum were prepared using LEICA CM 1850 cryotome at −20 °C and stored at −80 °C until use. For binding studies, slides were thawed for approx. 15 min at ambient temperature and were subsequently pre-incubated for 10 min at ambient temperature in buffer (120 mmol/L Tris HCl containing 5 mmol/L NaCl, 5 mmol/L KCl, 2.5 mmol/L CaCl2, 1 mmol/L MgCl2, pH 7.4). The preincubation buffer was then discarded. Subsequently, the slices were treated with incubation buffer containing [18F]nifene (148 kBq/mL) at 37 °C for 60 min. Competitive binding assay with different concentrations of unlabeled nifene (0.01, 0.1, and 1 μM) were carried out. Nonspecific binding was measured in the presence of 300 μmol of nicotine. After incubation, slides were washed twice (2 min each) with ice-cold incubation buffer, followed by a quick rinse in cold (0–5 °C) deionized water. The dried slides were apposed to phosphor screens and read by the Cyclone Phosphor Imaging System (Packard Instruments Co.). The amount of bound [18F]nifene in the autoradiograms was evaluated in various brain regions (as digital lights units (DLU]/mm2) using the OptiQuant acquisition and analysis program (Packard Instruments Co.).
2.8. Hualpha-Syn ((A53T) Mice
Male (n = 4) and female (n = 4), hemizygous Hualpha-Syn ((A53T) and no-carrier male(n = 4) and female (n = 4) mice were used in the study. All mice were injected [18F]nifene (PETNET solutions) intraperitoneally in normal saline (7.4 ± 0.7 MBq in 0.05–0.1 mL sterile saline) under 3% isoflurane (Patterson Veterinary, Loveland, CO, USA). Mice were then awake after [18F]nifene injections and free to move in their cages for 2 h. They were placed in the supine position in a mouse holder and anesthetized with 3% isoflurane for whole-body PET/CT imaging. A 15 min-long PET scans was acquired 2 h after [18F]nifene injections followed by a 10-min-long CT scan after the PET scan for attenuation correction and anatomical delineation of PET images. The Inveon Multimodality scanner was used for all combined PET/CT experiments.
2.9. Image Analysis
All in vivo images were analyzed using Inveon Research Workplace (IRW) software (version 4.2) (Siemens Medical Solutions, Knoxville, TN, USA) and PMOD Software (version 3.0) (PMOD Technologies, Zurich, Switzerland). Whole-body PET/CT images were analyzed using the IRW software for [
18F]nifene uptake and any other CT anomalies in the whole body images. For brain quantitative analysis, brain images were analyzed using PMOD, with PET images co-registered to a mouse brain MRI template [
28]. The magnitude of [
18F]nifene was expressed as standard uptake value (SUV) which was computed as the average [
18F]nifene activity in each volume of interest, VOI (in kBq/mL) divided by the injected dose (in MBq) times the body weight of each animal (in Kg). The SUV values were then statistically analyzed using students t-test and Hualpha-Syn ((A53T) PD mice were compared with non-carrier mice.
2.10. In Vitro Postmortem Human Brain Autoradiography
Human brain frontal cortex tissue from the 6 PD and 6 cognitively normal (CN) subjects were preincubated in Tris buffer (described above) for 15 min. The slides contained 1 to 3 brain sections each were placed in separate glass chambers (six slides per chamber). The preincubation buffer was discarded and then to the chambers, [18F]nifene in Tris buffer pH 7.4 (60 mL; 37 kBq/mL), was added and the chambers were incubated at 25 °C for 1 h. Nonspecific binding was measured in separate chambers in the presence of 300 μM nicotine. The slices were then washed with cold buffer twice, 3 min each time, Tris buffer and cold water for rinse. The brain sections were air dried, exposed overnight on a phosphor film, and then placed on the Phosphor Autoradiographic Imaging System/ Cyclone Storage Phosphor System (Packard Instruments Co.). Regions of interest (ROIs) were drawn on the slices and the extent of binding of [18F]nifene was measured in DLU/mm2 using the OptiQuant acquisition and analysis program (Packard Instruments Co.).
2.11. Immunohistochemistry
Immunostaining of all brain sections were carried out by University of California-Irvine, Pathology services using Ventana BenchMark Ultra protocols. Neighboring slices were immunostained for Ubiquitin (Cell Marque catalog no. 318A-18, Rocklin, CA, USA) and α-synuclein (EMD Millipore Corporation, lot No. 2985418, Burlington, MA, USA). All IHC stained slides were scanned using the Ventana Roche instrumentation and analyzed using QuPath.
4. Discussion
Among the various PET imaging agents for α4β2* nAChRs currently being used in humans, [
18F]nifene exhibits the fastest equilibration in vivo [
5]. Our previous studies in rats [
10], monkeys [
4,
11] and humans [
8] have validated interspecies similarity of in vivo behavior of [
18F]nifene. Previous whole body radiation dosimetry of [
18F]nifene in mice did not include mice brain studies in detail [
29]. With the development of several transgenic mice models of neurodegenerative disorders, availability of [
18F]nifene for evaluating changes in α4β2* nAChRs offers a unique tool. The goal in this work was, therefore, to develop methodology for [
18F]nifene studies in mice models and validate the utility of [
18F]nifene in PD.
Previous methods of radiosynthesis of [
18F]nifene involved the use of fluorine displacement of a nitro group in the precursor (
Figure 2). Purification by chromatographic separation of the nitro precursor from the fluorinated product was challenging due to their very close retention times (
Figure 2B,C). In order to improve the separation, the alternate trimethylammonium precursor was prepared (
Figure 2). The precursor was synthesized in two steps from
N-BOC-nifene, by displacement of the fluorine with dimethylamine followed by methylation of the tertiary amine using methyl trifluorosulfonic acid. Retention time of this trimethylammonium precursor was approximately 5 min (
Figure 2A), thus eluting much before the desired
N-BOC-[
18F]nifene product and enabling good purification. Radiochemical yields of the two precursors (nitro and trimethylamine) were comparable under reaction similar conditions of temperature and reaction times. The distinct advantage of the new precursor was the purification of the intermediate.
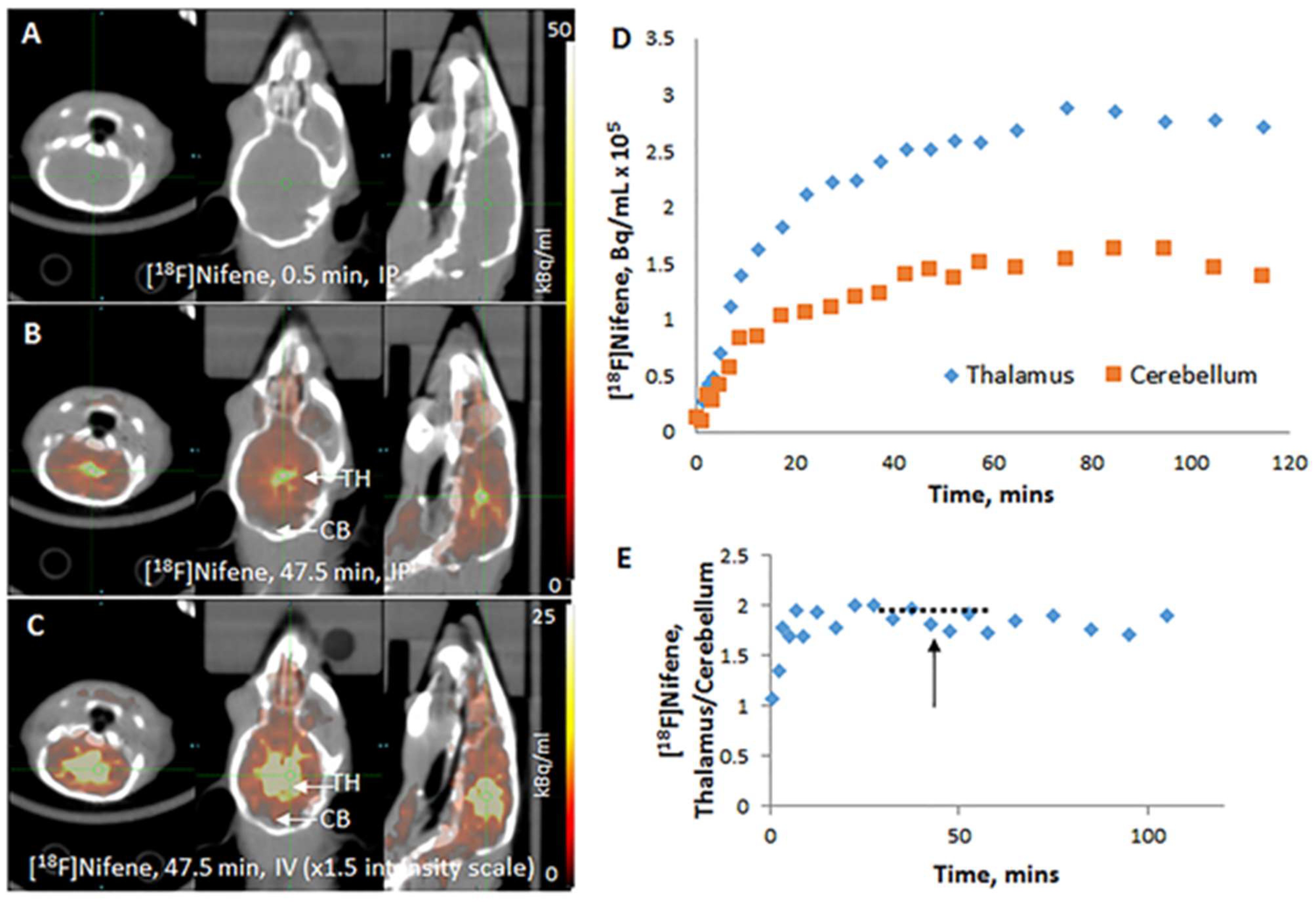
After intravenous administration of [
18F]nifene in the mouse, brain uptake was rapid (
Figure 3A). The unbound radiotracer cleared quickly from the thalamus and other receptor poor regions such as the cerebellum. Thalamus exhibited greater retention (
Figure 3B) and other extrathalamic regions were also seen to bind the radiotracer (
Figure 3C). Time–activity curves in the thalamus and cerebellum showed the rapid kinetics of [
18F]nifene and the greater retention in the thalamus (
Figure 3D). A ratio plot of thalamus to cerebellum showed a maximum of approximately 2.5 (
Figure 3E). A plateau of the ratio was observed between 30 and 60 min, after which there was a gradual decrease in the ratio. Distinct difference after intraperitoneal administration of [
18F]nifene in the mouse were observed compared to intravenous administration (
Figure 4). Initial images show no uptake of [
18F]nifene in the brain (
Figure 4A). The radiotracer is taken up gradually over time with greater binding observed in the thalamus lower levels in the cerebellum (
Figure 4B). Thalamus exhibited greater retention (
Figure 4B) and other extrathalamic regions were also seen to bind the radiotracer (
Figure 4C). Time–activity curves in the thalamus and cerebellum showed the slow uptake of [
18F]nifene and the greater retention in the thalamus (
Figure 4D). A ratio plot of thalamus to cerebellum showed a maximum of approximately 2 (
Figure 4E). A plateau of the ratio was observed between 30 and 60 min, after which there was a gradual decrease in the ratio.
Because of the need to carry out longitudinal PET studies in transgenic mice and the difficulty in repeat intravenous administrations, the intraperitoneal administration of [18F]nifene in mice is preferred. Our findings suggest a similarity in the time course of [18F]nifene in the brain where a static scan 30 to 60 min post intraperitoneal administration would be suitable. The thalamus to cerebellum ratio in the case of intraperitoneal administration was found to be about 20% lower compared to the intravenous administration.
Further detailed analysis of intraperitoneally administered [
18F]nifene bound to the mouse brain was carried out using co-registration of PET with mouse brain MR template (
Figure 5A–C). The in vivo measures were correlated with ex vivo brain slices of the same mouse (
Figure 5D,E). Thalamic and extrathalamic regions were confirmed using the PET-MR co-registered images, including the extracranial localization in the vicinity of the eyes (
Figure 5C). Ex vivo brain autoradiography showed more detailed localization of [
18F]nifene in various brain regions (
Figure 5). Thalamus had the highest levels followed by subiculum, striatum, frontal cortex and the lowest in the cerebellum. In vivo measures of thalamus, striatum, frontal cortex and cerebellum correlated well with the ex vivo measures suggesting reliability of in vivo measures for comparative, longitudinal studies in transgenic mice models. In vitro [
18F]nifene binding in mouse brain slices (
Figure 5G,H) correlated with ex vivo regional distribution. Unlabeled nifene progressively displaced [
18F]nifene and at 1 mM was able to completely displace from all brain regions confirming reversibility (
Figure 5I–K). Our previous studies in rats confirmed absence of any radiolabeled metabolites in the brain after [
18F]nifene administration [
10].
The Hualpha-Syn ((A53T) transgenic line of mice are of utility when studying Parkinson’s disease and various synucleinopathies. The transgenic mice used in the study express a A53T missense mutant form of human α-synuclein under the control of the murine prion promoter. The transgene yielded a 48,317 bp deletion in 2310039L15Rik by integrating into chromosome 10. The Hualpha-Syn ((A53T) mice exhibit the familial Parkinson’s disease- associated A53T missense mutant form of human α-synuclein (α-Syn), and express the A53T mutant α-Syn at a level sixfold that of the endogenous mice α-Syn. The age at which the hemizygous mice spontaneously develop neurodegenerative disease is between nine to 16 months of age. Neuronal abnormalities displayed by affected mice include pathological accumulations of α-Syn and ubiquitin. α-Syn-dependent neurodegeneration associated with increased/abnormal detergent-insoluble α-Syn and α-Syn aggregation is shown in brain regions as well. In the A35T α-Syn mutant mice, it was discovered that they attain intraneuronal inclusions, mitochondrial degeneration, and cell death in neocortex, brainstem, and spinal cord. In addition, they formed inclusions similar to Lewy bodies in neurons, and had profound deficits in their motor neurons, which could explain their paralysis [
22].
[
18F]Nifene binding in noncarrier mice brains followed the normal distribution as described above in C57BL\6 mice (
Figure 6A,B). Thalamus was the highest followed by subiculum, striatum and frontal cortex. Cerebellum was the lowest as expected. Two additional regions, namely anterior cingulate and hippocampus, were also analyzed. Binding in the hippocampus was low, while anterior cingulate was closer to levels of the frontal cortex. In the Hualpha-Syn ((A53T) PD mice brains, there was overall reduction of the binding of [
18F]nifene in most brain regions (
Figure 6D, E). Frontal cortex, anterior cingulate and striatum showed a decrease of more than 30%, while thalamus, subiculum and hippocampus were over 25% (
Figure 6E inset). The reduction in [
18F]nifene binding may be related to the α-synuclein aggregates in Hualpha-Syn ((A53T) PD brain slices. In [
18F]nifene PET/CT studies of non-carrier (
Figure 7A–C) and Hualpha-Syn ((A53T) PD mice (
Figure 7D–F), a similar reduction of [
18F]nifene binding was observed in various brain regions. A reduction of SUV of Hualpha-Syn ((A53T) PD compared to non-carrier mice (
Figure 7G) with some regions such as the cortex, caudate putamen and thalamus showing >20% decrease. The Hualpha-Syn ((A53T) PD mice have been shown to accumulate α-synuclein aggregates in these brain regions and spinal cord, potentially affecting other neurotransmitter receptor systems [
30], resulting in motoric and nonmotoric deficits. Our previous [
18F]FDG PET/CT studies in these Hualpha-Syn ((A53T) PD mice also observed significant metabolic deficits in these brain regions and spinal cord, contributing to hind limb muscle hypometabolism and leading to hind limb paralysis [
23].
Postmortem human brain sections of the anterior cingulate from well characterized subjects were confirmed as controls (
Figure 8A,B) and PD (
Figure 8D,E) by the absence or presence of Lewy bodies. Anti-ubiquitin stained Lewy bodies were found in the inner cortical layers (
Figure 8E) with dimensions of approximately 6–9 microns (
Figure 8E inset). In the control brains, there was no anti-ubiquitin staining confirming the absence of Lewy bodies (
Figure 8B and
Figure 9D). Binding of [
18F]nifene was observed in the grey matter regions within anterior cingulate (
Figure 8C). In the case of the PD brain, this anterior cingulate binding of [
18F]nifene was remarkably reduced. Even white matter binding of [
18F]nifene in the PD brains was reduced compared to the control brains. The presence of α-synuclein aggregates in the anterior cingulate of the PD brain slice is shown in
Figure 9A confirming the presence of Lewy neurites and Lewy bodies (
Figure 9B,C). Reduction of [
18F]nifene in the PD brain was significant compared to the control brains as seen in
Figure 9E. PET studies in PD subjects using [
18F]2-FA85380 have shown reduced levels of α4β2* nAChRs [
31]. Human PET studies using [
18F]nifene in PD subjects may be a valuable tool to study changes in the α4β2* nAChRs.