Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Light Treatments and Sample Collection
2.3. Color Measurement
2.4. Chlorophyll and Carotenoids Measurements
2.5. Total Anthocyanins Measurements
2.6. Fresh and Dry Weight Measurements
2.7. Nutritional Compounds Measurements
2.8. Antioxidant Activity Measurements
2.9. Antioxidant Components Measurements
2.10. Heat Map Analysis
2.11. Statistics Analysis
3. Results
3.1. Plant Growth and Biomass
3.2. Foliage Color and Pigment Contents of Lettuce
3.3. Antioxidant Sctivities of Lettuce
3.4. Antioxidant Compounds of Lettuce
3.5. Nutritional Qualities of Lettuce
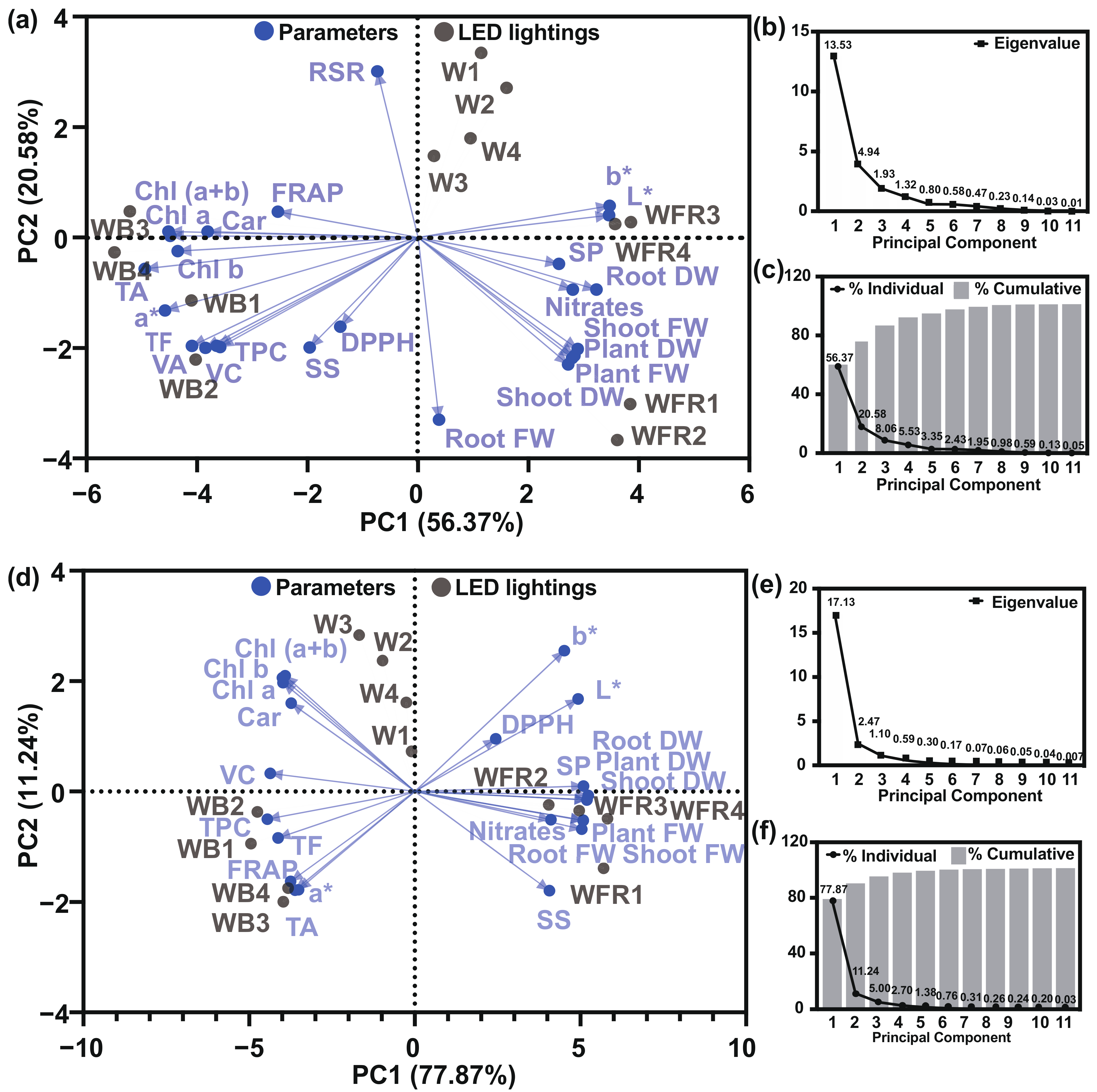
3.6. Principal Compound Analysis and Heatmap Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kozai, T. Plant factories with artificial lighting (PFALs): Benefits, problems, and challenge. In Smart Plant Factory; Springer: Singapore, 2018; pp. 15–29. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, Á.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. varcapitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Jones-Baumgardt, C.; Zheng, Y. Responses of yield and appearance quality of four Brassicaceae microgreens to varied blue light proportion in red and blue light-emitting diodes lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bantis, F.; Karamanoli, K.; Ainalidou, A.; Radoglou, K.; Constantinidou, H.I.A. Light emitting diodes (LEDs) affect morphological, physiological and phytochemical characteristics of pomegranate seedlings. Sci. Hortic. 2018, 234, 267–274. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Wang, H.; Ma, X.; Wang, B.; Wu, G.; Wang, H. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat. Commun. 2017, 8, 348. [Google Scholar] [CrossRef] [Green Version]
- Weinig, C.; Johnston, J.; German, Z.M.; Demink, L.M. Local and global costs of adaptive plasticity to density in Arabidopsis thaliana. Am. Nat. 2006, 167, 826–836. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Brutnell, T.P. The molecular analysis of the shade avoidance syndrome in the grasses has begun. J. Exp. Bot. 2007, 58, 3079–3089. [Google Scholar] [CrossRef] [Green Version]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, X.Z.; Guo, W.Z.; Wang, L.C.; Qiao, X.J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Zhen, S.; Van-Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, Y.Q.; Yang, Q.C.; Li, T. Overhead supplemental far-red light stimulates tomato growth under intra-canopy lighting with LEDs. J. Integr. Agric. 2019, 18, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.H.; Park, S.Y.; Oh, M.M. Supplemental irradiation with far-red light-emitting diodes improves growth and phenolic contents in Crepidiastrum denticulatum in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2017, 58, 357–366. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Rapisarda, P.; Fallico, B.; Izzo, R.; Maccarone, E. A simple and reliable method for determining anthocyanins in blood orange juices. Agrochimica 1994, 1, 157–164. [Google Scholar]
- Blakesley, R.W.; Boezi, J.A. Short communication: A new staining technique for proteins gels using Coomassie brilliant blue G250. Anal. Biochem. 1977, 82, 580–582. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Jiang, H.; Wu, L.; Zhang, Y.; Song, S.; Su, W.; Liu, H. End-of-day LED lightings influence the leaf color, growth and phytochemicals in two cultivars of lettuce. Agronomy 2020, 10, 1475. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Kuswandi, B.; Hidayat, M.A. A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol inhibition of lipid peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-n.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- De-Keyser, E.; Dhooghe, E.; Christiaens, A.; Van-Labeke, M.C.; Van-Huylenbroeck, J. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Hiyama, A.; Takemiya, A.; Munemasa, S.; Okuma, E.; Sugiyama, N.; Tada, Y.; Murata, Y.; Shimazaki, K.I. Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat. Commun. 2017, 8, 1284–1297. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Zhang, Y.T.; Liu, H.C.; Li, Y.M.; Liu, Y.L.; Hao, Y.W.; Lei, B.F. Supplemental blue light increases growth and quality of greenhouse pak choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [Green Version]
- Mickens, M.A.; Skoog, E.J.; Reese, L.E.; Barnwell, P.L.; Spencer, L.E.; Massa, G.D.; Wheeler, R.M. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Zhang, Y.T.; Zhang, Y.Q.; Bian, Z.H.; Fanourakis, D.; Yang, Q.C.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of temporally shifted irradiation of blue and red LED light on cos lettuce growth and morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Q.C. Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci. Hortic. 2018, 234, 220–226. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic responses of ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Yang, R.Y.; Hughes, J.; Easdown, W.J.; Holmer, R. The importance of vegetables in ensuring both food and nutritional security in attainment of the Millennium Development Goals. Food Secur. 2011, 3, 491–501. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Letchamo, W.; Hartman, T.; Gosslin, A.; Mamedov, N.A.; Craker, L. The accumulation of phenolic compounds in genetically selected Amaranthus hybridus is influenced by endophytic natural growth regulator. Int. J. Second. Metab. 2017, 5, 12–19. [Google Scholar] [CrossRef]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Ham, K.S.; Cho, J.Y.; Kang, S.G. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur. Food Res. Technol. 2009, 228, 903–911. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Liu, H.; Zhang, Y.; Hao, Y.; Song, S.; Lei, B. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zheng, D.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Effects of supplementary blue and UV-A LED lights on morphology and phytochemicals of Brassicaceae baby-leaves. Molecules 2020, 25, 5678. [Google Scholar] [CrossRef]
- Matysiak, B.; Kowalski, A. White, blue and red LED lighting on growth, morphology and accumulation of flavonoid compounds in leafy greens. Zemdirbyste 2019, 106, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.J.; Hu, W.L.; Li, J.X.; Chen, X.Y.; Wang, L.J. Increased accumulation of artemisinin and anthocyanins in Artemisia annua expressing the Arabidopsis blue light receptor CRY1. Plant Mol. Biol. Report. 2009, 27, 334–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, F.E. Vitamin C: An antioxidant agent. In Vitamin C; IntechOpen: London, UK, 2017; pp. 23–35. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kamboj, M.; Virender, V. A review on photometric methods for the quantitation of vitamin A. Microchem. J. 2021, 171, 106791. [Google Scholar] [CrossRef]
- Xie, B.; Wei, J.; Zhang, Y.; Song, S.; Su, W.; Sun, G.; Hao, Y.; Liu, H. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. Supplementary red light results in the earlier ripening of tomato fruit depending on ethylene production. Environ. Exp. Bot. 2020, 175, 104044. [Google Scholar] [CrossRef]


| Parameters | Lightings 1 | ||
|---|---|---|---|
| W | WFR | WB | |
| Single-band photon flux density (μmol·m−2·s−1) | |||
| Blue light (400–500 nm) | 31.41 | 29.66 | 72.25 |
| Green light (500–600 nm) | 47.92 | 46.51 | 36.34 |
| Red light (600–700 nm) | 170.73 | 168.96 | 137.22 |
| Far-red light (700–800 nm) | 3.30 | 13.24 | 3.59 |
| Integrated photon flux density 2 (μmol·m−2·s−1) | |||
| PPFD (400–700 nm) | 249.06 | 245.13 | 245.80 |
| YPFD (400–700 nm) | 223.73 | 219.76 | 212.42 |
| Radiation ratio | |||
| Red/Blue | 5.44 | 5.70 | 1.90 |
| Red/Green | 3.56 | 3.63 | 3.78 |
| Red/Far-red | 51.70 | 12.76 | 38.25 |
| Blue/Far-red | 9.51 | 2.24 | 20.14 |
| Daily light integral (mol·m−2·day−1) | |||
| 10 h | 9.00 | 8.82 | 8.85 |
| Fresh Weight (g) | Dry Weight (g) | Root/Shoot | |||||
|---|---|---|---|---|---|---|---|
| Plant | Shoot | Root | Plant | Shoot | Root | ||
| Yanzhi | |||||||
| W 1 | 53.41 ± 1.66b 2 | 47.99 ± 1.55b | 5.42 ± 0.44a | 2.68 ± 0.19b | 2.08 ± 0.17b | 0.60 ± 0.04a | 0.29 ± 0.02a |
| WFR | 77.93 ± 5.82a | 71.07 ± 4.45a | 6.86 ± 1.44a | 3.85 ± 0.53a | 3.15 ± 0.47a | 0.71 ± 0.07a | 0.23 ± 0.02b |
| WB | 50.64 ± 4.15b | 44.30 ± 3.77b | 6.35 ± 0.44a | 2.32 ± 0.32b | 1.85 ± 0.26b | 0.46 ± 0.05b | 0.25 ± 0.01b |
| Red Butter | |||||||
| W | 50.65 ± 1.81b | 46.44 ± 1.82b | 4.22 ± 0.12b | 2.52 ± 0.05b | 1.92 ± 0.05b | 0.60 ± 0.01b | 0.31 ± 0.01b |
| WFR | 97.81 ± 2.00a | 91.69 ± 1.96a | 6.12 ± 0.06a | 3.87 ± 0.24a | 3.18 ± 0.23a | 0.69 ± 0.01a | 0.22 ± 0.01c |
| WB | 41.96 ± 1.09c | 38.00 ± 1.00c | 3.96 ± 0.09c | 1.97 ± 0.03c | 1.42 ± 0.02c | 0.55 ± 0.01c | 0.39 ± 0.00a |
| Interaction 3 | |||||||
| Light quality (L) | *** | *** | *** | *** | *** | *** | *** |
| Cultivar (C) | * | *** | *** | NS | NS | NS | *** |
| L × C | *** | *** | NS | NS | NS | * | *** |
| L* 1 | a* | b* | |
|---|---|---|---|
| Yanzhi | |||
| W 2 | 39.18 ± 0.56b 3 | −5.26 ± 0.03c | 21.48 ± 0.21b |
| WFR | 40.89 ± 0.78a | −4.19 ± 0.10b | 23.51 ± 0.19a |
| WB | 29.75 ± 0.29c | 2.34 ± 0.15a | 7.68 ± 0.24c |
| Red Butter | |||
| W | 41.06 ± 0.51b | −10.28 ± 0.09b | 27.73 ± 0.80b |
| WFR | 43.45 ± 0.37a | −10.35 ± 0.04b | 29.33 ± 0.10a |
| WB | 36.71 ± 0.46c | −6.75 ± 0.11a | 19.10 ± 0.14c |
| Interaction 4 | |||
| Light quality (L) | *** | *** | *** |
| Cultivar (C) | *** | *** | *** |
| L × C | *** | *** | *** |
| Chlorophylls (mg·g−1) | Carotenoids (mg·g−1) | TA (mg·g−1) | |||
|---|---|---|---|---|---|
| Chl a 1 | Chl b | Chl (a + b) | |||
| Yanzhi | |||||
| W 2 | 0.84 ± 0.08b 3 | 0.26 ± 0.03b | 1.11 ± 0.11a | 0.19 ± 0.01ab | 6.72 ± 0.24b |
| WFR | 0.73 ± 0.10b | 0.24 ± 0.03b | 0.98 ± 0.14a | 0.18 ± 0.02b | 5.11 ± 0.32c |
| WB | 1.04 ± 0.09a | 0.33 ± 0.03a | 1.38 ± 0.13a | 0.21 ± 0.01a | 14.53 ± 0.73a |
| Red Butter | |||||
| W | 1.26 ± 0.15a | 0.38 ± 0.05a | 1.66 ± 0.20a | 0.42 ± 0.09ab | 0.24 ± 0.02b |
| WFR | 0.97 ± 0.09b | 0.29 ± 0.02b | 1.27 ± 0.11b | 0.32 ± 0.03b | 0.24 ± 0.02b |
| WB | 1.30 ± 0.08a | 0.40 ± 0.03a | 1.69 ± 0.10a | 0.47 ± 0.06a | 0.60 ± 0.08a |
| Interaction 4 | |||||
| Light quality (L) | *** | *** | *** | ** | *** |
| Cultivar (C) | *** | *** | *** | *** | *** |
| L × C | NS | NS | NS | NS | *** |
| DPPH 1 (%) | FRAP (mmol·g−1) | |
|---|---|---|
| Yanzhi | ||
| W 2 | 91.92 ± 0.62a 3 | 0.02 ± 0.00a |
| WFR | 92.29 ± 0.87a | 0.02 ± 0.00a |
| WB | 92.91 ± 1.28a | 0.02 ± 0.00a |
| Red Butter | ||
| W | 90.49 ± 0.91a | 0.01 ± 0.00b |
| WFR | 91.19 ± 0.40a | 0.01 ± 0.00b |
| WB | 90.10 ± 1.31a | 0.02 ± 0.00a |
| Interaction 4 | ||
| Light quality (L) | NS | *** |
| Cultivar (C) | *** | *** |
| L × C | NS | *** |
| TPC1 (mgGAE·g−1) | TF (mg·g−1) | VC (mg·g−1) | VA (nmol·g−1) | |
|---|---|---|---|---|
| Yanzhi | ||||
| W 2 | 1.07 ± 0.09b 3 | 1.76 ± 0.26b | 0.83 ± 0.07b | 5.23 ± 0.14c 2 |
| WFR | 1.10 ± 0.11b | 1.91 ± 0.18b | 0.84 ± 0.05b | 7.15 ± 0.29b |
| WB | 1.32 ± 0.10a | 2.58 ± 0.13a | 0.95 ± 0.05a | 10.43 ± 0.06a |
| Red Butter | ||||
| W | 0.68 ± 0.03b | 2.10 ± 0.06b | 0.43 ± 0.03a | 7.29 ± 0.34c |
| WFR | 0.39 ± 0.01c | 1.69 ± 0.34b | 0.33 ± 0.02b | 9.41 ± 0.51b |
| WB | 0.95 ± 0.02a | 2.81 ± 0.15a | 0.47 ± 0.01a | 11.05 ± 0.23a |
| Interaction 4 | ||||
| Light quality (L) | *** | *** | *** | *** |
| Cultivar (C) | *** | NS | *** | *** |
| L × C | *** | * | NS | *** |
| Soluble Proteins (mg·g−1) | Soluble Sugars (mg·g−1) | Nitrates (mg·g−1) | |
|---|---|---|---|
| Yanzhi | |||
| W 1 | 11.50 ± 0.50a | 30.33 ± 3.39a | 0.57 ± 0.05b |
| WFR | 12.04 ± 0.66a | 30.41 ± 5.90a | 0.65 ± 0.04a |
| WB | 10.69 ± 0.92a | 33.63 ± 3.66a | 0.52 ± 0.02b |
| Red Butter | |||
| W | 7.46 ± 0.43b | 12.47 ± 2.30b | 0.97 ± 0.06b |
| WFR | 9.34 ± 0.42a | 23.63 ± 1.34a | 1.07 ± 0.04a |
| WB | 6.19 ± 0.47c | 15.57 ± 1.58b | 0.95 ± 0.04b |
| Interaction 3 | |||
| Light quality (L) | *** | * | *** |
| Cultivar (C) | *** | *** | *** |
| L × C | * | ** | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. https://doi.org/10.3390/molecules26237405
Li Y, Wu L, Jiang H, He R, Song S, Su W, Liu H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules. 2021; 26(23):7405. https://doi.org/10.3390/molecules26237405
Chicago/Turabian StyleLi, Yamin, Linyuan Wu, Haozhao Jiang, Rui He, Shiwei Song, Wei Su, and Houcheng Liu. 2021. "Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory" Molecules 26, no. 23: 7405. https://doi.org/10.3390/molecules26237405
APA StyleLi, Y., Wu, L., Jiang, H., He, R., Song, S., Su, W., & Liu, H. (2021). Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules, 26(23), 7405. https://doi.org/10.3390/molecules26237405








