Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols
Abstract
1. Introduction
2. Results and Discussion
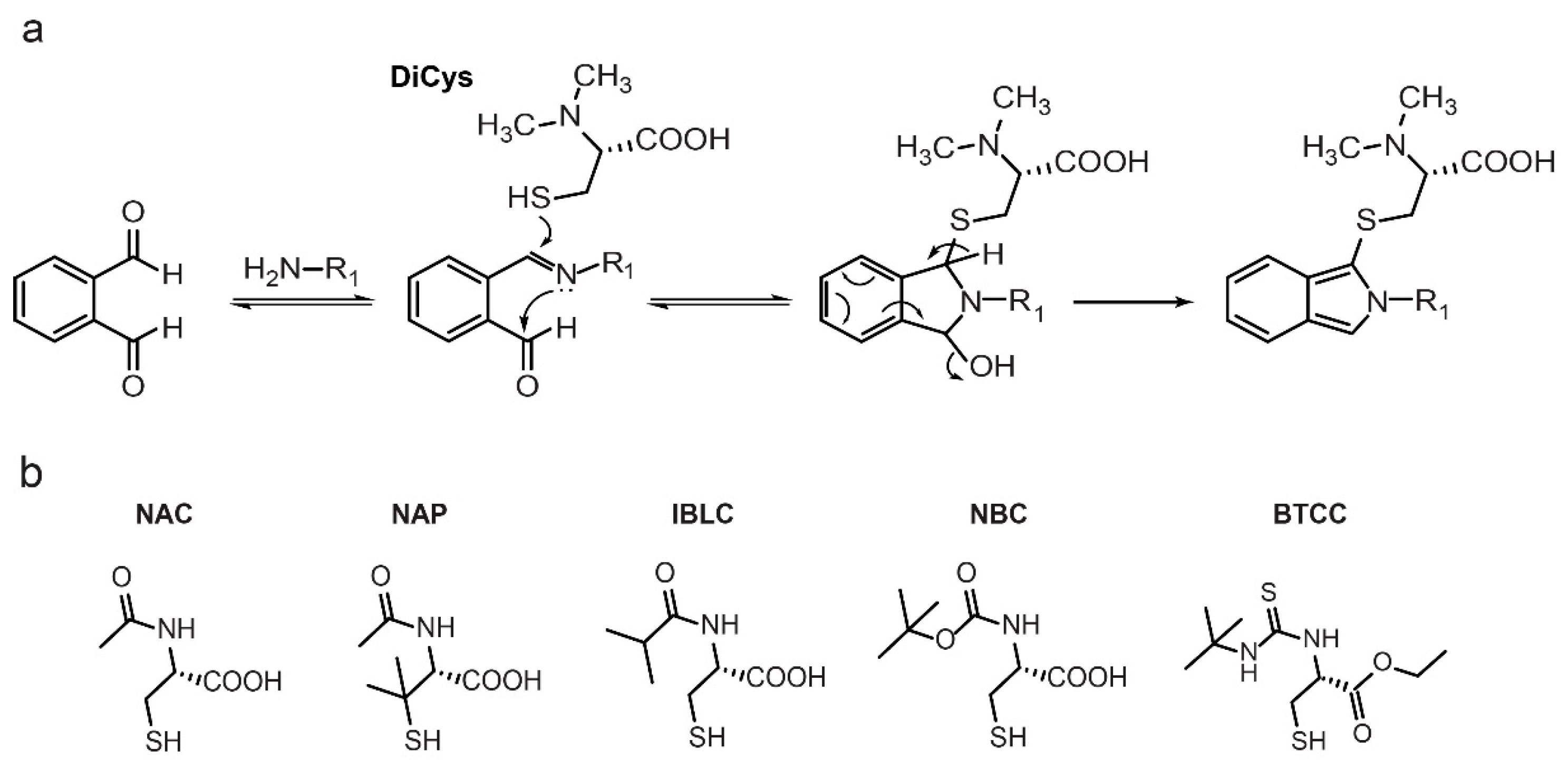
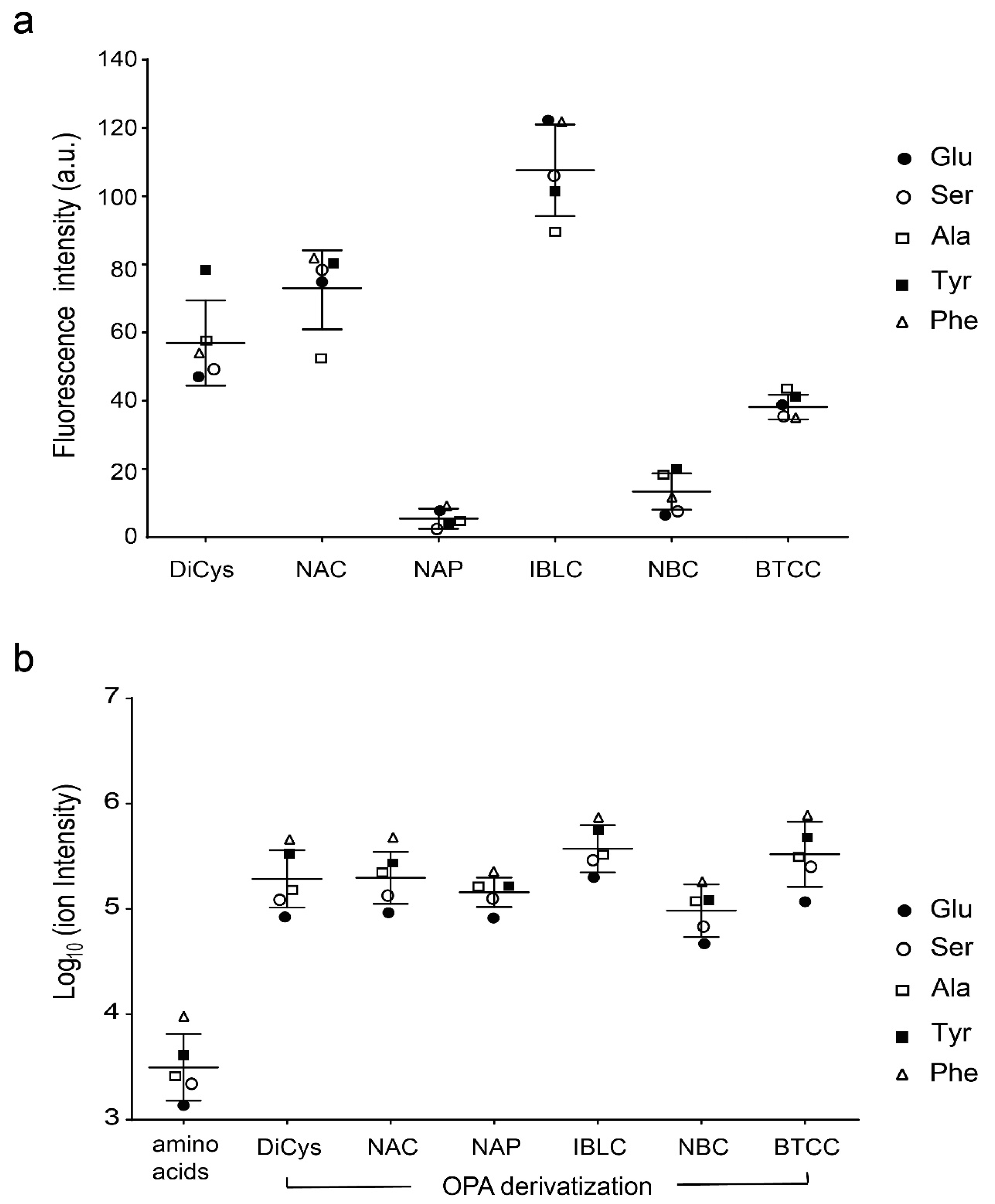
2.1. Stability and Fluorescence of DiCys Derivatives
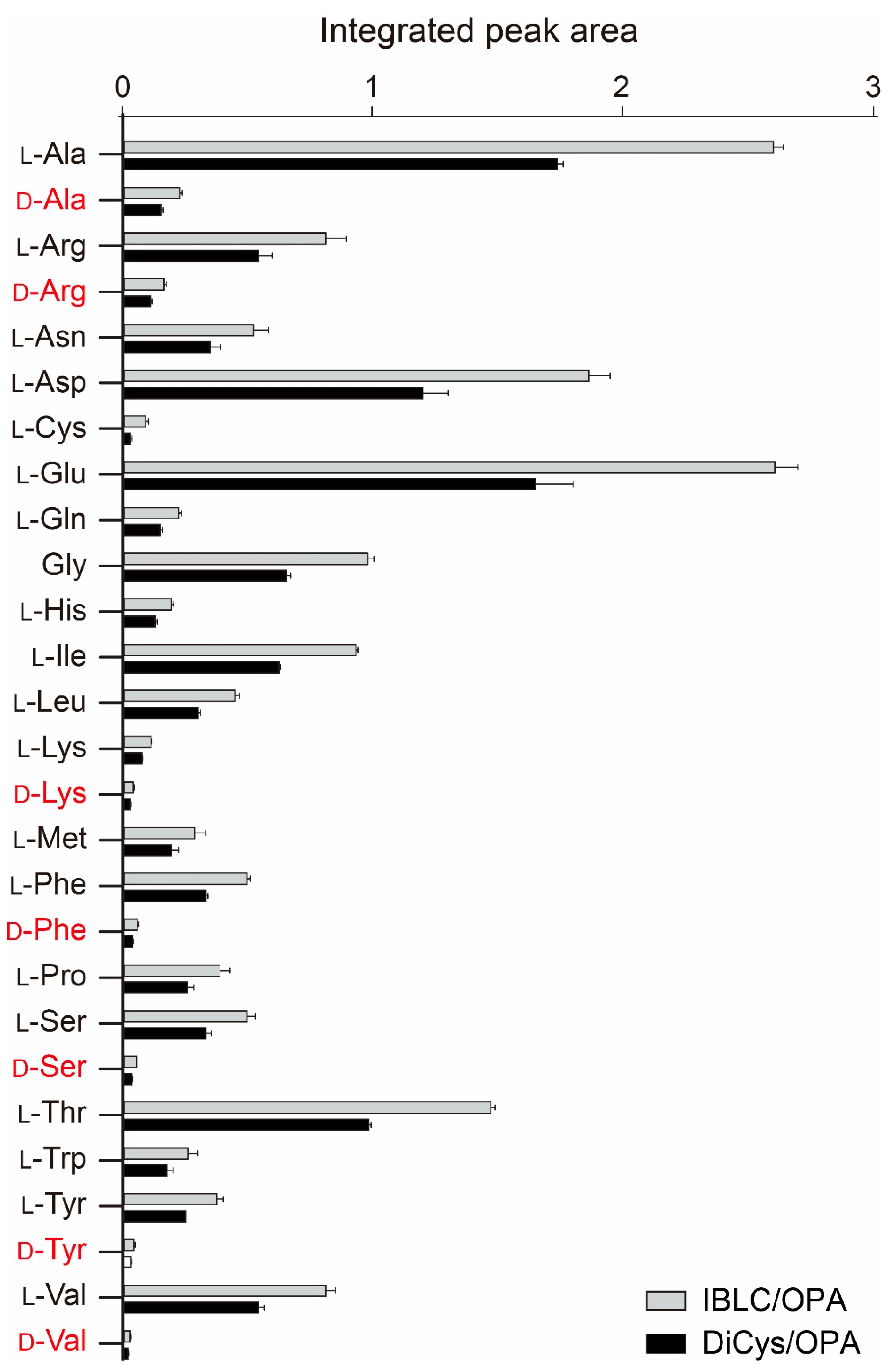
2.2. Separation of Enantiomers
2.3. Ionization Efficiency and MS/MS Properties
2.4. Comparing DiCys/OPA against Marfey’s Reagents
2.5. Enantiomer Identification in Rice Water with DiCys/OPA
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of N,N-Dimethyl-l-Cysteine
3.3. Rice Water Preparation
3.4. Derivatization Reactions
3.5. LC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Weckwerth, W. Metabolomics: An integral technique in systems biology. Bioanalysis 2010, 2, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Sevin, D.C.; Kuehne, A.; Zamboni, N.; Sauer, U. Biological insights through nontargeted metabolomics. Curr. Opin. Biotechnol. 2015, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 2016, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Fuhrer, T.; Zamboni, N. High-throughput discovery metabolomics. Curr. Opin. Biotechnol. 2015, 31, 73–78. [Google Scholar] [CrossRef]
- Zampieri, M.; Sekar, K.; Zamboni, N.; Sauer, U. Frontiers of high-throughput metabolomics. Curr. Opin. Chem. Biol. 2017, 36, 15–23. [Google Scholar] [CrossRef]
- Huan, T.; Wu, Y.; Tang, C.; Lin, G.; Li, L. DnsID in MyCompoundID for rapid identification of dansylated amine- and phenol-containing metabolites in LC-MS-based metabolomics. Anal. Chem. 2015, 87, 9838–9845. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, K.; Li, L. 5-Diethylamino-naphthalene-1-sulfonyl chloride (DensCl): A novel triplex isotope labeling reagent for quantitative metabolome analysis by liquid chromatography mass spectrometry. Anal. Chem. 2013, 85, 11532–11539. [Google Scholar] [CrossRef]
- SG, B.G.; Gowda, D.; Liang, C.; Li, Y.; Kawakami, K.; Fukiya, S.; Yokota, A.; Chiba, H.; Hui, S.P. Chemical Labeling Assisted Detection and Identification of Short Chain Fatty Acid Esters of Hydroxy Fatty Acid in Rat Colon and Cecum Contents. Metabolites 2020, 10, 398. [Google Scholar]
- Toyo’oka, T. Derivatization-based High-throughput Bioanalysis by LC-MS. Anal. Sci. 2017, 33, 555–564. [Google Scholar]
- Koga, R.; Yoshida, H.; Nohta, H.; Hamase, K. Multi-dimensional hplc analysis of metabolic related chiral amino acids-method development and biological/clinical applications. Chromatography 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Pandey, R.; Collins, M.; Lu, X.; Sweeney, S.R.; Chiou, J.; Lodi, A.; Tiziani, S. Novel Strategy for Untargeted Chiral Metabolomics using Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Anal. Chem. 2021, 93, 5805–5814. [Google Scholar] [CrossRef]
- Takayama, T.; Mochizuki, T.; Todoroki, K.; Min, J.Z.; Mizuno, H.; Inoue, K.; Akatsu, H.; Noge, I.; Toyo’oka, T. A novel approach for LC-MS/MS-based chiral metabolomics fingerprinting and chiral metabolomics extraction using a pair of enantiomers of chiral derivatization reagents. Anal. Chim. Acta 2015, 898, 73–84. [Google Scholar] [CrossRef]
- Mochizuki, T.; Todoroki, K.; Inoue, K.; Min, J.Z.; Toyo’oka, T. Isotopic variants of light and heavy l-pyroglutamic acid succinimidyl esters as the derivatization reagents for dl-amino acid chiral metabolomics identification by liquid chromatography and electrospray ionization mass spectrometry. Anal. Chim. Acta 2014, 811, 51–59. [Google Scholar] [CrossRef]
- Takayama, T.; Kuwabara, T.; Maeda, T.; Noge, I.; Kitagawa, Y.; Inoue, K.; Todoroki, K.; Min, J.Z.; Toyo’oka, T. Profiling of chiral and achiral carboxylic acid metabolomics: Synthesis and evaluation of triazine-type chiral derivatization reagents for carboxylic acids by LC-ESI-MS/MS and the application to saliva of healthy volunteers and diabetic patients. Anal. Bioanal. Chem. 2015, 407, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Hamase, K.; Morikawa, A.; Etoh, S.; Tojo, Y.; Miyoshi, Y.; Zaitsu, K. Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal. Sci. 2009, 25, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Koga, R.; Oyama, T.; Han, H.; Ueno, K.; Masuyama, K.; Itoh, Y.; Hamase, K. HPLC analysis of naturally occurring free d-amino acids in mammals. J. Pharm. Biomed. Anal. 2012, 69, 42–49. [Google Scholar] [CrossRef]
- Bastings, J.J.; van Eijk, H.M.; Olde Damink, S.W.; Rensen, S.S. d-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Jin, D.; Lee, Y.-I. Recent development on spectroscopic methods for chiral analysis of enantiomeric compounds. Appl. Spectrosc. Rev. 2009, 44, 267–316. [Google Scholar] [CrossRef]
- Fanali, C.; D’Orazio, G.; Gentili, A.; Fanali, S. Analysis of enantiomers in products of food interest. Molecules 2019, 24, 1119. [Google Scholar] [CrossRef]
- Hui, M.; Cheung, S.-W.; Chin, M.-L.; Chu, K.-C.; Chan, R.C.-Y.; Cheng, A.F.-B. Development and application of a rapid diagnostic method for invasive Candidiasis by the detection of d-/l-arabinitol using gas chromatography/mass spectrometry. Diagn. Microbiol. Infect. Dis. 2004, 49, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Takayama, T.; Mizuno, H.; Toyo’oka, T.; Akatsu, H.; Inoue, K.; Todoroki, K. Isotope Corrected Chiral and Achiral Nontargeted Metabolomics: An Approach for High Accuracy and Precision Metabolomics Based on Derivatization and Its Application to Cerebrospinal Fluid of Patients with Alzheimer’s Disease. Anal. Chem. 2019, 91, 4396–4404. [Google Scholar] [CrossRef]
- Guranda, D.T.; Kudryavtsev, P.A.; Khimiuk, A.Y.; Švedas, V.K. Efficient enantiomeric analysis of primary amines and amino alcohols by high-performance liquid chromatography with precolumn derivatization using novel chiral SH-reagent N-(R)-mandelyl-(S)-cysteine. J. Chromatogr. A 2005, 1095, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Molnár-Perl, I. HPLC of amino acids as o-phthalaldehyde derivatives. J. Chromatogr. Libr. 2005, 70, 163–198. [Google Scholar]
- Molnár-Perl, I. Advancement in the derivatizations of the amino groups with the o-phthaldehyde-thiol and with the 9-fluorenylmethyloxycarbonyl chloride reagents. J. Chromatogr. B 2011, 879, 1241–1269. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Nakahashi, A.; Yamane, K.; Nakahashi, S.; Murai, Y.; Siddegowda, A.K.C.; Hammam, M.A.S.; Monde, K. Facile Chemoselective Strategy toward Capturing Sphingoid Bases by a Unique Glutaraldehyde-Functionalized Resin. ACS Omega 2018, 3, 753–759. [Google Scholar] [CrossRef]
- Yokoyama, T.; Tokuda, M.; Amano, M.; Mikami, K. Simultaneous determination of primary and secondary d- and l-amino acids by reversed-phase high-performance liquid chromatography using pre-column derivatization with two-step labelling method. Biosci. Biotechnol. Biochem. 2017, 81, 1681–1686. [Google Scholar] [CrossRef]
- Brückner, H.; Wittner, R.; Godel, H. Amino acid analysis by derivatization with o-phthaldialdehyde and chiral thiols. In Amino Acids; Lubec, G., Rosenthal, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 143–151. [Google Scholar]
- Fitznar, H.P.; Lobbes, J.M.; Kattner, G. Determination of enantiomeric amino acids with high-performance liquid chromatography and pre-column derivatisation with o-phthaldialdehyde and N-isobutyrylcysteine in seawater and fossil samples (mollusks). J. Chromatogr. A 1999, 832, 123–132. [Google Scholar] [CrossRef]
- Brückner, H.; Zivny, S. High-performance liquid chromatographic resolution of (R, S)-α-alkyl-α-amino acids as diastereomeric derivatives. Amino Acids 1993, 4, 157–167. [Google Scholar] [CrossRef]
- Nimura, N.; Fujiwara, T.; Watanabe, A.; Sekine, M.; Furuchi, T.; Yohda, M.; Yamagishi, A.; Oshima, T.; Homma, H. A novel chiral thiol reagent for automated precolumn derivatization and high-performance liquid chromatographic enantioseparation of amino acids and its application to the aspartate racemase assay. Anal. Biochem. 2003, 315, 262–269. [Google Scholar] [CrossRef]
- Chernobrovkin, M.; Shapovalova, E.; Guranda, D.; Kudryavtsev, P.; Švedas, V.; Shpigun, O. Chiral high-performance liquid chromatography analysis of α-amino acid mixtures using a novel SH reagent—N-R-mandelyl-l-cysteine and traditional enantiomeric thiols for precolumn derivatization. J. Chromatogr. A 2007, 1175, 89–95. [Google Scholar] [CrossRef]
- Lóki, K.; Varga-Visi, É.; Albert, C.; Csapó, J. Separation and determination of the tryptophan enantiomers. Acta Univ. Sapientiae 2008, 1, 61–71. [Google Scholar]
- Hsu, J.L.; Chen, S.H. Stable isotope dimethyl labelling for quantitative proteomics and beyond. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150364. [Google Scholar] [CrossRef] [PubMed]
- Kovanich, D.; Cappadona, S.; Raijmakers, R.; Mohammed, S.; Scholten, A.; Heck, A.J. Applications of stable isotope dimethyl labeling in quantitative proteomics. Anal. Bioanal. Chem. 2012, 404, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Scott, C.; Trepman, E. Fluorescence properties of o-phthaldialdehyde derivatives of amino acids. Biochim. Biophys. Acta Protein Struct. 1979, 576, 440–455. [Google Scholar] [CrossRef]
- Gurram, I.; Kavitha, M.; Nagabhushnam, M.; Bonthagara, B.; Reddy, D.N. Overview of validation, basic concepts and analytical method process validation. Indian J. Pharm. Sci. 2017, 4, 1665–1680. [Google Scholar]
- Sabir, A.; Moloy, M.; Bhasin, P. HPLC Method Development and Validation: A Review. Int. Res. J. Pharm. 2015, 4, 39–46. [Google Scholar] [CrossRef]
- Lkhagva, A.; Shen, C.-C.; Leung, Y.-S.; Tai, H.-C. Comparative study of five different amine-derivatization methods for metabolite analyses by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1610, 460536. [Google Scholar] [CrossRef]
- Ayon, N.J.; Sharma, A.D.; Gutheil, W.G. LC-MS/MS-based separation and quantification of Marfey’s reagent derivatized proteinogenic amino acid dl-stereoisomers. J. Am. Soc. Mass Spectrom. 2018, 30, 448–458. [Google Scholar] [CrossRef]
- Bhushan, R.; Brückner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, H.; Gah, C. High-performance liquid chromatographic separation of dl-amino acids derivatized with chiral variants of Sanger’s reagent. J. Chromatogr. A 1991, 555, 81–95. [Google Scholar] [CrossRef]
- Marfey, P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1, 5-difluoro-2, 4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Inamasu, S.; Ikuyama, R.; Fujisaki, Y.; Sugimoto, K.I. The effect of rinse water obtained from the washing of rice (YU-SU-RU) as a hair treatment. J. Soc. Cosmet. Chemists Japan 2010, 44, 29–32. [Google Scholar] [CrossRef][Green Version]
- Kalman, D.S. Amino acid composition of an organic brown rice protein concentrate and isolate compared to soy and whey concentrates and isolates. Foods 2014, 3, 394–402. [Google Scholar] [CrossRef]
- Wong, H. Rice water in treatment of infantile gastroenteritis. Lancet 1981, 2, 102–103. [Google Scholar] [CrossRef]
- Chung, L. A fluorescamine assay for membrane protein and peptide samples with non-amino-containing lipids. Anal. Biochem. 1997, 248, 195–201. [Google Scholar] [CrossRef]
- Müller, C.; Fonseca, J.R.; Rock, T.M.; Krauss-Etschmann, S.; Schmitt-Kopplin, P. Enantioseparation and selective detection of d-amino acids by ultra-high-performance liquid chromatography/mass spectrometry in analysis of complex biological samples. J. Chromatogr. A 2014, 1324, 109–114. [Google Scholar] [CrossRef]
- Kawai, M.; Sekine-Hayakawa, Y.; Okiyama, A.; Ninomiya, Y. Gustatory sensation of l-and d-amino acids in humans. Amino Acids 2012, 43, 2349–2358. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, J.; Suzuki, M. Emerging Role of d-Amino Acid Metabolism in the Innate Defense. Front. Microbiol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed]


| Amino Acids | d/l Ratio |
|---|---|
| Ala | 0.09 |
| Arg | 0.31 |
| Lys | 0.30 |
| Phe | 0.09 |
| Ser | 0.10 |
| Tyr | 0.12 |
| Val | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lkhagva, A.; Tai, H.-C. Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols. Molecules 2021, 26, 7416. https://doi.org/10.3390/molecules26247416
Lkhagva A, Tai H-C. Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols. Molecules. 2021; 26(24):7416. https://doi.org/10.3390/molecules26247416
Chicago/Turabian StyleLkhagva, Ankhbayar, and Hwan-Ching Tai. 2021. "Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols" Molecules 26, no. 24: 7416. https://doi.org/10.3390/molecules26247416
APA StyleLkhagva, A., & Tai, H.-C. (2021). Dimethylcysteine (DiCys)/o-Phthalaldehyde Derivatization for Chiral Metabolite Analyses: Cross-Comparison of Six Chiral Thiols. Molecules, 26(24), 7416. https://doi.org/10.3390/molecules26247416





