Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types
Abstract
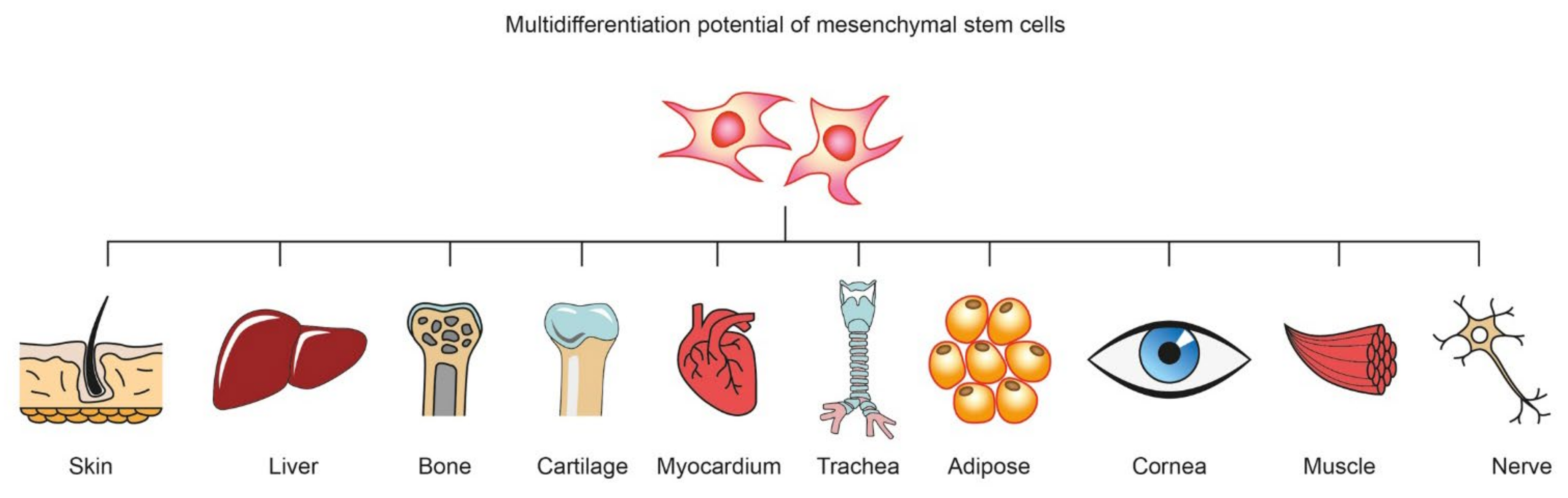
:1. Introduction
2. Characterisation of Dental Pulp Stem Cells
2.1. Surface Markers
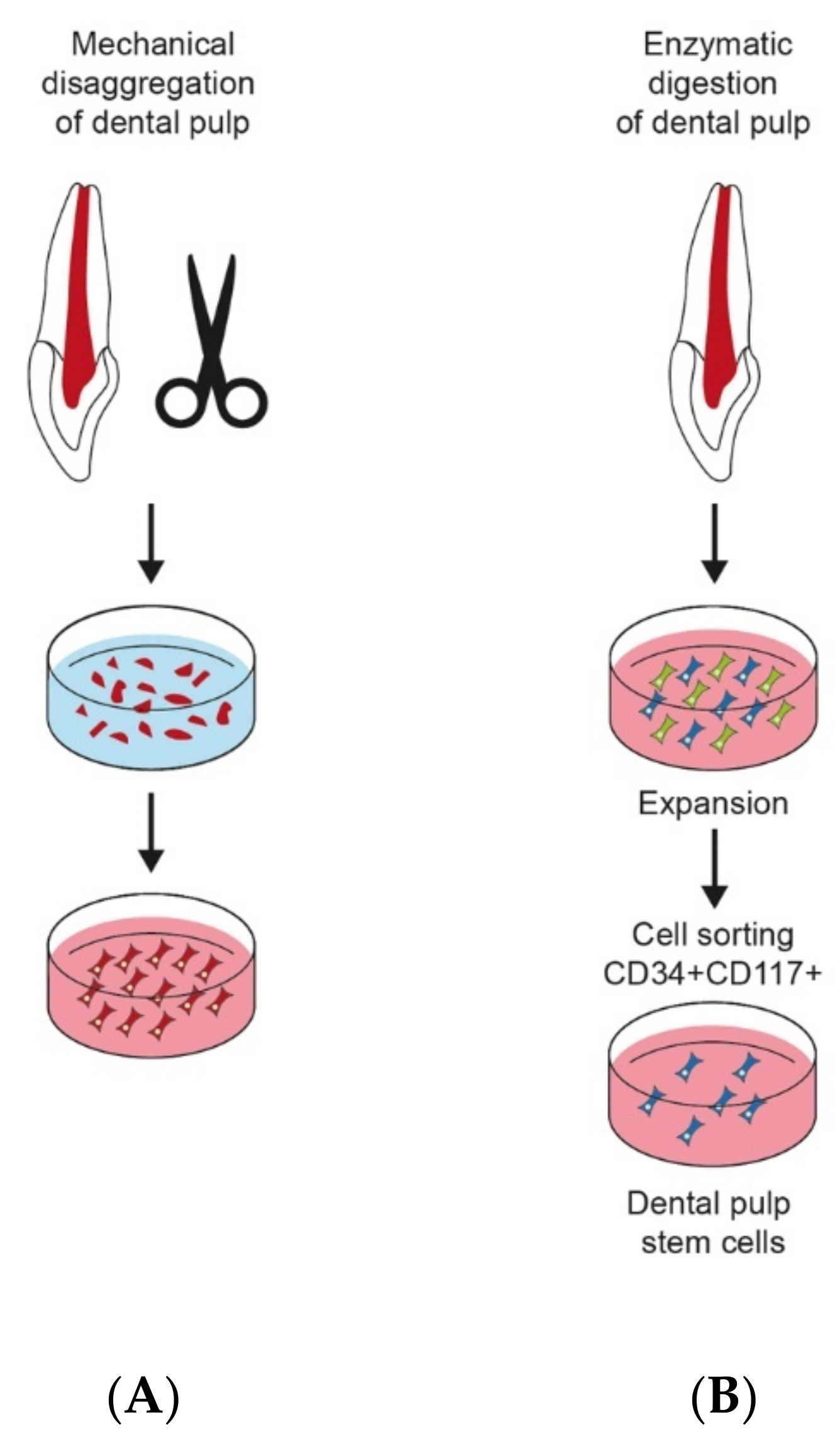
2.2. Isolation of DPSCs
2.3. DPSC Culture
2.4. Methods of Stem Cells Characterisation
- -
- Inverted phase-contrast microscopy images of the morphologies and behaviour of acquired cells
- -
- Quantitative real-time polymerase chain reaction (qRT-PCR) provides a flexible, sensitive and scalable method for analysing the gene expression profile of any cell population. This method is used to duplicate the DNA fragments. With the help of the polymerase chain reaction (PCR) technique, we can obtain billions of copies of a specific DNA sequence in a short time. Then, we can determine its length and exact structure. The quantitative real-time polymerase chain reaction (qRT-PCR) method enables the measurement of the number of new copies of the reaction product at any point in time (in real time). It is a very sensitive technique that enables the detection of even one copy of the studied gene. It differs from traditional PCR in that it analyses the increase of the PCR reaction products after each reaction cycle, not only after the last one. Due to its specificity, the qPCR method has become a valuable diagnostic tool. By this method, we can identify numerous human MSC-characteristic genes [58].
- -
- Flow cytometry based on fluorochrome-conjugated antibodies against specific markers on the cell surface. Flow cytometry enables the analysis of several parameters for each cell, cell fragments, or other artifacts as the colour intensity, size and fluorescence of the tested cells. The results of the diagnosis are histograms, which allow us to visualise the populations of cells with specific characteristics in the tested sample. Cytometers equipped with a device for sorting cells in an electric field make it possible to evaluate some functional parameters of cells and isolation-appropriate cells [59].
- -
- Immunofluorescence is used to confirm the presence or absence of protein expression in stem cells. Complexes resulting from combining antibodies with antigens are detected using fluorescent dyes.
- -
- Protein extraction and immunoblotting: a technique that uses specific antibodies to identify separated proteins based on size by gel electrophoresis.
2.5. Cryopreservation
3. Characterisation of Scaffolds and Growth Factors
4. Materials and Methods
5. Results
5.1. Cell Sources
5.2. Scaffolds
5.3. Growth Factors
5.4. Osteogenic Differentiation
5.5. Xenotransplantation
5.6. Allotransplantation
5.7. Autotransplantation
| Xenotransplantation (From Human to Animal) | |||||||
|---|---|---|---|---|---|---|---|
| Case No. | Aim | Cell Source | Host | Scaffold/Cell Sheet | Growth Factor | Results | Article |
| 1. | To explore the potential roles and molecular mechanisms of DPSCs in crushed nerve recovery. | human DPSCs extracted third molars or orthodontic teeth (15–25 years) | 32 adult male SD rats had nerve crush injury | cell sheets and N-DPSC | epidermal growth factor basic fibroblast growth factor | DPSCs are inclined to differentiate into neural cells. Could help crushed nerves with functional recovery and anatomical repair in vivo. Thus, DPSCs or N-DPSCs could be a promising therapeutic cell source for peripheral nerve repair and regeneration | [77] |
| 2. | Comparison of the bone formation capacity of DPSCs and ADSCs in vitro and in vivo. | hDPSCS (third molars) from 20–25-year-old individuals; ADSCs from 25–35-year-olds during liposuction. | 15 rats mandibular bone defect | alkaline phosphate (ALP) | Indicated the extensive potential of the DPSCs in tissue repair and regeneration. ADSCs exhibited greater osteogenic differentiation potential, higher expression of osteoblast marker genes and greater mineral deposition | [78] | |
| 3. | Comparison of the regeneration characteristics of cell sheets derived from dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs) and stem cells of the apical papilla (SCAPs). | Human (DPSC, PDLSCs) and (SCAPs)—impacted third molars | subcutaneously into the dorsal surfaces of 5 10-week-old mice | cell sheet | vitamin C, hydroxyapatite/tricalcium phosphate (HA/TCP) | Although in vitro DPSC, PDLSC and SCAP cell sheets have similar characteristics, their regenerative characteristics in vivo are different, with each showing potential application for regeneration of different tissues. Dental pulp stem cell sheet formed a loose connective tissue, rich in blood vessels, similar to dental pulp tissue, suggesting that DPSC sheet could be more suitable for dental pulp or vascular rich tissue regeneration. | [79] |
| 4. | Evaluation the effect of cell injection and cell sheet transplantation on periodontal regeneration in a swine model. | human DPSCs | 12 pigs were used to generate periodontitis lesions of the first molars for a total of 24 defects | HDPSC sheet group hDPSC injection group | Vc xenobiotic-free cell culture reagents | Xenogeneic DPSC sheets and DPSC injection can be appropriate therapies for periodontal bone and soft tissue regeneration | [80] |
| 5. | DPSCs and human umbilical vein endothelial cells (HUVECs) were used to evaluate the biological effects of SAP-based scaffolds. | hDPSC premolars, third molars (18–25 years) | 35 rats | Extracellular matrix (ECM)-like biomimetic hydrogels composed of self-assembling peptides (SAPs) scaffold with SAPs | Morphogenic signals in the form of growth factors (GFs) | DPSCs grown on this composite scaffold stimulating pulp recovery and dentin regeneration in vivo | [81] |
| 6. | Identification of the optimal dental source of MSCs through a biological and functional comparison of gingival (GMSCs) and dental pulp stem cells (DPSCs) focusing mainly on their angiogenic potential | human MSCs from the dental pulp of the third molars and gingival tissues of the same patient | 24 NSG mice | Matrigel implants | endothelial cell growth medium | GMSCs displayed a higher capacity to proliferate, migrate and form angiogenic tubules compared with DPSCs in vitro and in vivo | [82] |
| 7. | Assessment viability of these 3D DPSC constructs for dental pulp regeneration through in vitro and in vivo studies | DPSCs from human adult third molars | DPSC were inserted into the human root canal, and then transplanted into the subcutaneous space of 6 mice | Rod-shaped 3D cell construct | OM for odontoblastic differentiation | DPSC constructs possess self-organizing ability and can be used for novel dental pulp regeneration therapy; fabrication of a scaffold-free, rod-shaped cell construct composed of DPSCs, using thermoresponsive hydrogel | [83] |
| 8. | Whether medium modification improves the odontogenic differentiation of human dental pulp stem cells (DPSC) in vitro and in vivo | DPSC human impacted third molar teeth | subcutaneous dorsal surface of the mice | hydroxyapatite tricalcium phosphate scaffold | bone morphogenetic protein 2 (BMP2) | Odontogenic differentiation of the isolated and characterised human DPSC was improved with medium modification by the addition of BMP2 in vitro and in vivo | [84] |
| 9. | Peptide hydrogel PuraMatrix™ was used as a scaffold system to investigate the role of dental pulp stem cells (DPSCs) in triggering angiogenesis and the potential for regenerating vascularised pulp in vivo | DPSCs from extracted sound third molars from humans (18 to 25 years) | Root segments were implanted in the subcutaneous space of the dorsum of 20 5- to 7-week-old mice | peptide hydrogel PuraMatrix™ | - | Importance of a microenvironment that supports cell–cell interactions and cell migration, which contribute to successful dental pulp regeneration | [85] |
| 10. | Comparison of the biological properties of aged MDPSCs versus young MDPSCs | DPSCs from human third molars were collected from younger (19–30 years, n = 6) and older (44–70 years, n = 6) | SCID Mice (ischemic hidlimb) SCID Mice (subcutaneous) | Tooth roots, collagen TE | - | The regenerative potential of MDPSCs is independent of age, demonstrating an immense utility for clinical applications by autologous cell transplantation in dental pulp regeneration and ischemic diseases | [86] |
| 11. | Investigation of the potential of human dental pulp stem cells (hDPSCs) and human amniotic fluid stem cells (hAFSCs) to differentiate toward a skeletal myogenic lineage using several different protocols | human DPSCs (enclosed third molar of teenage subjects) hAFSCs | mdx/SCID mice (gastrocnemius muscles (GMs) | Intramuscular injection of pre-differentiated DPSCs in myogenic medium | - | Promoted angiogenesis and reduced fibrosis, improvement of pathological features of dystrophic skeletal muscle tissues, regeneration of muscles in Duchenne muscular dystrophy | [87] |
| 12. | Investigation of the therapeutic potential of intravenous and Intrapancreatic transplantation of human dental pulp stem cells in a rat model of streptozotocin-induced type 1 diabetes | DPSCs from human impacted third molars | 40 rats | hDPSCs were injected into the pancreas or tail vein after the induction of diabetes in nude mice | - | Human dental pulp stem cells can migrate and survive within streptozotocin-injured pancreas and induce antidiabetic effects through the differentiation and replacement of lost β-cells and paracrine-mediated pancreatic regeneration | [88] |
| 13. | Engineering sizable three-dimensional cartilage-like constructs using stem cells isolated from human dental pulp stem cells (DPSCs) | DPSCs from human premolars extracted for orthodontic treatment | 10 mice (8–10 weeks) | poly-l-lactic acid/polyethylene glycol (PLLA/PEG) electrospun fiber scaffolds | growth factor β3 (TGFβ3) | Immuno-selected DPSCs can be successfully differentiated toward chondrogenic lineage; it may be useful in future treatment of cartilage defects | [89] |
| 14. | How human mesenchymal stem cells differentiate after birth into endothelial cells that make up blood vessels | human permanent teeth (DPSC) or deciduous teeth (SHED) | MSCs seeded in human tooth slice/scaffolds were transplanted 8 mice | poly-L-lactic acid (PLLA) scaffold | vasculogenic differentiation medium, i.e., endothelial cell growth medium (EGM2-MV, Lonza) supplemented with rhVEGF165. | VEGF signalling through the canonical Wnt/β-catenin pathway defines the vasculogenic fate of postnatal mesenchymal stem cells dental pulp stem cells can differentiate into endothelial cells that form blood vessels | [90] |
| 15. | Illuminate the role of hsa_circ_0026827 in human dental pulp stem cells (DPSCs) during osteoblast differentiation. | human DPSCs | 15 mice | Bio-Oss Collagen scaffolds | osteogenic medium | hsa_circ_0026827 promotes osteoblast differentiation of DPSCs | [98] |
| 16. | Investigation of whether the combination of Bio-Oss scaffold with BMSCs and DMSCs promotes improved bone regeneration and osteogenesis-related protein expression in a rabbit calvarial defect model | human DPSCs and BMSCs | Rabbit calvarial defects | xenografts bio-oss | In the in vivo studies, the bone volume density in DPSCs group was significantly greater than that in the empty control or Bio-Oss only group | [99] | |
| 17. | Comparison of multiphase region-specific microscaffolds (polycarprolactione-hydroxylapatite) with spatiotemporal delivery of bioactive cues for integrated periodontium regeneration. | human DPSCs, PDLSCs, and ABSCs from 18–39-year-old patients | 20 mice (dorsum’s midsagittal plane) | Polycarprolactione-hydroxylapatite (90:10 wt%) scaffolds | GF Recombinant human amelogenin, connective tissue growth factor, and bone morphogenetic protein-2 | DPSC appears to differentiate into putative dentin/cementum, PDL and alveolar bone complex by scaffold’s biophysical properties and spatially released bioactive cues | [100] |
| 18. | To investigate the localisation of transplanted DPSCs in a mouse fracture model | human DPSCs | 27 mice (calvarial defect model) | - | helioxanthin derivative 4-(4-methoxyphenyl)pyrido[40,30:4,5]thieno[2,3-b]pyridine-2-carboxamide (TH)) and osteogenic medium | OM + TH-treated DPSCs promoted fracture healing. Moreover, transplanted DPSCs had localised to the fracture site and were directly involved in fracture healing. | [101] |
| 19. | Investigation the expression and biological function of human β-defensin 4 (HBD4) in dental pulp stem cells (DPSC) and explored its potential as a pulp capping agent | human DPSCs | 15 8-week-old male Wistar rats (holes in the centre of the bilateral maxillary first molar surface to expose the pulp chamber) | gelatin sponge | osteogenic induction medium adipogenic induction medium cartilage induction medium | DPSC (with expression and biological function of human β-defensin 4 HBD4) controlled the degree of pulp inflammation in a rat model of reversible pulpitis and induced the formation of restorative dentin. DPSC may be a useful pulp capping agent for use in vital pulp therapy VPT. | [102] |
| 20. | Comparison of the stemness and differentiation potential of ACCs and DPSCs of human immature permanent teeth with the aim of determining a more suitable source of stem cells for regeneration of the dentin-pulp complex | human DPSCs 13 from permanent teeth of 12 children aged 6–18 years | 15 mice subcutaneous pockets made in 5-week-old male | biphasic calcium phosphate | osteogenic medium | In the in vivo study, ACCs and DPSCs formed amorphous hard tissue using macroporous biphasic calcium phosphate particles. Regarding regeneration of the dentin-pulp complex, the coronal pulp can be a suitable source of stem cells considering its homogenous lineages of cells and favorable osteo/odontogenic differentiation potential. | [103] |
| 21. | Exploration of the survival, differentiation and immunomodulatory ability of transplanted cells in the extreme inflammatory environment, and to investigate tissue regenerative capability and possible corresponding mechanisms of transplanted cells after spinal cord injury | human (18–22 years) DPSCs SHEDs | 32 male Wistar rats (10th spinal cord was completely transected) | natural and artificial scaffold | medium with ascorbic acid | DFSC demonstrated the potential in repairing the completely transected spinal cord and promoting functional recovery after injury | [104] |
| 22. | Evaluation clinical, histological and radiological osseous regeneration in a critical-sized bilateral cortico-medullary osseous defect in model rabbits from New Zealand after receiving a hydroxyapatite matrix and polylactic polyglycolic acid (HA/PLGA) implanted with human dental pulp stem cells (DPSCs) | human DPSCs extracted teeth for orthodontic reasons | 8 rabbits with critical-sized bilateral cortico-medullary osseous defect | hydroxyapatite matrix and polylactic polyglycolic acid (HA/PLGA)/DPSC matrix | BMP | HA/PLGA/DPSC scaffold was an effective in vivo method for mandibular bone regeneration | [105] |
| 23. | Determination of the effects of in vitro odontogenic/cementogenic differentiation on the in vivo tissue regeneration of (DPSCs) and (PDLSCs) | human DPSC from 16 human teeth and PDLSCs | subcutaneously transplanted into the dorsal surface of 5-week-old male mice (n = 45) | scaffold macroporous biphasic calcium phosphate | odontogenic/cementogenic medium | Predifferentiated DPSCs and PDLSCS generated hard tissue closer to dentin and higher-quality and greater amounts of tissue for dental regeneration than undifferentiated | [108] |
| 24. | Differentiation of SHED and DPSCs into islet cells and assessment of their insulin secretory capacity in vitro and in vivo | SHED and DPSCs were obtained from human teeth (5–40 years old) | Balb/C 40 male mice, 6–8 weeks old | immuno-isolatory biocompatible macro-capsules | polyurethane-polyvinylpyrrolidone semiinterpenetrating network | Differentiation DPSCs to islet cells aggregates (ICA) similar to pancreatic islet cells. T source of human tissue that could be used for management of diabetes type 1. | [109] |
| Allotransplantation (Animal Transplantation within the Same Species) | |||||||
|---|---|---|---|---|---|---|---|
| Case No. | Aim | Cell Source | Host | Scaffold/Cell Sheet | Growth Factor | Results | Article |
| 1. | Exploration of the therapeutic effects of DPSCs on acute radiation-induced oesophageal injury | rat DPSC isolated from the incisors | 33 rats with acute radioactive oesophageal injuries induced by radioactive 125I seeds in vivo | The OriCellTM osteogenesis differentiation kit was used to induce osteogenic differentiation. P3 SCs were cultured in osteogenic differentiation medium Adipogenesis was induced by using the OriCellTM adipogenic differentiation kit (injected with DPSCs (1 × 107 cells) | The results demonstrated that transplanted DPSCs, which trans-differentiated into esophageal stem cells in vivo, could repair the damaged esophageal tissue | [91] | |
| 2. | Investigation of the therapeutic potential of DPSCs for ischemic vascular diseases and opportunity for neural regeneration | DPSCs was harvested from the incisors of 4-week-old male SD rats | 24 rats with middle cerebral artery occlusion (MCAO) | Intravenous infusion of DPSCs | Neuroprotective effect on brain ischemia rats, by reducing the infarct volume and enhancing the neurological function recovery after cerebral ischemic injury | [92] | |
| 3. | Verification of DPSCs proliferation and osteogenic differentiation in a three-dimensional cell culture using SPG-178-Gel | DPSCs isolated from the dental pulp of extracted incisors of six-week-old male Sprague-Dawley (SD) rats | 24 h-week-old male Sprague-Dawley (SD) rats with the calvarial defect | self-assembling peptide hydrogel, SPG-178-Gel, | Osteogenic induction medium containing recombinant human bone morphogenetic protein-4 (rhBMP-4) in a two-dimensional cell culture | In conclusion, DPSCs + SPG-178-Gel can be a suitable tool for bone formation in vivo and in vitro | [93] |
| 4. | Evaluation of the osteogenic effects of dense collagen gel scaffolds seeded with rat DPSC (rDPSC) implanted in a rat critical-sized calvarial defect model | DPSC isolated from the molars of 4-day Wistar rats | 30 rats with critical-size calvarial defect model | dense collagen gel scaffolds | Bone mineral density and bone micro-architectural parameters were significantly increased when DPSC-seeded scaffolds were used | [94] | |
| 5. | Comparison of the osteogenic differentiation of bone marrow-derived mesenchymal stromal cells and dental pulp-derived stromal cells (DPSCs) in vitro and in a pig calvaria critical-size bone defect model | DPSCs isolated from the premolar from the upper and lower pig jaw, BMSCs was aspirated from the proximal tibia | 28 pigs with critical-size calvarial defect model | a three-dimensional (3D) polycaprolactone (PCL)–hyaluronic acid–tricalcium phosphate (HT–PCL) scaffold. | DPSCs exhibited a higher osteogenic potential compared with BMSCs both in vitro and in vivo, making it a potential cell source for future bone tissue engineering | [95] | |
| 6. | Assessment of the therapeutic potential of DPSCs transplant in the case of diabetic polyneuropathy | DPSCs isolated from the dental pulp of extracted incisors of Sprague-Dawley rats | 10 points of normal and diabetic rats | Transplantation of DPSCs could be a promising tool for the treatment of diabetic neuropathy | [125] | ||
| 7. | Evaluation of the therapeutic potential of mDPSCs in important complications of diabetes, namely pancreatic damage, renal function alterations and diabetic peripheral neuropathy | DPSCs DPSCs isolated from the incisor teeth of male EGFP transgenic C57BL/6 mice | 12 diabetic mice | Endovenous transplantation | Improved pancreatic damage, renal function and painful neuropathy | [126] | |
| (A) | |||||||
| Autotransplantation (Animal Autologous Transplantation) | |||||||
| Case No. | Aim | Cell Source | Host | Scaffold/Cell Sheet | Growth Factor | Results | Article |
| 1. | The demonstration for the first time of complete pulp regeneration in the root canal, after pulpectomy, in dogs | Dog CD105+ DPSCs | 5 dogs after pulpectomy in mature teeth | a collagen scaffold | stromal cell-derived factor-1 (SDF-1) | Complete pulp regeneration with neurogenesis and vasculogenesis occurred | [96] |
| 2. | Evaluation of effects of dental pulp stem cells (DPSCs) on regeneration of a defect experimentally created in the periodontium of a canine model | DPSCs isolated from 2 maxillary premolar dog teeth | 20 dogs | Bio-Oss | DPSCs are capable of promoting periodontal regeneration | [97] | |
| 3. | Demonstration of the neuronal differentiation of DPSC from murine incisors | DPSCs isolated from murine incisor teeth | murine | mouse-specific fibroblast growth factor-2 (FGF-2) mouse-specific nerve growth factor (NGF) | Generated neuronal-like cells from murine incisor DPSC to an immature stage of development. Our findings encourage the use of mDPSC to develop mouse models of autologous neural therapeutic transplantations for pre-clinical studies. | [106] | |
| 4. | Evaluation of the capacity of a Tissue-engineered bone complex of recombinant human bone morphogenetic protein 2 (rhBMP-2)-mediated rabbit dental pulp stem cells (DPSCs) and nano-hydroxyapatite/collagen/poly(L-lactide) (nHAC/PLA) to reconstruct critical-size alveolar bone defects in New Zealand rabbit | DPSCs from New Zealand white rabbit | 36 rabbits with critical-size alveolar bone defects | scaff-nano-hydroxyapatite/collagen/poly(L-lactide) (nHAC/PLA) | bone morphogenetic protein 2 (rhBMP-2)-mediated dental pulp stem cells (DPSCs) | The rhBMP-2 promoted osteogenic capability of DPSCs as a potential cell source for periodontal bone regeneration. DPSCs might be a better alternative to autologous bone for the clinical reconstruction of periodontal bone defects. | [107] |
| (B) | |||||||
| Autotransplantation (Human Autologous Transplantation) | |||||||
| Case No. | Aim | Cell Source | Host | Scaffold/Cell Sheet | Growth Factor | Results | Article |
| 1. | Evaluation of the safety, potential efficacy and feasibility of autologous transplantation of MDPSCs in pulpectomised teeth | DPSCs isolated from discarded teeth | 5 patients with irreversible pulpitis | atelocollagen | granulocyte colony-stimulating factor (G-CSF) | Pulp regeneration, functional dentin formation in three of the five patients | [39] |
| 2. | Trying to isolate of DPSCs-IPs from two patients and to evaluate the feasibility and the effect of reconstructing periodontal intrabone defects in each patient | DPSCs-IPs derived from inflammatory dental pulp tissues | 2 patientswith cells engrafted into the periodontal defect area in the root furcation. | β-tricalcium phosphate (β-TPC | - | Regeneration of new intrabony defect | [131] |
| 3. | The description of the clinical and radiographic regenerative potential of autologous DPSCs in the treatment of human uncontained intraosseous defects | DPSCs collected from deciduous teeth | 1 patient | - | The defect was completely filled with bonelike tissue | [132] | |
| 4. | To show that implantation of autologous tooth stem cells from deciduous teeth regenerated dental pulp with an odontoblast layer, blood vessels and nerves | DPSCs isolated from deciduous teeth | 40 patients with pulp necrosis after traumatic dental injuries | extracellular matrix | - | Regeneration of dental pulp tissue containing sensory nerves | [133] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnan, M.; Sharma, A.; Saraswathy, S.; Tiwari, B.; Ganganahalli, G.; Londhe, S.; Singh, A.K.; Nair, V. Mesenchymal stem cells from orthodontic premolar teeth. Med. J. Armed Forces India 2020, 76, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Wang, F.S.; Chang, C.; Ippolito, G.; Petrosillo, N.; Agrati, C.; Azhar, E.I.; El-Kafrawy, S.A.; Osman, M.; Zitvogel, L.; et al. Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy—Achieving global consensus and visibility for cellular host-directed therapies. Int. J. Infect. Dis. 2020, 96, 431–439. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, M.; Iohara, K. Immunomodulation and Regeneration Properties of Dental Pulp Stem Cells: A Potential Therapy to Treat Coronavirus Disease 2019. Cell Transplant. 2020, 29, 1–9. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Demarco, F.F.; Conde, M.C.M.; Cavalcanti, B.N.; Casagrande, L.; Sakai, V.T.; Nör, J.E. Dental pulp tissue engineering. Braz. Dent. J. 2011, 22, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Frank, R.M.; Nalbandian, J. Structure and Ultrastructure of the Dental Pulp. Teeth 1976, 5, 249–307. [Google Scholar] [CrossRef]
- Horst, O.V.; Horst, J.A.; Samudrala, R.; Dale, B.A. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 2011, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of Growth Factors on Dental Stem/Progenitor Cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadaghiani, L.; Gleeson, H.B.; Youde, S.; Waddington, R.J.; Lynch, C.D.; Sloan, A.J. Growth factor liberation and DPSC response following dentine conditioning. J. Dent. Res. 2016, 95, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Iezzi, I.; Pagella, P.; Mattioli-Belmonte, M.; Mitsiadis, T.A. The effects of ageing on dental pulp stem cells, the tooth longevity elixir. Eur. Cells Mater. 2019, 37, 175–185. [Google Scholar] [CrossRef]
- Hanafy, A.K.; Shinaishin, S.F.; Eldeen, G.N.; Aly, R.M. Nano hydroxyapatite & mineral trioxide aggregate efficiently promote odontogenic differentiation of dental pulp stem cells. Open Access Maced. J. Med. Sci. 2018, 6, 1727–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, M.; Chiego, D.J.; Heys, D.R. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch. Oral Biol. 1990, 35, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yelick, P.C. Vital Pulp Therapy—Current Progress of Dental Pulp Regeneration and Revascularization. Int. J. Dent. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, I.; Hollý, D.; Vojtaššák, J.; Böhmer, D.; Polák, Š.; Danišovič, L. Morphological characterization of in vitro expanded human dental pulp-derived stem cells. Biologia 2011, 66, 706–711. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Botero, T.M. Dental Stem Cells and Their Sources. Dent. Clin. N. Am. 2012, 56, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Junco, B.A.; Villicaña, C. Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation. Tissue Eng. Regen. Med. 2017, 14, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Elaine, E. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Marchionni, C.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Di Tullio, A.; Costa, R.; Bagnara, G.P. Angiogenic potential of human Dental Pulp Stromal (STEM) Cells. Int. J. Immunopathol. Pharmacol. 2009, 22, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mikami, Y.; Ishii, Y.; Watanabe, N.; Shirakawa, T.; Suzuki, S.; Irie, S.; Isokawa, K.; Honda, M.J. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011, 20, 901–913. [Google Scholar] [CrossRef]
- Carinci, F.; Papaccio, G.; Laino, G.; Palmieri, A.; Brunelli, G.; D’Aquino, R.; Graziano, A.; Lanza, V.; Scapoli, L.; Martinelli, M.; et al. Comparison between genetic portraits of osteoblasts derived from primary cultures and osteoblasts obtained from human pulpar stem cells. J. Craniofac. Surg. 2008, 19, 616–625. [Google Scholar] [CrossRef]
- Laino, G.; D’Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A new population of human adult dental pulp stem cells: A useful source of living autologous fibrous bone tissue (LAB). J. Bone Miner. Res. 2005, 20, 1394–1402. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Scacco, S.; Perillo, L.; Scarano, A.; Aityan, S.K.; Contaldo, M.; Nguyen, K.C.D.; Santacroce, L.; Syed, J.; et al. A comparative study on different stemness gene expression between dental pulp stem cells vs. Dental bud stem cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1626–1633. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Jahani, M.A.; Jahani, M.; Mirzaii-Dizgah, I.; Ali-Moghaddam, K. In vitro isolation of stem cells derived from human dental pulp. Clin. Transplant. 2010, 24, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Jasper, E. PDF hosted at the Radboud Repository of the Radboud University Nijmegen Article information. J. Stat. Softw. 2008, 18, 3–6. [Google Scholar]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Shi, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [Green Version]
- Karaöz, E.; Doǧan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.Ç.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Pivoriuunas, A.; Surovas, A.; Borutinskaite, V.; Matuzevicčius, D.; Treigyte, G.; Savickiene, J.; Tunaitis, V.; Aldonyte, R.; Jarmalavicčiuute, A.; Suriakaite, K.; et al. Proteomic analysis of stromal cells derived from the dental pulp of human exfoliated deciduous teeth. Stem Cells Dev. 2010, 19, 1081–1093. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dörfer, C.E. Toll-like receptor expression profile of human dental pulp stem/progenitor cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.H.; Yang, J.C.; Wang, C.W.; Lee, S.Y. Dental Stem Cells and Tooth Banking for Regenerative Medicine. J. Exp. Clin. Med. 2010, 2, 111–117. [Google Scholar] [CrossRef]
- Huang, A.H.C.; Chen, Y.K.; Chan, A.W.S.; Shieh, T.Y.; Lin, L.M. Isolation and Characterization of Human Dental Pulp Stem/Stromal Cells From Nonextracted Crown-fractured Teeth Requiring Root Canal Therapy. J. Endod. 2009, 35, 673–681. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, B.C.; Zhou, D.; Wu, X.; Yang, F.C.; Byers, M.A.; Chu, T.M.G.; Hockema, J.J.; Woods, E.J.; Goebel, W.S. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng.-Part C Methods 2008, 14, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh, R.; Eslaminejad, M.B.; Aflatoonian, R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J. Vis. Exp. 2012, 69, 4372. [Google Scholar] [CrossRef] [Green Version]
- Alkhalil, M.; Smajilagić, A.; Redžić, A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med. Glas. (Zenica) 2015, 12, 27–32. [Google Scholar] [PubMed]
- Woods, E.J.; Perry, B.C.; Hockema, J.J.; Larson, L.; Zhou, D.; Goebel, W.S. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009, 59, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Chimenti, I.; Gaetani, R.; Barile, L.; Forte, E.; Ionta, V.; Angelini, F.; Frati, G.; Messina, E.; Giacomello, A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol. Biol. 2012, 879, 327–338. [Google Scholar] [CrossRef]
- Alsulaimani, R.S.; Ajlan, S.A.; Aldahmash, A.M.; Alnabaheen, M.S.; Ashri, N.Y. Isolation of dental pulp stem cells from a single donor and characterization of their ability to differentiate after 2 years of cryopreservation. Saudi Med. J. 2016, 37, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.J.; Sonoyama, W.; Chen, J.; Park, S.H. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006, 324, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Fujimoto, K.; Goto, N.; Kanawa, M.; Kawamoto, T.; Pan, H.; Srivatanakul, P.; Rakdang, W.; Pornprasitwech, J.; Saskianti, T.; et al. Characteristic expression of MSX1, MSX2, TBX2 and ENTPD1 in dental pulp cells. Biomed. Rep. 2015, 3, 566–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoof, M.; Yaghoobi, M.M.; Derakhshani, A.; Kamal-Abadi, A.M.; Ebrahimi, B.; Abbasnejad, M.; Shokouhinejad, N. A modified efficient method for dental pulp stem cell isolation. Dent. Res. J. 2014, 11, 244–250. [Google Scholar]
- Alipour, R.; Sadeghi, F.; Hashemi-Beni, B.; Zarkesh-Esfahani, S.H.; Heydari, F.; Mousavi, S.B.; Adib, M.; Narimani, M.; Esmaeili, N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int. J. Prev. Med. 2010, 1, 164–171. [Google Scholar] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Kubo, H.; Shimizu, M.; Taya, Y.; Kawamoto, T.; Michida, M.; Kaneko, E.; Igarashi, A.; Nishimura, M.; Segoshi, K.; Shimazu, Y.; et al. Identification of mesenchymal stem cell (MSC)-transcription factors by microarray and knockdown analyses, and signature molecule-marked MSC in bone marrow by immunohistochemistry. Genes Cells 2009, 14, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K. Regeneration of dental pulp by stem cells. Adv. Dent. Res. 2011, 23, 313–319. [Google Scholar] [CrossRef]
- Lindemann, D.; Werle, S.B.; Steffens, D.; Garcia-Godoy, F.; Pranke, P.; Casagrande, L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch. Oral Biol. 2014, 59, 970–976. [Google Scholar] [CrossRef]
- Malekfar, A.; Valli, K.S.; Kanafi, M.M.; Bhonde, R.R. Isolation and Characterization of Human Dental Pulp Stem Cells from Cryopreserved Pulp Tissues Obtained from Teeth with Irreversible Pulpitis. J. Endod. 2016, 42, 76–81. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, S.; Rattan, V. Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank. 2015, 16, 513–522. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Tsang, V.L.; Sah, R.L.; Bhatia, S.N. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab A Chip 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, F.; Inchingolo, F.; Dipalma, G.; Postiglione, F.; Fulle, S.; Scarano, A. Synthetic scaffold/dental pulp stem cell (DPSC) tissue engineering constructs for bone defect treatment: An animal studies literature review. Int. J. Mol. Sci. 2020, 21, 9765. [Google Scholar] [CrossRef] [PubMed]
- Bratt-Leal, A.M.; Carpenedo, R.L.; Ungrin, M.D.; Zandstra, P.W.; McDevitt, T.C. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 2011, 32, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.F. Control of stem cell fate by engineering their micro and nanoenvironment. World J. Stem Cells 2015, 7, 37. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Gentleman, E.; Polak, J.M. Scaffolds for stem cells to encourage the development of functional replacement tissue. Mater. Today 2006, 9, 26–33. [Google Scholar] [CrossRef]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental pulp stem cells response on the nanotopography of scaffold to regenerate dentin-pulp complex tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rust, K.R.; Singleton, G.T.; Wilson, J.; Antonelli, P.J. Bioglass middle ear prosthesis: Long-term results. Am. J. Otol. 1996, 17, 371–374. Available online: http://europepmc.org/abstract/MED/8817012 (accessed on 1 December 2021). [PubMed]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro differentiation potential of mesenchymal stem cells. Transfus. Med. Hemotherapy 2008, 35, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Göpferich, A.; Schmalz, G. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Wang, D.-R.; Wang, Y.-H.; Pan, J.; Tian, W.-D. Neurotrophic effects of dental pulp stem cells in repair of peripheral nerve after crush injury. World J. Stem Cells 2020, 12, 1196–1213. [Google Scholar] [CrossRef]
- Jin, Q.; Yuan, K.; Lin, W.; Niu, C.; Ma, R.; Huang, Z. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2020, 47, 66–72. [Google Scholar] [CrossRef]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. Rgd-and vegf-mimetic peptide epitope-functionalized self-assembling peptide hydrogels promote dentin-pulp complex regeneration. Int. J. Nanomed. 2020, 15, 6631–6647. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Brizuela, C.; Khoury, M. Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential. Cell Transplant. 2018, 27, 967–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Atalayin, C.; Tezel, H.; Dagci, T.; Yavasoglu, N.U.K.; Oktem, G. Medium modification with bone morphogenetic protein 2 addition for odontogenic differentiation. Braz. Oral Res. 2016, 30, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [Green Version]
- Horibe, H.; Murakami, M.; Iohara, K.; Hayashi, Y.; Takeuchi, N.; Takei, Y.; Kurita, K.; Nakashima, M. Isolation of a stable subpopulation of Mobilized Dental Pulp Stem Cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS ONE 2014, 9, e98553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Miyabe, M.; Ito, M.; Makino, E.; Kanada, S.; Saiki, T.; Ohno, T.; et al. Transplantation of human dental pulp stem cells ameliorates diabetic polyneuropathy in streptozotocin-induced diabetic nude mice: The role of angiogenic and neurotrophic factors. Stem Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.; Rabie, A.B.M. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy 2013, 15, 712–725. [Google Scholar] [CrossRef]
- Zhang, Z.; Nör, F.; Oh, M.; Cucco, C.; Shi, S.; Nör, J.E. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells 2016, 34, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, Y.; Feng, Z.; Zhang, F.; Liu, Z.; Sun, X.; Ruan, M.; Liu, M.; Jin, S. Therapeutic effect of dental pulp stem cell transplantation on a rat model of radioactivity-induced esophageal injury. Cell Death Dis. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, Y.; Li, H.; Wang, R.; Yang, D.; Li, B.; Cao, X.; Fu, J. Transplanted Dental Pulp Stem Cells Migrate to Injured Area and Express Neural Markers in a Rat Model of Cerebral Ischemia. Cell. Physiol. Biochem. 2018, 45, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, J.; Naruse, K.; Nagai, Y.; Kan, S.; Nakamura, N.; Hata, M.; Omi, M.; Hayashi, T.; Kawai, T.; Matsubara, T. Efficacy of a self-Assembling peptide hydrogel, spg-178-gel, for bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng. Part A 2017, 23, 1394–1402. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.; Tvedesøe, C.; Rölfing, J.H.D.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.E.; Chen, M.; Baas, J.; Le, D.Q.S.; Bünger, C.E. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. Sicot-J 2016, 2, 16. [Google Scholar] [CrossRef]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Zhu, L.; Pan, J.; Shen, Z.; Yang, Z.; Wang, J.; Bai, X.; Lin, Y.; Tao, J. hsa_circ_0026827 Promotes Osteoblast Differentiation of Human Dental Pulp Stem Cells Through the Beclin1 and RUNX1 Signaling Pathways by Sponging miR-188-3p. Front. Cell Dev. Biol. 2020, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Yamakawa, D.; Kawase-Koga, Y.; Fujii, Y.; Kanno, Y.; Sato, M.; Ohba, S.; Kitaura, Y.; Kashiwagi, M.; Chikazu, D. Effects of helioxanthin derivative-treated human dental pulp stem cells on fracture healing. Int. J. Mol. Sci. 2020, 21, 9158. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kim, S.; Jeon, M.; Jung, U.W.; Lee, J.H.; Choi, H.J.; Choi, J.E.; Song, J.S. Evaluation of the Apical Complex and the Coronal Pulp as a Stem Cell Source for Dentin-pulp Regeneration. J. Endod. 2020, 46, 224–231. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef]
- Gutiérrez-Quintero, J.G.; Durán Riveros, J.Y.; Martínez Valbuena, C.A.; Pedraza Alonso, S.; Munévar, J.; Viafara-García, S. Critical-sized mandibular defect reconstruction using human dental pulp stem cells in a xenograft model-clinical, radiological, and histological evaluation. Oral Maxillofac. Surg. 2020, 24, 485–493. [Google Scholar] [CrossRef]
- Ellis, K.M.; O’Carroll, D.C.; Lewis, M.D.; Rychkov, G.Y.; Koblar, S.A. Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Res. Ther. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.C.; E, L.L.; Wang, D.S.; Su, F.; Wu, X.; Shi, Z.P.; Lv, Y.; Wang, J.Z. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(l-lactide). Tissue Eng. Part A 2011, 17, 2417–2433. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Jeon, M.; Lee, H.S.; Kim, S.; Kim, S.O.; Lee, J.H.; Song, J.S. Effects of In Vitro Osteogenic Induction on In Vivo Tissue Regeneration by Dental Pulp and Periodontal Ligament Stem Cells. J. Endod. 2015, 41, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Kanafi, M.M.; Rajeshwari, Y.B.; Gupta, S.; Dadheech, N.; Nair, P.D.; Gupta, P.K.; Bhonde, R.R. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 2013, 15, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Datta, I.; Bhadri, N.; Shahani, P.; Majumdar, D.; Sowmithra, S.; Razdan, R.; Bhonde, R. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 2017, 19, 1208–1224. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Kozhuharova, A.; Tanaka, T.; Okada, M.; Mitev, V.; Fukuda, M.; Imai, T. Pancreatic differentiation of human dentStrober, W. 2001. ‘Trypan Blue Exclusion Test of Cell Viability’. Current Protocols in Immunology/Edited by John, E. Coligan ... [et Al.] Appendix 3:Appendix 3B.acl pulp CD117+ stem cells. Regen. Med. 2013, 8, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Torrente, Y.; Belicchi, M.; Sampaolesi, M.; Pisati, F.; Meregalli, M.; D’Antona, G.; Tonlorenzi, R.; Porretti, L.; Gavina, M.; Mamchaoui, K.; et al. Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Investig. 2004, 114, 182–195. [Google Scholar] [CrossRef]
- Palladino, M.; Gatto, I.; Neri, V.; Straino, S.; Smith, R.C.; Silver, M.; Gaetani, E.; Marcantoni, M.; Giarretta, I.; Stigliano, E.; et al. Angiogenic impairment of the vascular endothelium: A novel mechanism and potential therapeutic target in muscular dystrophy. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2867–2876. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Asakura, Y.; Hirai, H.; Watanabe, S.; Tastad, C.; Fong, G.H.; Ema, M.; Call, J.A.; Lowe, D.A.; Asakura, A. Flt-1 haploinsufficiency ameliorates muscular dystrophy phenotype by developmentally increased vasculature in mdx mice. Hum. Mol. Genet. 2010, 19, 4145–4159. [Google Scholar] [CrossRef] [Green Version]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic Properties of Human Dental Pulp Stem Cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef] [Green Version]
- Hilkens, P.; Fanton, Y.; Martens, W.; Gervois, P.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014, 12, 778–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, A.; Shi, S.; Zannettino, A.C.W.; Fujii, N.; Gronthos, S.; Koblar, S.A. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells 2009, 27, 2229–2237. [Google Scholar] [CrossRef]
- Brittberg, M.; Nilsson, A.; Lindahl, A.; Ohlsson, C.; Peterson, L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin. Orthop. Relat. Res. 1996, 326, 270–283. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rando, T.A. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 2011, 193, 257–266. [Google Scholar] [CrossRef]
- Khan, M.; Mohsin, S.; Khan, S.N.; Riazuddin, S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J. Cell Mol. Med. 2011, 15, 1515–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [Green Version]
- Samsonraj, R.M.; Dudakovic, A.; Zan, P.; Pichurin, O.; Cool, S.M.; Van Wijnen, A.J. A Versatile Protocol for Studying Calvarial Bone Defect Healing in a Mouse Model. Tissue Eng.-Part C Methods 2017, 23, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Tosaki, T.; Miyabe, M.; Kojima, N.; Kubo, K.; Ozawa, S.; Maeda, H.; et al. Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: Therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res. Ther. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, E.T.; da Cruz, G.S.; de Almeida, T.F.; de Souza, B.S.F.; Kaneto, C.M.; Vasconcelos, J.F.; dos Santos, W.L.C.; Ribeiro-dos-Santos, R.; Villarreal, C.F.; Soares, M.B.P. Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant. 2013, 22, 2345–2354. [Google Scholar] [CrossRef]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Ozawa, S.; Nukada, H.; Tsukamoto, M.; Sango, K.; Himeno, T.; Kamiya, H.; et al. Transplantation of dental pulp stem cells improves long-Term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.A.; Martins, J.F.P.; Maria, D.A.; Wenceslau, C.V.; De Souza, D.M.; Kerkis, A.; Câmara, N.O.S.; Balieiro, J.C.C.; Kerkis, I. Immature Dental Pulp Stem Cells Showed Renotropic and Pericyte-Like Properties in Acute Renal Failure in Rats. Cell Med. 2015, 7, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mendonça Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofac. Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, S.; Nan, X.; Wei, H.; Shi, J.; Li, A.; Gou, J. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues Rocky Tuan; Timothy O’Brien. Stem Cell Res. Ther. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimetti, M.; Ferrarotti, F.; Cricenti, L.; Mariani, G.M.; Romano, F. Autologous dental pulp stem cells in periodontal regeneration: A case report. nt. J. Periodontics Restor. Dent. 2014, 34 (Suppl. 3), s27–s33. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [Green Version]
- Feigin, K.; Shope, B. Regenerative endodontics. J. Vet. Dent. 2017, 34, 161–178. [Google Scholar] [CrossRef]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Rezaei-Tavirani, M.; Farhadihosseinabadi, B.; Zali, H.; Niknejad, H. Human amniotic mesenchymal stem cells to promote/suppress cancer: Two sides of the same coin. Stem Cell Res. Ther. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Alcayaga-Miranda, F.; Illanes, S.E.; Figueroa, F.E. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front. Immunol. 2014, 5, 205. [Google Scholar] [CrossRef] [Green Version]
- Cairo, M.S.; Wagner, J.E. Placental and/or umbilical cord blood: An alternative source of hematopoietic stem cells for transplantation. Blood 1997, 90, 4665–4678. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.A.; Crawford, A.; English, A.; Henshaw, K.; Mundy, J.; Corscadden, D.; Chapman, T.; Emery, P.; Hatton, P.; McGonagle, D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008, 58, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniowski, T.; Zawadzka-Knefel, A.; Skośkiewicz-Malinowska, K. Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types. Molecules 2021, 26, 7423. https://doi.org/10.3390/molecules26247423
Staniowski T, Zawadzka-Knefel A, Skośkiewicz-Malinowska K. Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types. Molecules. 2021; 26(24):7423. https://doi.org/10.3390/molecules26247423
Chicago/Turabian StyleStaniowski, Tomasz, Anna Zawadzka-Knefel, and Katarzyna Skośkiewicz-Malinowska. 2021. "Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types" Molecules 26, no. 24: 7423. https://doi.org/10.3390/molecules26247423
APA StyleStaniowski, T., Zawadzka-Knefel, A., & Skośkiewicz-Malinowska, K. (2021). Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types. Molecules, 26(24), 7423. https://doi.org/10.3390/molecules26247423






