Influence of Mixed Imide Composition and Thermal Annealing on Ionic Liquid Uptake and Conductivity of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes
Abstract
:1. Introduction
2. System Design
3. Results
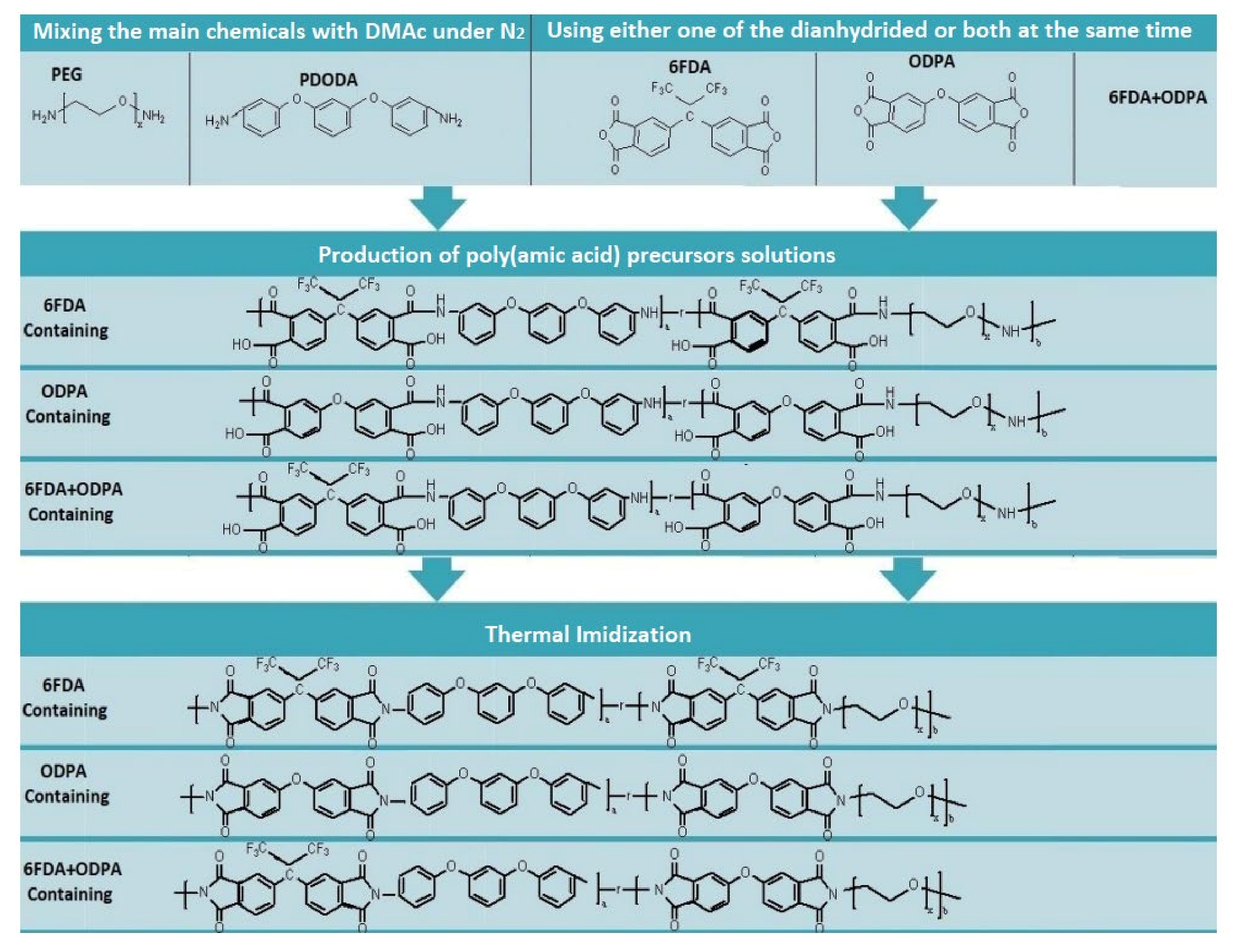
3.1. Synthesis of Polyimide
3.2. Material Characterization
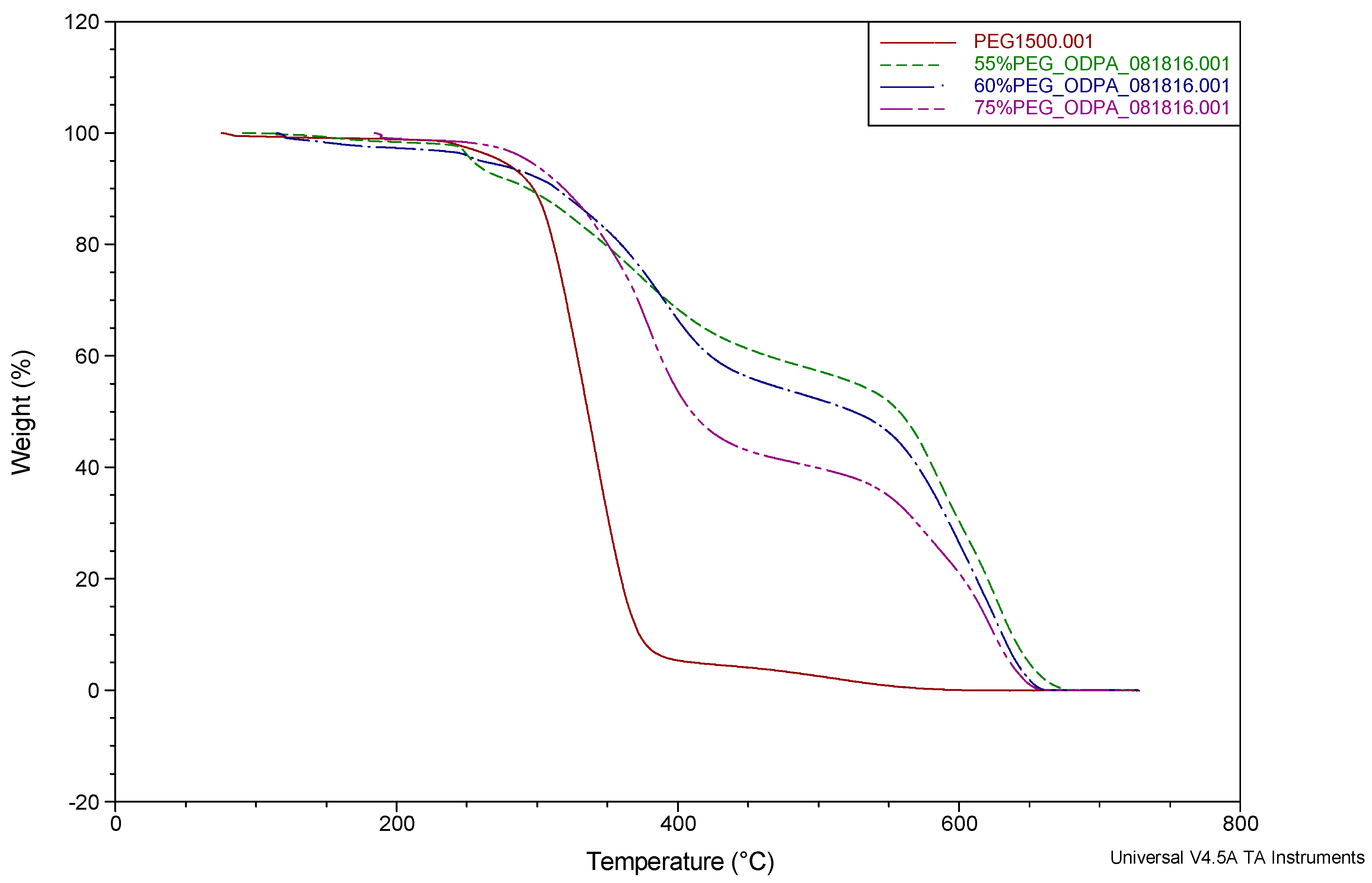
3.2.1. Thermal Stability of PI-PEG Systems
3.2.2. Morphology of Polymers
3.2.3. Ionic Liquid and Water Uptake
3.2.4. Conductivity
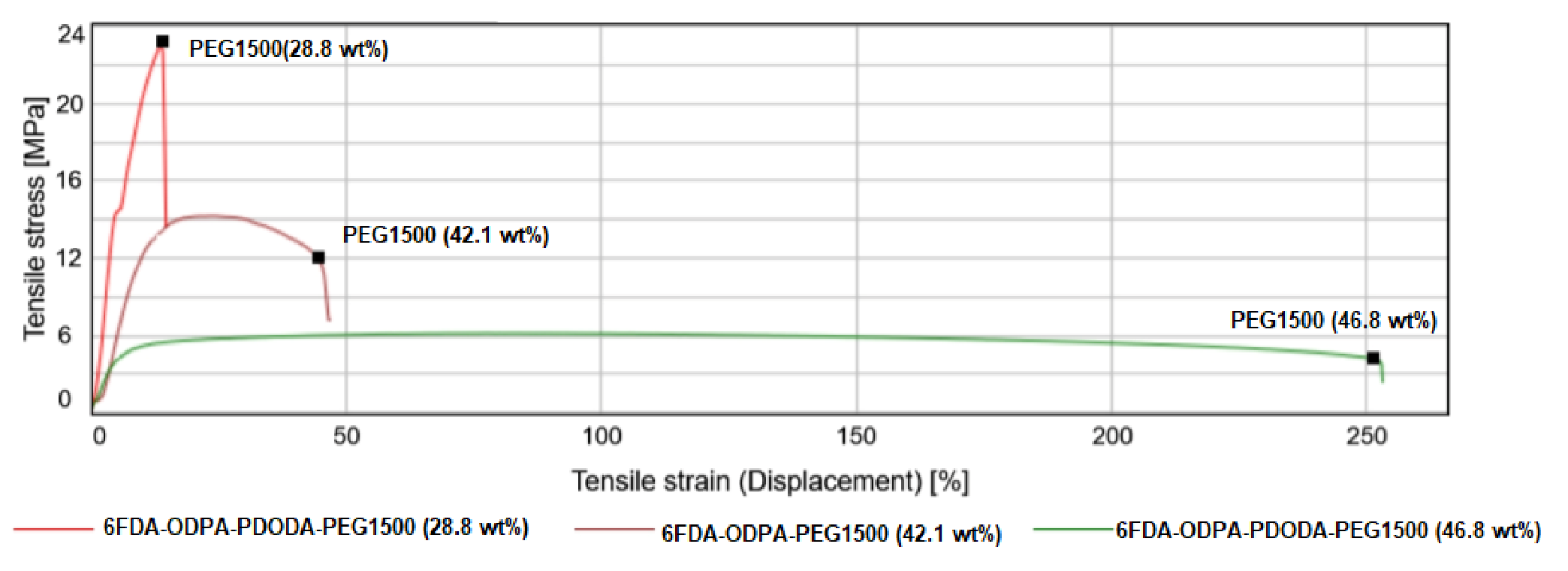
3.2.5. Tensile Test
4. Discussion
5. Materials and Methods
5.1. Materials
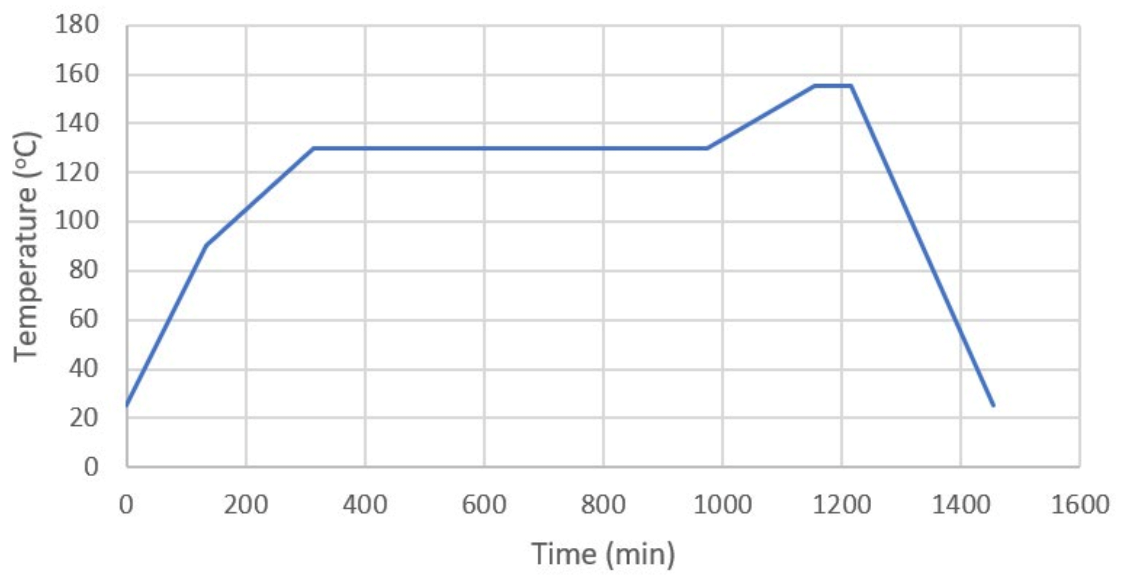
5.2. Synthesis
5.3. Characterization
5.3.1. FTIR
5.3.2. TG
5.3.3. DSC
5.4. Ionic Liquid and Water Uptake
5.5. Conductivity
5.6. Tensile
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–100. [Google Scholar]
- National Energy Technology Laboratory. Fuel Cell Handbook, 7th ed.; EG&G Technical Services, Inc.: Morgantown, WV, USA, 2004.
- Viesters, D.; Melece, L. Advantages and Disadvantages of Biofuels: Observations in Latvia, Engineering for Rural Development. In Proceedings of the 13th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 29–30 May 2014; pp. 210–215. [Google Scholar]
- Baker, K.; Hug, G.; Li, X. Optimal integration of intermittent energy sources using distributed multi-step optimization. In Proceedings of the IEEE Power and Energy Society General Meeting, San Diego, CA, USA, 1–8 July 2012; pp. 1–8. [Google Scholar]
- Available online: https://www.ge.com/sites/default/files/GE_FuelCells.pdf (accessed on 24 November 2018).
- Hardman, S.; Chandan, A.; Steinberger-Wilckens, R. Fuel cell added value for early market applications. J. Power Sour. 2015, 287, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Ralph, T.R. Proton exchange membrane fuel cells progress in cost reduction of the key components. Platin. Met. Rev. 1997, 41, 102–113. [Google Scholar]
- Dunwoody, D.; Leddy, J. Proton Exchange Membranes: The View Forward and Back. Electrochem. Soc. Interface 2005, 14, 37–39. [Google Scholar] [CrossRef]
- Blomen, L.J.M.J.; Mugerwa, M.N. (Eds.) Fuel Cell Systems; Springer: New York, NY, USA, 1993; p. 614. [Google Scholar]
- Srinivasan, S.; Dave, B.B.; Murugesamoorthi, K.A.; Parthasarathy, A.; Appleby, A.J. Overview of Fuel Cell Technology. In Fuel Cell Systems; Blomen, L.J.M.J., Mugerwa, M.N., Eds.; Plenum: New York, NY, USA, 1993; pp. 37–72. [Google Scholar]
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. J. Power Sour. 2001, 103, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Coletta, E.; Toney, M.F.; Frank, C.W. Influences of liquid electrolyte and polyimide identity on the structure and conductivity of polyimide–poly(ethylene glycol) materials. J. Appl. Polym. Sci. 2015, 132, 41675–41687. [Google Scholar] [CrossRef]
- Shu, Y. Preparation and properties of polyethylene glycol-based composite phase change materials. Adv. Mat. Res. 2014, 1004–1005, 546–549. [Google Scholar]
- Majumdar, R.; Alexander, K.S.; Riga, A.T. Physical characterization of polyethylene glycols by thermal analytical technique and the effect of humidity and molecular weight. Pharmazie 2010, 65, 343–347. [Google Scholar]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K.; Kujawa, J. A Review on Ionic Liquids-Based Membranes for Middle and High Temperature Polymer Electrolyte Membrane Fuel Cells (PEM FCs). Int. J. Mol. Sci. 2021, 22, 5430. [Google Scholar] [CrossRef]
- Lu, J.; Tang, H.; Xu, C.; Jiang, A.P. Nafion membranes with ordered mesoporous structure and high water retention properties for fuel cell applications. J. Mater. Chem. 2012, 22, 5810–5819. [Google Scholar] [CrossRef]
- Barclay Satterfield, M.; Majsztrik, P.W.; Ota, H.; Benziger, J.B.; Bocarsly, A.B. Mechanical properties of Nafion and titania/Nafion composite membranes for polymer electrolyte membrane fuel cells. J. Poly. Sci. 2006, 44, 2327–2345. [Google Scholar] [CrossRef] [Green Version]
- Eslami, B.; López-Guerra, E.A.; Raftari, M.; Solares, S.D. Evolution of nano-rheological properties of Nafion® thin films during pH modification by strong base treatment: A static and dynamic force spectroscopy study. J. App. Phy. Sci. 2016, 119, 165301. [Google Scholar] [CrossRef]
- Malhotra, S.; Datta, R. Membrane-supported nonvolatile acidic electrolytes allow higher temperature operation of proto-exchange membrane fuel cells. J. Electrochem. Soc. L. 1997, 144, 23–26. [Google Scholar] [CrossRef]
- Doyle, M.; Choi, S.; Proulx, G. High-temperature proton conducting membranes based on perfluorinated ionomer membrane-ionic liquid composites. J. Electrochem. Soc. 2000, 147, 34–37. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Palombari, R. Inorgano-organic proton conducting membranes for fuel cells and sensors at medium temperatures. J. Membr. Sci. 2000, 172, 233–239. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Payne, J.T. Perfluorosulfonate ionomer]/silicate hybrid membranes via base-catalyzed in situ sol-gel processes for tetraethylorthosilicate. J. Membr. Sci. 2000, 168, 39–51. [Google Scholar] [CrossRef]
- Nakano, T.; Nagaoka, S.; Kawakami, H. Preparation of novel sulfonated block copolyimides for proton conductivity membranes. Polym. Adv. Technol. 2005, 16, 753–757. [Google Scholar] [CrossRef]
- Hatori, H.; Kobayashi, T.; Hanzawa, Y.; Yamada, Y.; Limura, Y.; Kimura, T.; Shiraishi, M. Mesoporous carbon membranes from polyimide blended with poly(ethylene glycol). J. Appl. Polym. Sci. 2001, 79, 836–841. [Google Scholar] [CrossRef]
- Chen, E.Q.; Jing, A.J.; Weng, X.; Huang, P.; Lee, S.W.; Cheng, S.Z.; Hsiao, B.S.; Yeh, F. In situ observation of low molecular weight poly(ethylene oxide) crystal melting, recrystallizetion. Polymer 2003, 44, 6051–60588. [Google Scholar] [CrossRef]
- Chen, E.Q.; Weng, X.; Zhang, A.; Mann, I.; Harris, F.W.; Cheng, S.Z.D. Primary nucleation in polymer crystallization. Macromol. Rapid. Commun. 2001, 22, 611–615. [Google Scholar] [CrossRef]
- Hayamizu, K.; Sugimoto, K.; Akiba, E.; Corporation, Y.; Price, W.S. An NMR and ionic conductivity study of ion dynamics in liquid poly (ethylene oxide)-based electrolytes doped with LiN(SO2CF3)2. J. Phys. Chem B. 2002, 106, 547–554. [Google Scholar] [CrossRef]
- Coletta, E.; Toney, M.F.; Frank, C.W. Impacts of Polymer-polymer Interactions and Interfaces on the structure and conductivity of PEG-containing polyimides doped with ionic liquid. Polymers 2014, 55, 6883–6895. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water Soluble Polymers as Proton Exchange Membranes for Fuel Cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Ho, W.W. Recent developments in fuel-processing and proton-exchange membranes for fuel cells. Polym. Int. 2011, 60, 26–41. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Deluca, N.W.; Elabd, Y.A. Polymer electrolyte membranes for the direct methanol fuel cell: A review. J. Polym. Sci. Part. B Polym. Phys. 2006, 44, 22001–22025. [Google Scholar] [CrossRef]
- Roziere, J.; Jones, D.J. Non-Fluorinated polymer metarials for protone xchange membrane fuel cells. Annu. Rev. 2003, 33, 503–555. [Google Scholar]
- Coletta, E.; Toney, M.F.; Frank, C.W. Effects of Aromatic Regularity on the Structure and Conductivity of Polyimide-Poly(ethylene glycol) Materials Doped with Ionic Liquid. J. Polym. Sci. Part. B Polym. Phys. 2015, 53, 509–521. [Google Scholar] [CrossRef]
- Eastman, S.A.; Kim, S.; Page, K.; Rowe, B.W.; Kang, S. Effect of confinement on structure, water solubility and water transport in Nafion thin films. Macromolecules 2012, 45, 7920–7930. [Google Scholar] [CrossRef]
- Kim, S.; Dura, J.; Page, K.; Soles, C. Interfacial morphology and water transport of proton-conducting membranes in fuel cell. Macromolecules 2013, 46, 5630–5637. [Google Scholar] [CrossRef]
- Page, K.; Kusoglu, A.; Stafford, C.; Kim, S.; Kline, R.; Weber, A. Confinement-drive increase in ionomer thin-film modulus. Nano. Lett. 2014, 14, 2299–2304. [Google Scholar] [CrossRef]
- Davis, E.; Stafford, C.; Page, K. Elucidating water transport mechanisms in Nafion thin films. ACS Macro. Lett. 2014, 3, 1029–1035. [Google Scholar] [CrossRef]
- Woo, E.; Coletta, E.; Holm, A.; Mun, J.; Toney, M.F.; Yoon, D.Y.; Frank, C.W. Polyimide-PEG Segmented Block Copolymer Membranes with Hight Proton Conductivity by Improving Bicontinuous Nanostructure of ionic-Doped Films. Macromol. Chem. Phys. 2019, 220, 1900006. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Laupheimer, M.; Sottmann, T.; Schweins, R.; Stubenrauch, C. Studying orthogonal self-assembled systems: Microstructure of gelled bicontinuous microemulsions. Soft Matter. 2014, 10, 8744. [Google Scholar] [CrossRef]
- Jung, C.; Jikei, M.; Kakimoto, M. Synthesis of polyamic acid possessing second-order nonlinear optical properties and its application to Langmuir–Blodgett films. J. Opt. Soc. Am. B 1998, 15, 471–476. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.; Chen, W.; Yi, C.; Xu, Z. Preparation of poly(amic acid) and polyimide via microwave-assisted polycondensation of aromatic dianhydrides and diamines. Macromol. Symp. 2008, 261, 148–156. [Google Scholar] [CrossRef]
- Liu, P. Preparation and characterizations of poly(amic acid) grafted silica nanoparticles. Iran. Polym. J. 2005, 14, 968–972. [Google Scholar]
- Yang, J.; Lee, M.A. Water-soluble polyimide precursor: Synthesis and characterization of poly(amic acid) salt. Macromol. Res. 2004, 12, 263–268. [Google Scholar] [CrossRef]
- Marcos-Fernandez, A.; Tena, A.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J.; Palacio, L.; Pradanos, P.; Hernandez, A. Physical properties of films made of copoly(ether-imide)s with long poly(ethylene oxide) segments. Eur. Polym. J. 2010, 46, 2352–2364. [Google Scholar] [CrossRef] [Green Version]
- Tena, A.; Marcos-Fernandez, A.; Palacio, L.; Pradanos, P.; Lozano, A.E.; de Abajo, J.; Hernandez, A. On the influence of the proportion of PEO in thermally controlled phase segregation of copoly (ether-imide) s for gas separation. J. Membr. Sci. 2013, 434, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Tena, A.; Marcos-Fernandez, A.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J.; Palacio, L.; Pradanos, P.; Hernandez, A. Thermally treated copoly (ether-imide) s made from bpda and alifatic plus aromatic diamines. Gas separation properties with different aromatic diamimes. J. Membr. Sci. 2012, 387, 54–65. [Google Scholar] [CrossRef]
- Tena, A.; de la Viuda, M.; Palacio, L.; Pradanos, P.; Marcos Fernandez, A.; Lozano, A.E.; Hernandez, A. Prediction of gas permeability of block-segregated polymeric membranes by an effective medium model. J. Membr. Sci. 2014, 453, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Tena, A.; Marcos-Fernandez, A.; Palacio, L.; Cuadrado, P.; Pradanos, P.; de Abajo, J.; Lozano, A.E.; Hernandez, A. Phase segregation and gas separation properties of thermally treated copoly (ether-imide) from an aromatic dianhydride, an aromatic diamine, and various aliphatic diamines. Ind. Eng. Chem. Res. 2012, 51, 3766–3775. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Chung, T.S. Synthesis and characterization of poly (ethylene oxide) containing copolyimides for hydrogen purification. Polymers 2010, 51, 4077–4086. [Google Scholar] [CrossRef]
- Huertas, R.M.; Doherty, C.M.; Hill, A.J.; Lozano, A.E.; de Abajo, J.; de la Campa, J.G.; Maya, E.M. Preparation and gas separation properties of partially pyrolyzed membranes (PPMs) derived from copolyimides containing polyethylene oxide side chains. J. Membr. Sci. 2012, 409–410, 200–211. [Google Scholar] [CrossRef]
- Maya, E.M.; Munoz, D.M.; Lozano, A.E.; De Abajo, J.; De La Campa, J.G. Fluorenyl cardo copolyimides containing poly(ethylene oxide) segments: Synthesis, characterization, and evaluation of properties. J. Polym. Sci. Part. A Polym. Chem. 2008, 46, 8170–8178. [Google Scholar] [CrossRef]
- Georgiev, A.; Dimov, D.; Spassova, E.; Assa, J.; Dineff, P.; Danev, G. Chemical and Physical of polyimides: Biomedical and Engineering Applications. Intech. Open 2012, 65–84. [Google Scholar]
- Lazareva, Y.N.; Vidyakin, M.N.; Alentiev, A.Y.; Yablokova, M.Y.; Kuznetsov, A.A.; Ronova, I.A. Transport properties of polyimides derived from benzophenonetetracarboxylic dianhydride and other diamines. Polym. Sci. Ser. A 2009, 51, 1068–1074. [Google Scholar] [CrossRef]
- Low, B.T.; Xiao, Y.; Chung, T.S. Amplifying the molecular sieving capability of polyimide membranes via coupling of diamine networking and molecular architecture. Polymer 2009, 50, 3250–3258. [Google Scholar] [CrossRef]
- Fu, Q.; Livengood, B.P.; Shen, C.C.; Lin, F.L.; Harris, F.W.; Cheng, S.Z.D.; Hsiao, B.S.; Yeh, F. Crystallization and phase behavior in nylon 6/aromatic polyimide triblock copolymers. Macromol. Chem. Phys. 1998, 199, 1107–1118. [Google Scholar] [CrossRef]
- Strunskus, Y.; Grunze, M. Polyimides—Fundamentals and Applications; Crosh, M., Mittal, K., Eds.; Marcel Dekker: New York, NY, USA, 1994; pp. 187–205. [Google Scholar]
- Abadie, M.J.; Rusanov, A.L. Practical Guide to Polyimides; Smithers Rapra Technology Limited: Shawbury, UK, 2007; pp. 45–77. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides: Thermally Stable Polymers, 2nd ed.; Plenum: New York, NY, USA, 1987; pp. 56–187. [Google Scholar]
- Okamoto, K.; Umeo, N.; Okamyo, S.; Tanaka, K.; Kita, H. Selective Permeation of Carbon Dioxide over Nitrogen through Polyethyleneoxide-Containing Polyimide Membranes. Chem. Lett. 1993, 225–228. [Google Scholar] [CrossRef]
- Morikawa, A.; Miyata, F.; Nishimura, J. Synthesis and properties of polyimides from 1,4-bis(4-amino-2-phenylphenoxy) benzene and 4,4′-bis(4-amino-2-phenylphenoxy) biphenyl. J. High. Perform. Polym. 2012, 24, 783–792. [Google Scholar] [CrossRef]
- Pandiyan, S.; Brown, D.; Van Der Vegt, N.F.A.; Neyertz, S. Atomistic models of three fluorinated polyimides in the amorphous state. J. Polym. Sci. Part. B Polym. Phys. 2009, 47, 1166–1180. [Google Scholar] [CrossRef]
- Ritter, N.; Senkovska, I.; Kaskel, S.; Weber, J. Intrinsically Microporous Poly(imide)s: Structure−Porosity Relationship Studied by Gas Sorption and X-ray Scattering. Macromolecules 2011, 44, 2025–2033. [Google Scholar] [CrossRef]
- Shimazu, A.; Miyazaki, T.; Ikeda, K. Interpretation of d-spacing determined by wide angle X-ray scattering in 6FDA-based polyimide by molecular modeling. J. Membr. Sci. 2000, 166, 113–118. [Google Scholar] [CrossRef]




| PEG-PI Systems. | PEG wt% | 6FDA wt% + ODPA wt% | PDODA wt% |
|---|---|---|---|
| 6FDA-PDODA-PEG1500 | 28.8 | 48 + 0 | 23.2 |
| 42.1 | 30 + 0 | 27.9 | |
| 46.8 | 38 + 0 | 15.2 | |
| ODPA-PDODA-PEG1500 | 28.8 | 0 + 48 | 23.2 |
| 42.1 | 0 + 30 | 27.9 | |
| 46.8 | 0 + 38 | 15.2 | |
| 6FDA+ODPA-PDODA-PEG1500 (50/50 wt% of dianhydrides) | 28.8 | 24 + 24 | 23.2 |
| 42.1 | 15 + 15 | 27.9 | |
| 46.8 | 19 + 19 | 15.2 |
| 6FDA wt% (in All Materials) | ODPA wt% (in All Materials) | |
|---|---|---|
| 6FDA + ODPA-PDODA-PEG1500 | 30 | 0 |
| 22.5 | 7.5 | |
| 19 | 19 | |
| 7.5 | 22.5 | |
| 0 | 30 |
| Material (PEG1500: 42.1 wt%) | Liquid (at 25 °C) | wU (%) |
|---|---|---|
| 6FDA-PDODA-PEG1500 | Water | 22 ± 10 |
| ODPA-PDODA-PEG1500 | Water | 45 ± 12 |
| 6FDA + ODPA-PDODA-PEG1500 | Water | 42 ± 10 |
| Material (PEG1500: 42.1 wt%) | IL (at 25 °C) | ILU (%) |
|---|---|---|
| 6FDA-PDODA-PEG1500 | EAN | 106 ± 14 |
| ODPA-PDODA-PEG1500 | EAN | 80 ± 9 |
| 6FDA + ODPA-PDODA-PEG1500 | EAN | 89 ± 11 |
| Material | Conductivity (mS/cm) (at 60 °C and RH 70%) |
|---|---|
| 6FDA-PDODA-PEG1500 a (PEG1500: 50 wt%) | 80 ± 15 |
| ODPA-PDODA-PEG1500 b (PEG1500: 50 wt%) | 16 ± 4 |
| 6FDA-PDODA-PEG1500 c (PEG1500: 42.1 wt%) | 60 ± 20 |
| 6FDA + ODPA,-PDODA-PEG1500 d (PEG1500: 42.1 wt%) | 84 ± 17 |
| SBC Membranes | No Thermal Annealing Processes | 1 h Thermal Annealing Process | ||
|---|---|---|---|---|
| Conductivities of Undoped Membrane Films (mS/cm) | Conductivities of Doped Membrane Films in EAN Ionic Liquid (mS/cm) | Conductivities of Undoped Membrane Films (mS/cm) | Conductivities of Doped Membrane Films in EAN Ionic Liquid (mS/cm) | |
| 6FDA (30 wt%)- PDODA-PEG1500 | 34 × 10–4 | 81 | 20 × 10–3 | 334 |
| 6FDA (22.5 wt%)-ODPA (7.5 wt%) -PDODA- PEG1500 | 49 × 10–4 | 73 | 30 × 10–3 | 152 |
| 6FDA (15 wt%)-ODPA (15 wt%)-PDODA-PEG1500 | 8 × 10–4 | 57 | 15 × 10–3 | 307 |
| 6FDA (7.5 wt%)-ODPA (22.5 wt%) -PDODAPEG1500 | 4 × 10–4 | 42 | 6 × 10–3 | 285 |
| ODPA (30 wt%)-PDODA-PEG1500 | 6 × 10–4 | 23 | 22 × 10–3 | 128 |
| Thermally Untreated SBC Membranes | Thermally Annealed SBC Membranes | |||
|---|---|---|---|---|
| Tensile Stress at Maximum Force [MPa] | Young’s Modulus [MPa] | Tensile Stress at Maximum Force [MPa] | Young’s Modulus [MPa] | |
| 6FDA-PDODA-PEG1500 (28.8 wt%) | 14.82 | 274.21 | 8.15 | 92.26 |
| 6FDA-PDODA-PEG1500 (42.1 wt%) | 9.25 | 147.13 | 6.5 | 45.6 |
| 6FDA-PDODA-PEG1500 (46.8 wt%) | 7.57 | 28.91 | 4.87 | 25.58 |
| ODPA-PDODA-PEG1500 (28.8 wt%) | - | - | 5.57 | 63.19 |
| ODPA-PDODA-PEG1500 (42.1 wt%) | 12.97 | 222.73 | 10.8 | 63.17 |
| ODPA-PDODA-PEG1500 (46.8 wt%) | 2.87 | 79.68 | 4.04 | 24.22 |
| 6FDA-ODPA-PDODA-PEG1500 (28.8 wt%) | 23.21 | 289.02 | 10.45 | 155.89 |
| 6FDA-ODPA-PDODA-PEG1500 (42.1 wt%) | 15.19 | 119.9 | 6.71 | 79.83 |
| 6FDA-ODPA-PDODA-PEG1500 (46.8 wt%) | 5.11 | 67.03 | 3.63 | 41.78 |
| Thermally Untreated SBC Membranes | ||
|---|---|---|
| Tensile Stress at Maximum Force [MPa] | Young’s Modulus [MPa] | |
| 6FDA (30 wt%)-ODPA (0 wt%)-PDODA-PEG1500 | 9.62 | 147.05 |
| 6FDA (22.5 wt%)-ODPA (7.5 wt%)-PDODA-PEG1500 | 11.67 | 166.92 |
| 6FDA (15 wt%)-ODPA (15 wt%)-PDODA-PEG1500 | 18.21 | 150.43 |
| 6FDA (7.5 wt%)-ODPA (22.5 wt%)-PDODA-PEG1500 | 14.53 | 152.18 |
| 6FDA (0 wt%)-ODPA (30 wt%)-PDODA-PEG1500 | 17.43 | 220.33 |
| Nafion 115 | 32.46 | 288.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciftcioglu, G.A.; Frank, C.W. Influence of Mixed Imide Composition and Thermal Annealing on Ionic Liquid Uptake and Conductivity of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes. Molecules 2021, 26, 7450. https://doi.org/10.3390/molecules26247450
Ciftcioglu GA, Frank CW. Influence of Mixed Imide Composition and Thermal Annealing on Ionic Liquid Uptake and Conductivity of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes. Molecules. 2021; 26(24):7450. https://doi.org/10.3390/molecules26247450
Chicago/Turabian StyleCiftcioglu, Gokcen A., and Curtis W. Frank. 2021. "Influence of Mixed Imide Composition and Thermal Annealing on Ionic Liquid Uptake and Conductivity of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes" Molecules 26, no. 24: 7450. https://doi.org/10.3390/molecules26247450





