An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide
Abstract
:1. Introduction
2. Results
2.1. Initial In Vitro Characterization and Assay Selection
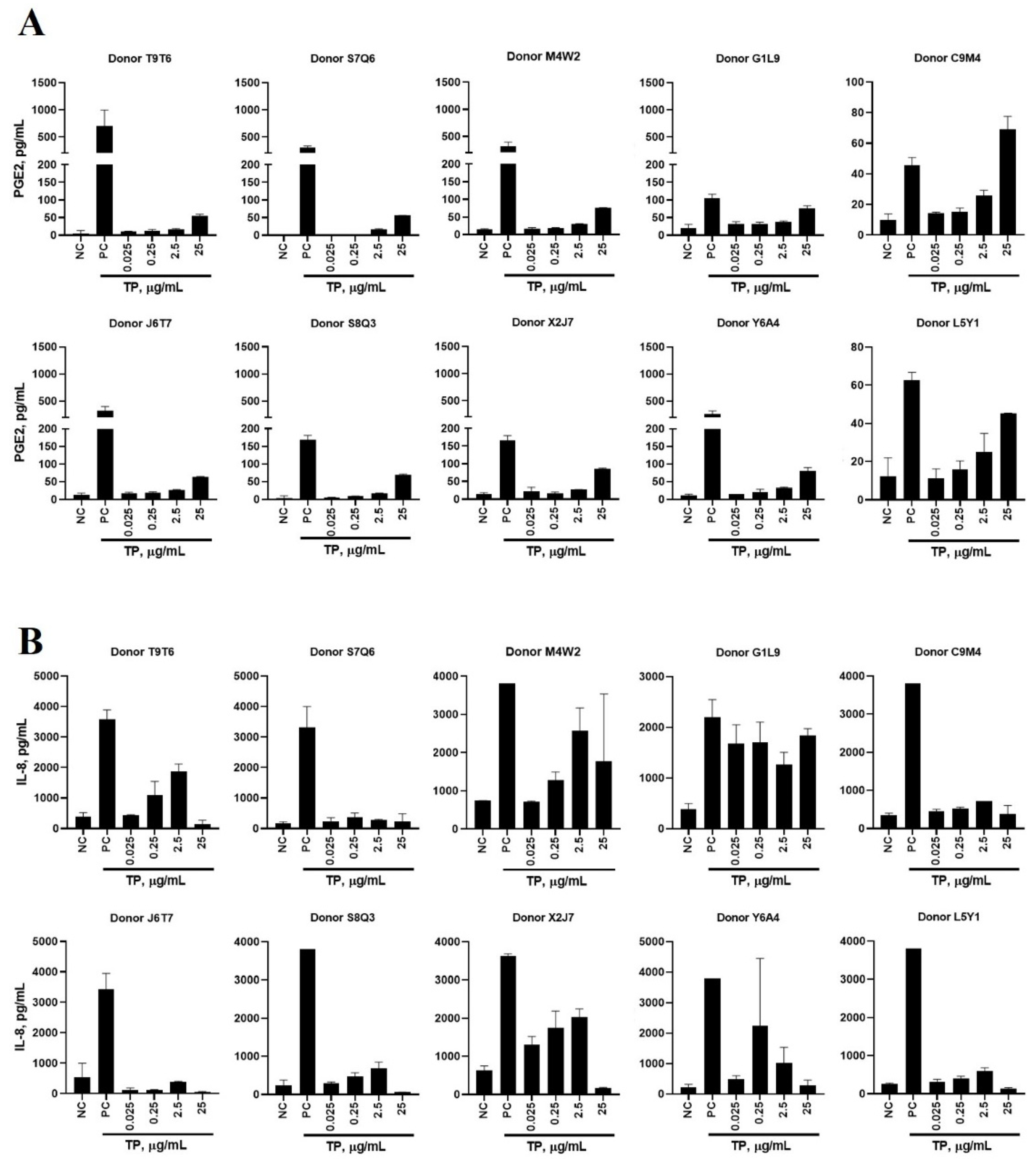
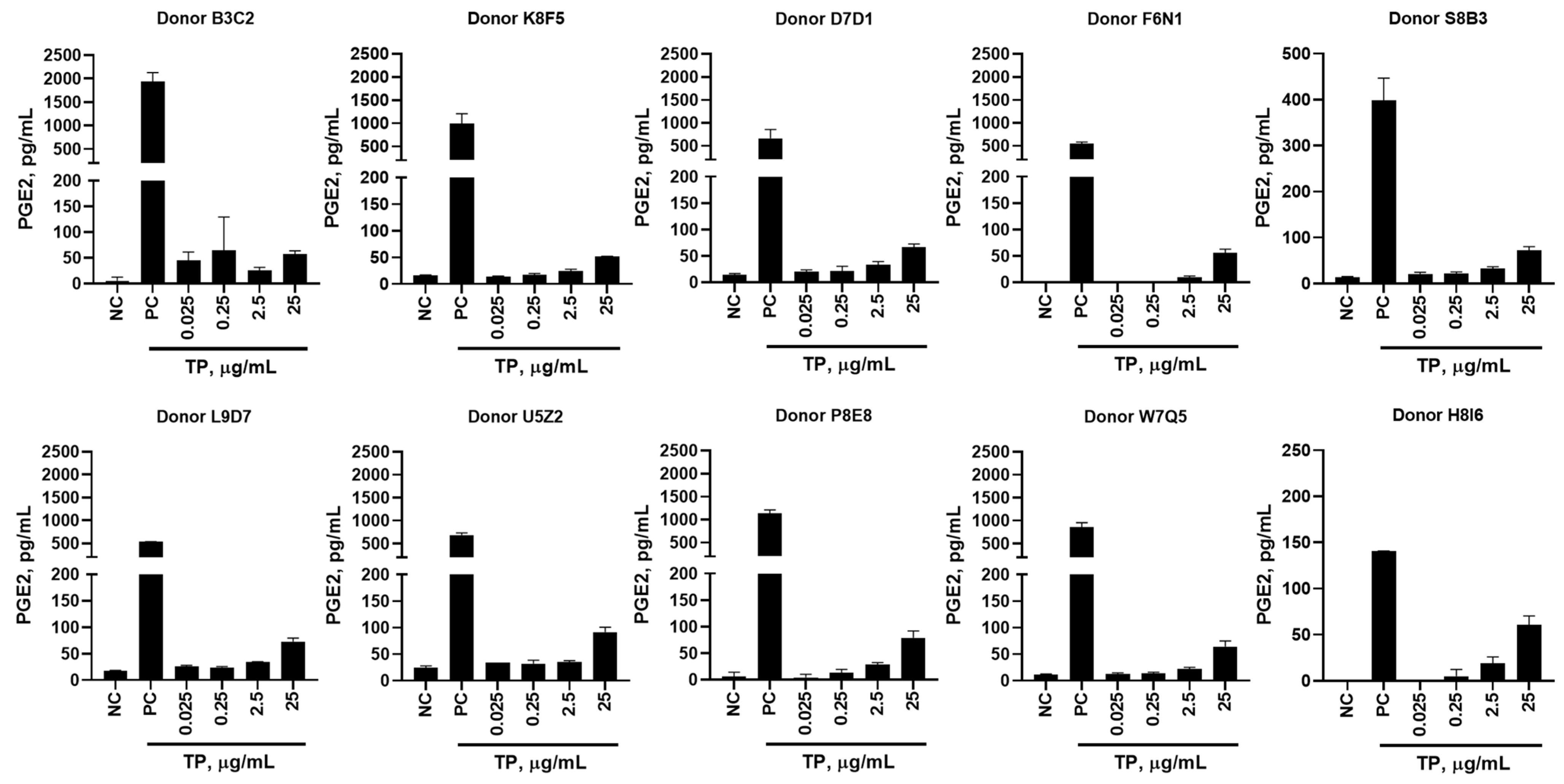
2.2. In Vitro Cytokine Responses to Teriparatide
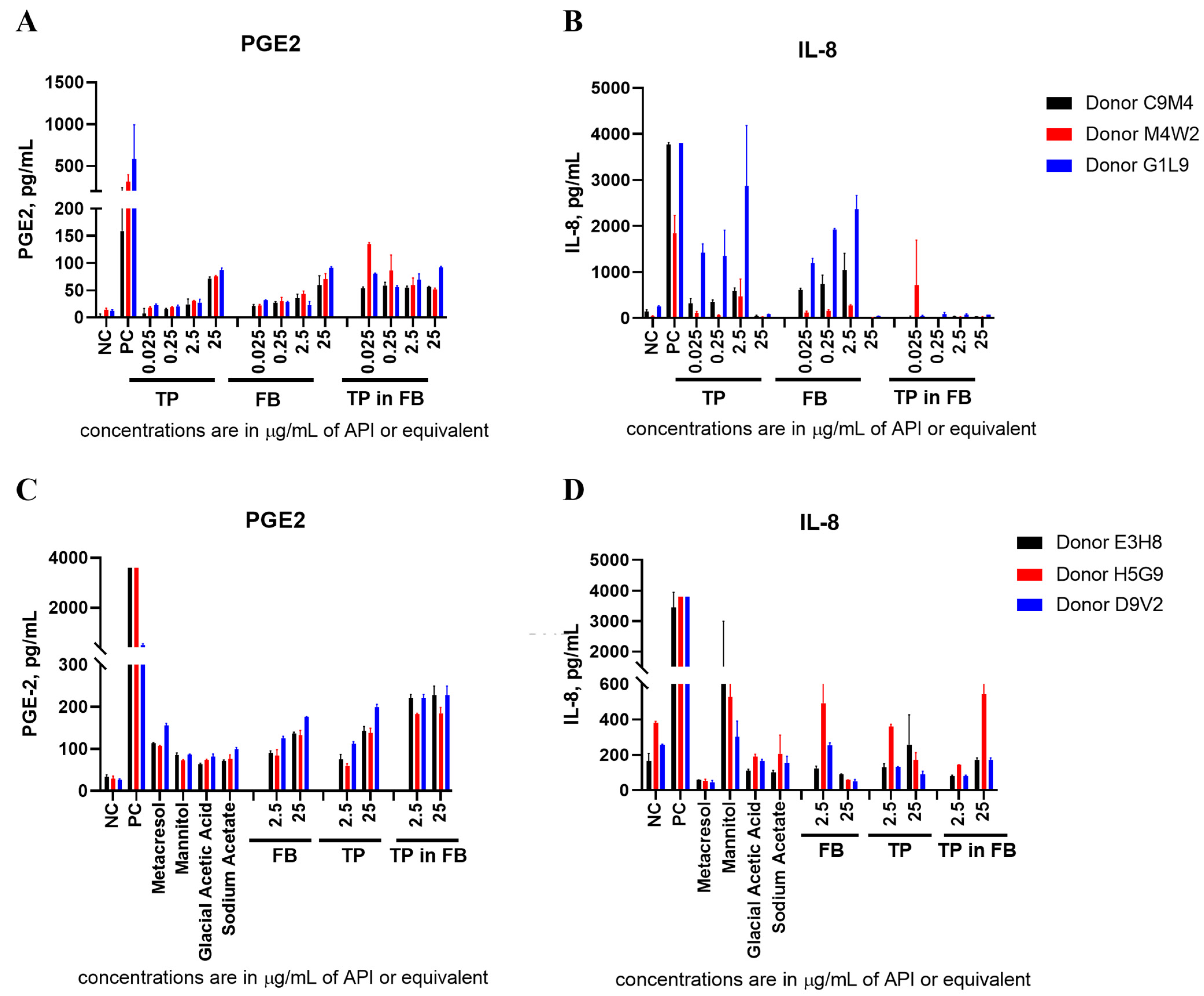
2.3. Teriparatide Effects on Cytokine Expression Are Due to the Formulation Buffer (FB)
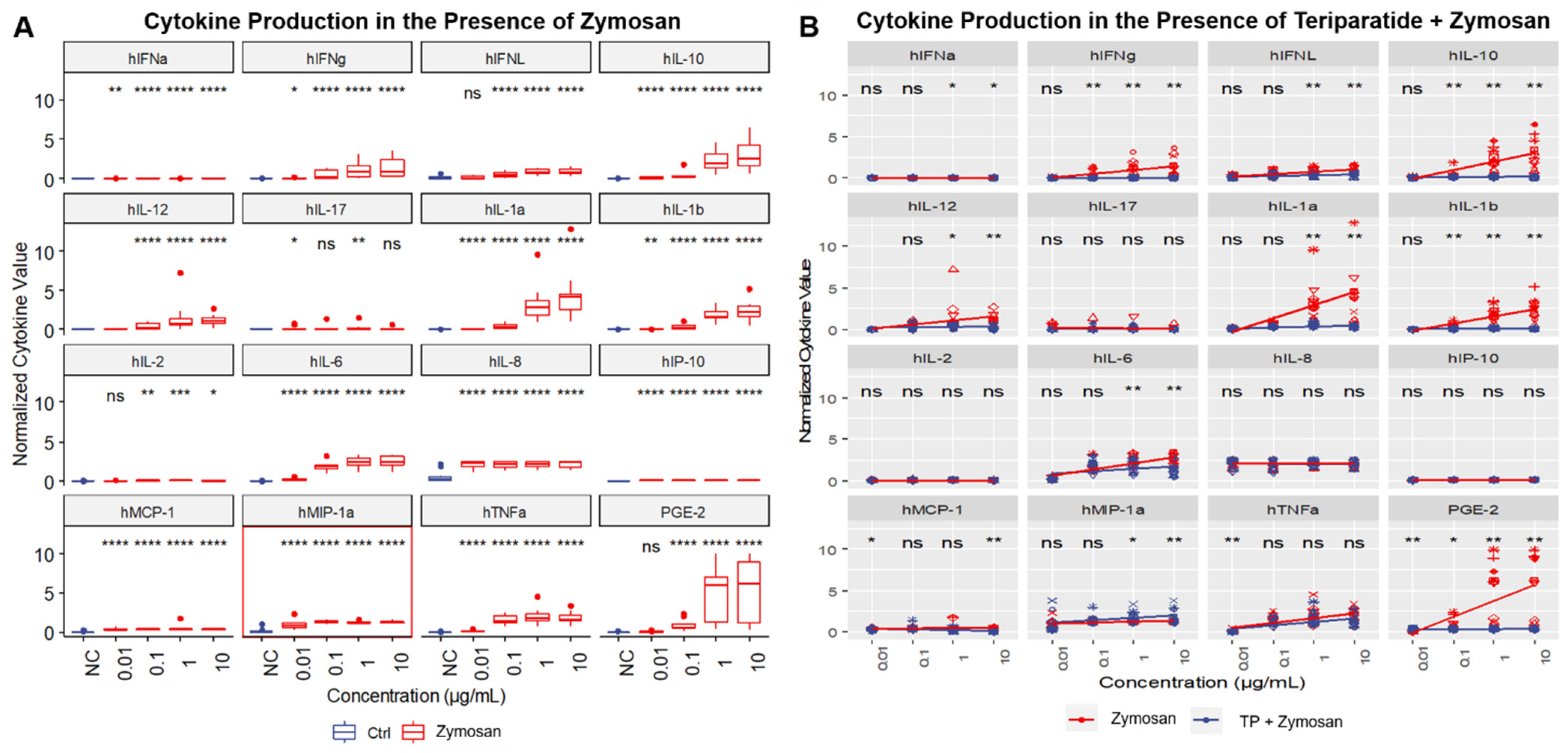
2.4. In Vitro Cytokine Responses to Individual IIRMIs
2.5. Identification of Signature Cytokines
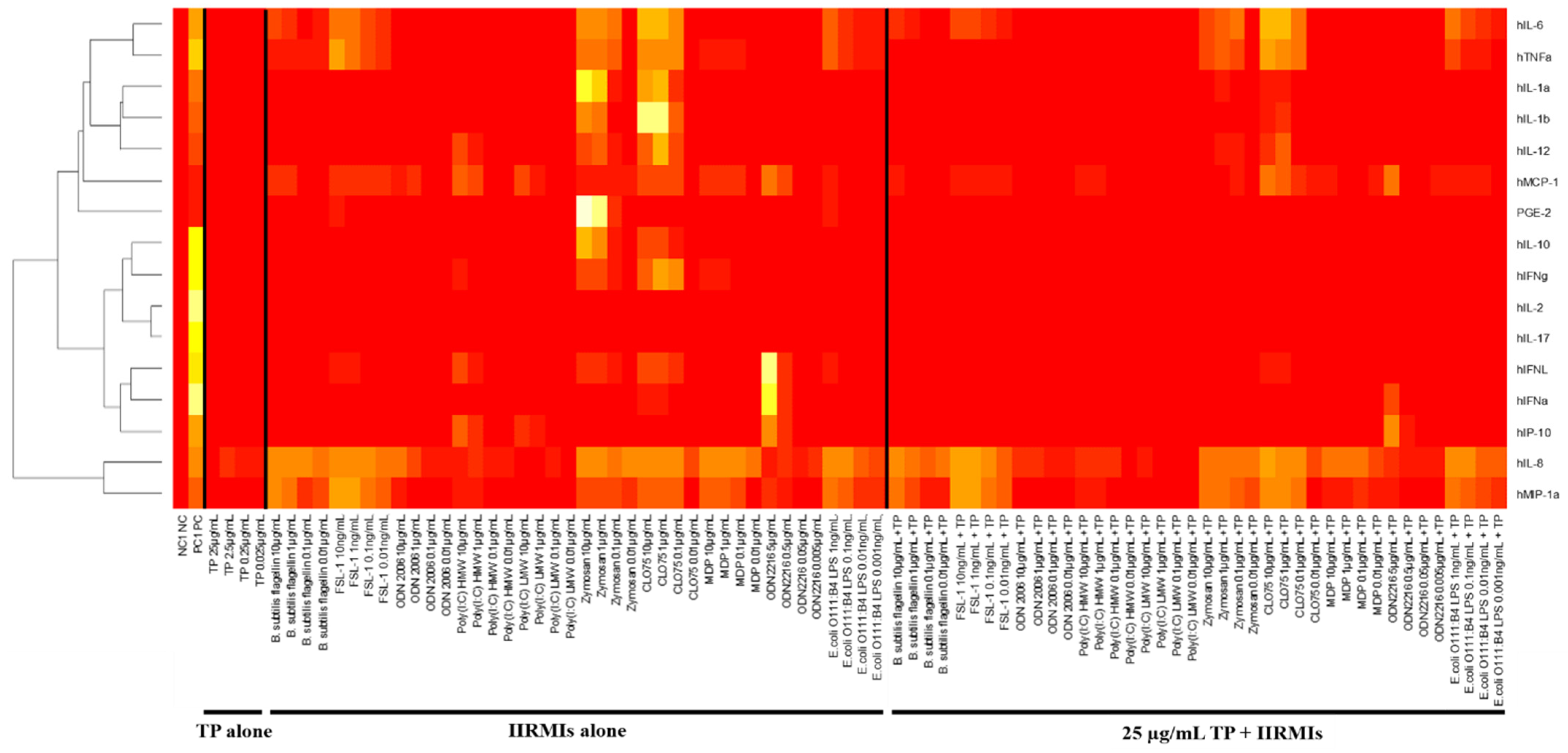
2.6. Selection of the Cytokine Panel Specific to Teriparatide and Individual IIRMIs
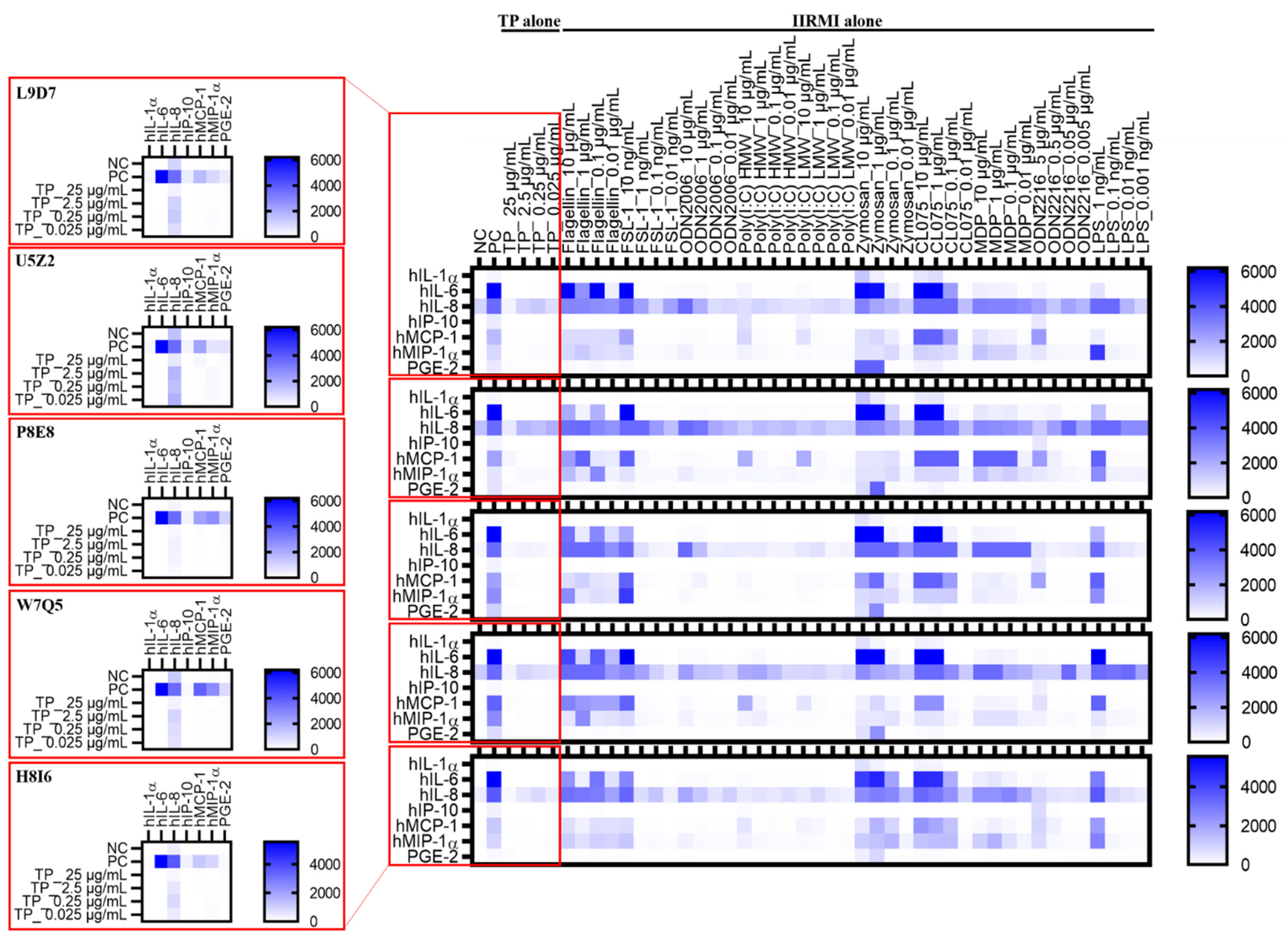
2.7. Teriparatide Affects Expression of IIRMI-Induced Cytokines
2.8. Teriparatide Effects on IIRMI-Induced Cytokines Are Due to the Formulation Buffer (FB)
2.9. Donor’s Genetic Background Determines the Magnitude of Cytokine Response to IIRMIs
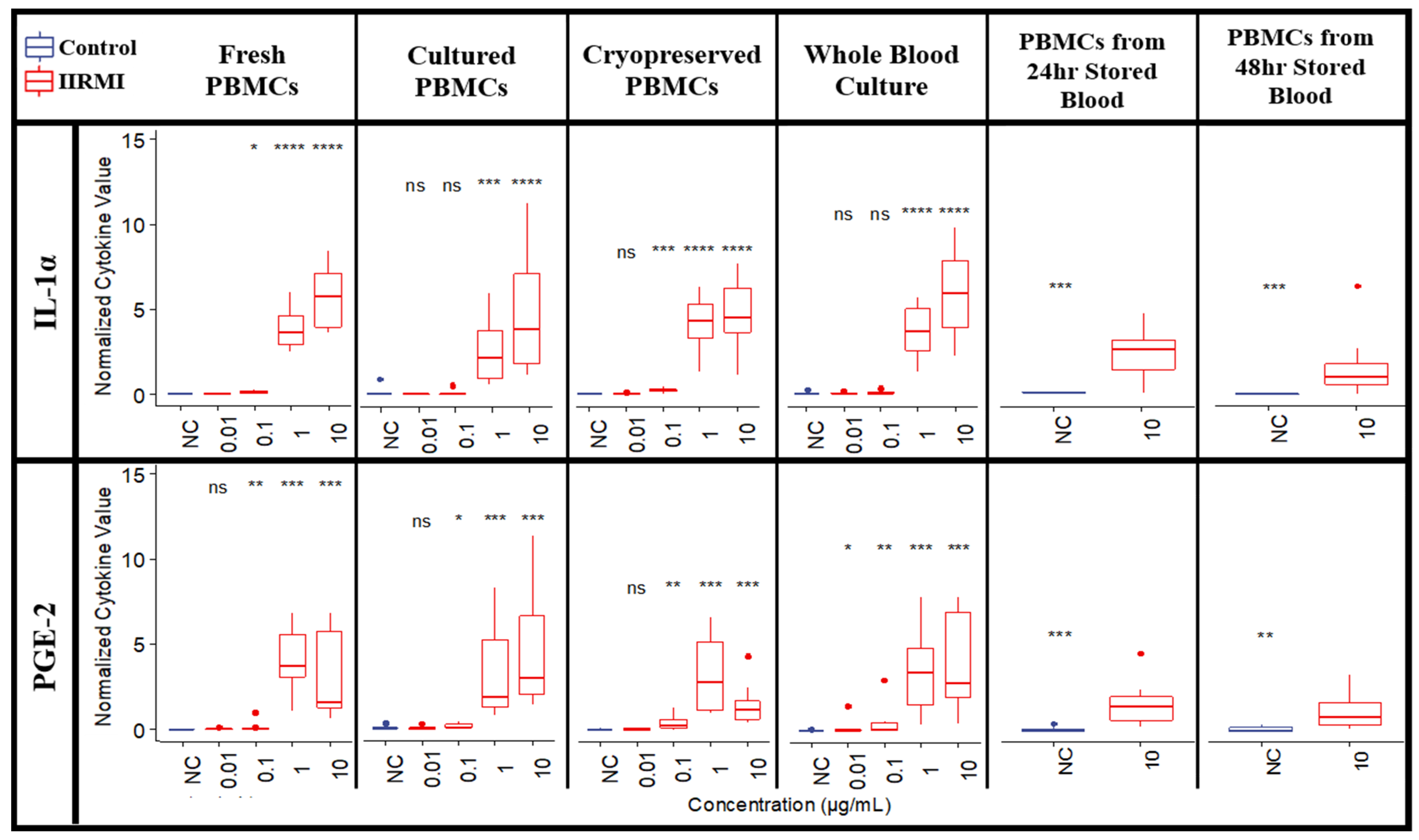
2.10. Influence of Assay Logistics on Cytokine Responses to TP and IIRMIs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Innate Immune Response Modulating Impurities
4.3. Endotoxin Detection
4.4. β-Glucan Detection
4.5. Donor Blood
4.6. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Culture
4.7. PBMC Cryopreservation
4.8. Whole Blood Cell (WBC) Culture
4.9. Teriparatide Cytotoxicity Analysis
4.10. Leukocyte Proliferation
4.11. Complement Activation
4.12. Cytokine Production
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Raw, A.; Yu, L.; Lionberger, R.; Ya, N.; Verthelyi, D.; Rosenberg, A.; Kozlowski, S.; Webber, K.; Woodcock, J. Scientific considerations in the review and approval of generic enoxaparin in the United States. Nat. Biotechnol. 2013, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lozier, J.; Johnson, G.; Kirshner, S.; Verthelyi, D.; Pariser, A.; Shores, E.; Rosenberg, A. Neutralizing antibodies to therapeutic enzymes: Considerations for testing, prevention and treatment. Nat. Biotechnol. 2008, 26, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Regenmortel, M.H. Antigenicity and immunogenicity of synthetic peptides. Biologicals 2001, 29, 209–213. [Google Scholar] [CrossRef]
- Jawa, V.; Cousens, L.P.; Awwad, M.; Wakshull, E.; Kropshofer, H.; De Groot, A.S. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin. Immunol. 2013, 149, 534–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, M.; Goldsmith, D.; Schellekens, H. Immunogenicity of biopharmaceuticals. Nephrol. Dial. Transpl. 2006, 21 (Suppl. 5), v9–v12. [Google Scholar] [CrossRef]
- Büttel, I.C.; Chamberlain, P.; Chowers, Y.; Ehmann, F.; Greinacher, A.; Jefferis, R.; Kramer, D.; Kropshofer, H.; Lloyd, P.; Lubiniecki, A.; et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals 2011, 39, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P.; Mire-Sluis, A.R. An overview of scientific and regulatory issues for the immunogenicity of biological products. Dev. Biol. 2003, 112, 3–11. [Google Scholar]
- Shankar, G.; Shores, E.; Wagner, C.; Mire-Sluis, A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol. 2006, 24, 274–280. [Google Scholar] [CrossRef]
- Haile, L.A.; Puig, M.; Kelley-Baker, L.; Verthelyi, D. Detection of innate immune response modulating impurities in therapeutic proteins. PLoS ONE 2015, 10, e0125078. [Google Scholar] [CrossRef]
- Haile, L.A.; Puig, M.; Polumuri, S.K.; Ascher, J.; Verthelyi, D. In Vivo Effect of Innate Immune Response Modulating Impurities on the Skin Milieu Using a Macaque Model: Impact on Product Immunogenicity. J. Pharm. Sci. 2017, 106, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Polumuri, S.K.; Haile, L.A.; Ireland, D.D.C.; Verthelyi, D. Aggregates of IVIG or Avastin, but not HSA, modify the response to model innate immune response modulating impurities. Sci. Rep. 2018, 8, 11477. [Google Scholar] [CrossRef] [Green Version]
- Verthelyi, D.; Wang, V. Trace levels of innate immune response modulating impurities (IIRMIs) synergize to break tolerance to therapeutic proteins. PLoS ONE 2010, 5, e15252. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, J.; Cherney, B.; Lubinecki, A.; Ma, S.; Marszal, E.; Mire-Sluis, A.; Nikolai, T.; Novak, J.; Ragheb, J.; Simak, J. Meeting report on protein particles and immunogenicity of therapeutic proteins: Filling in the gaps in risk evaluation and mitigation. Biologicals 2010, 38, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology E-Book; Elsevier Health Sciences: London, UK, 2014. [Google Scholar]
- Neun, B.W.; Cedrone, E.; Potter, T.M.; Crist, R.M.; Dobrovolskaia, M.A. Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations. Molecules 2020, 25, 3367. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef]
- Castellheim, A.; Lindenskov, P.H.; Pharo, A.; Fung, M.; Saugstad, O.D.; Mollnes, T.E. Meconium is a potent activator of complement in human serum and in piglets. Pediatr. Res. 2004, 55, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szebeni, J.; Wassef, N.M.; Rudolph, A.S.; Alving, C.R. Complement activation by liposome-encapsulated hemoglobin in vitro: The role of endotoxin contamination. Artif. Cells Blood Substit. Immobil. Biotechnol. 1995, 23, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Kammerer, D.; Streit, B.; Licht, A.H. Phenolic excipients of insulin formulations induce cell death, pro-inflammatory signaling and MCP-1 release. Toxicol. Rep. 2015, 2, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, L.; Dinarello, C.A. Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp. Cell Res. 1997, 231, 354–362. [Google Scholar] [CrossRef]
- Malek, A.M.; Goss, G.G.; Jiang, L.; Izumo, S.; Alper, S.L. Mannitol at clinical concentrations activates multiple signaling pathways and induces apoptosis in endothelial cells. Stroke 1998, 29, 2631–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [Green Version]
- Ozato, K.; Tsujimura, H.; Tamura, T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 2002, 33, S66–S75. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, T.M.; Desouza, K.; Fahey, J.V.; Beagley, K.W.; Wira, C.R. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 2004, 112, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Wu, Z.; Ren, S.; Wei, Y.; Gao, M.; Randolph, G.J.; Qu, C. TLR8 agonists stimulate newly recruited monocyte-derived cells into potent APCs that enhance HBsAg immunogenicity. Vaccine 2010, 28, 6273–6281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neun, B.W.; Dobrovolskaia, M.A. Detection of Endotoxin in Nano-formulations Using Limulus Amoebocyte Lysate (LAL) Assays. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Neun, B.W.; Dobrovolskaia, M.A. NCL Method STE-1.2: Detection and Quantification of Gram Negative Bacterial Endotoxin Contamination in Nanoparticle Formulations by Kinetic Turbidity LAL Assay. Available online: https://ncl.cancer.gov/sites/default/files/protocols/NCL_Method_STE-1.2.pdf (accessed on 15 October 2020).
- Neun, B.W.; Dobrovolskaia, M.A. NCL Method STE-4: Detection and Quantification of β-(1,3)-D-Glucan Contamination in Nanoparticle Formulations by Factor C Depleted LAL (Glucatell®) Assay. Available online: https://ncl.cancer.gov/sites/default/files/NCL_Method_STE-4.pdf (accessed on 15 October 2020).
- Potter, T.M.; Neun, B.W.; Rodriguez, J.C.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Pro-inflammatory Cytokine and Type II Interferon Induction by Nanoparticles. Methods Mol. Biol. 2018, 1682, 173–187. [Google Scholar] [CrossRef]
- Potter, T.M.; Cedrone, E.; Neun, B.W.; Dobrovolskaia, M.A. NCL Method-10: Preparation of Human Whole Blood and Peripheral Blood Mononuclear Cell Cultures to Analyze Nanoparticle Potential to Induce Cytokines In Vitro. Available online: https://ncl.cancer.gov/sites/default/files/NCL_Method_ITA-10.pdf (accessed on 15 October 2020).
- Nexcelom. Viability Using Acridine Orange/Propidium Lodide. Available online: https://www.nexcelom.com/applications/cellometer/viability/viability-using-ao-pi/ (accessed on 15 October 2020).
- Potter, T.M.; Cedrone, E.; Skoczen, S.L.; Neun, B.W.; Dobrovolskaia, M.A. NCL Method ITA-6.1: Leukocyte Proliferation Assay. Available online: https://ncl.cancer.gov/sites/default/files/NCL_Method_ITA-6.1.pdf (accessed on 15 October 2020).
- Neun, B.W.; Cedrone, E.; Dobrovolskaia, M.A. NCL Method STE 5.2: Analysis of Complement Activation by Single-Plex EIA or Multiplex ELISA. Available online: https://ncl.cancer.gov/sites/default/files/NCL_Method_ITA-5.2.pdf (accessed on 15 October 2020).
- Hempel, J.C.; Poppelaars, F.; Gaya da Costa, M.; Franssen, C.F.; de Vlaam, T.P.; Daha, M.R.; Berger, S.P.; Seelen, M.A.; Gaillard, C.A. Distinct in vitro Complement Activation by Various Intravenous Iron Preparations. Am. J. Nephrol. 2017, 45, 49–59. [Google Scholar] [CrossRef]
- Jackman, J.A.; Mészáros, T.; Fülöp, T.; Urbanics, R.; Szebeni, J.; Cho, N.J. Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: Foundation of a validatable immune toxicity model. Nanomedicine 2016, 12, 933–943. [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of complement-activating particles in aqueous solutions of Taxol: Possible role in hypersensitivity reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]

| Purpose | Assay Type (NCL Protocol) | Main Findings |
|---|---|---|
| Endotoxin Detection | LAL (STE-1.2) | Endotoxin contamination is below the assay LLOQ |
| β-Glucan Detection | Glucatell (STE-4) | β-glucan contamination is below the assay LLOQ |
| Cell Viability/Teriparatide Cytotoxicity | AO/PI staining | >85% viability for TP <25 μg/mL ~60% viability for 50 μg/mL TP 25 μg/mL TP chosen for future experiments |
| Leukocyte Proliferation | CBA (ITA-6) | TP did not induce leukocyte proliferation IIRMIs induced low levels of leukocyte proliferation TP suppressed IIRMI-induced leukocyte proliferation The assay is not chosen for future studies |
| Complement Activation | ELISA (ITA-5.2) | TP resulted in complement activation Levels of IIRMIs contamination in drug product are insufficient for the complement activation The assay is not chosen for future studies |
| IIRMI | PRR | Signature Cytokine | Lowest Conc. of IIRMI at Which Signature Cytokine Is Detected | Other Cytokines Statistically Higher than the Baseline at the Lowest IIRMI Conc. that Induced Signature Cytokine |
|---|---|---|---|---|
| B. subtilis flagellin | TLR5 | IL-1β | 0.01 µg/mL | IFNα, IL-10, IL-1α, IL-2, IL-6, IL-8, IL-1, MCP-1, MIP-1α, TNFα |
| FSL-1 | TLR2/TLR6 | IL-1α | 10 pg/mL | IFNα, IFNγ, IFNλ, IL-10, IL-12, IL-1b, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1α, TNFα, PGE-2 |
| ODN2006 Class B | TLR9 | IFNα | 1 µg/mL | IFNγ, IL-1α, IL-10, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1α, TNFα |
| Poly(I:C) HMW | TLR3 | IP-10 | 0.1 µg/mL | IFNα, IFNγ, IFNλ, IL-10, IL-12, IL-1α, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1α, TNFα |
| Poly(I:C) LMW | TLR3 | MCP-1 | 1 µg/mL | IFNγ, IL-12, IL-6, IP-10, MIP-1α |
| Zymosan | TLR2/Dectin 1 | MIP-1α | 0.01 µg/mL | IFNα, IFNγ, IFNλ, IL-10, IL-12, IL-17, IL-1α, IL-1β, IL-6, IL-8, IP-10, MCP-1, TNFα |
| CLO75 | TLR8 | IL-10 | 0.01 µg/mL | IL-1α, IL-1β, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1α, TNFα |
| MDP | NOD2 | IL-8 | 0.01 µg/mL | IFNα, IL-10, IL-12, IL-6, IP-10, MCP-1, MIP-1α, TNFα |
| ODN2216 | TLR9 | IL-6 | 0.005 µg/mL | IL-6, IL-8 |
| E. coli O111:B4 LPS | TLR4 | IL-1α | 1 pg/mL | IFNα, IFNγ, IL-10, IL-12, IL-1β, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1α, TNFα, PGE-2 |
| IIRMI | IFNα | IFNγ | IFNλ | IL-10 | IL-12 | IL-17 | IL-1α | IL-1β | IL-2 | IL-6 | IL-8 | IP-10 | MCP-1 | MIP-1α | TNFα | PGE-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis flagellin | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | FALSE |
| FSL-1 | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | FALSE |
| Zymosan | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE |
| E. coli O111:B4 LPS | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | FALSE |
| ODN2006 | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | TRUE | FALSE | FALSE | FALSE |
| Poly(I:C) HMW | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE |
| Poly(I:C) LMW | FALSE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE |
| CLO75 | FALSE | FALSE | FALSE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE |
| ODN2216 | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | FALSE | FALSE | FALSE |
| MDP | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | TRUE | TRUE | TRUE | FALSE | FALSE | FALSE | FALSE |
| IIRMI | IIRMI-Induced Cytokines Affected by TP |
|---|---|
| B. subtilis flagellin | IFNα, IL-1α, IL-1β, IL-6, MIP-1α, TNFα, PGE-2 * |
| FSL-1 | IFNα, IFNγ, IFNλ, IP-10, IL-1α, IL-1β, IL-2, IL-6, TNFα |
| ODN2006 Class B | IFNα, IP-10, TNFα, PGE-2 * |
| Poly(I:C) HMW | IFNγ, IFNλ, IL-12, IP-10, MIP-1α, TNFα, PGE-2 * |
| Poly(I:C) LMW | IFNγ, IP-10, MCP-1, MIP-1α, TNFα, PGE-2 * |
| Zymosan | IFNα, IFNγ, IFNλ, IL-10, IL-12, IL-1α, IL-1β, IL-6, MCP-1, MIP-1α *, PGE-2 |
| CLO75 | IFNα, IFNγ, IFNλ, IL-10, IL-1α, IL-1β, IP-10, PGE-2 * |
| MDP | IL-1α, IL-1β, MIP-1α, TNFα, PGE-2 * |
| ODN2216 | IFNα, IFNγ, IFNλ, IL-1α, MIP-1α, TNFα, PGE-2 * |
| E. coli O111:B4 LPS | IFNγ, IFNλ, IL-1β |
| Reagent | PRR | Final Concentrations per mL |
|---|---|---|
| B. subtilis flagellin | TLR5 | 10 µg, 1 µg, 100 ng, 10 ng |
| FSL-1 | TLR2/TLR6 | 10 ng, 1 ng, 100 pg, 10 pg |
| ODN2006 Class B | TLR9 | 10 µg, 1 µg, 100 ng, 10 ng |
| Poly(I:C) HMW | TLR3 | 10 µg, 1 µg, 100 ng, 10 ng |
| Poly(I:C) LMW | TLR3 | 10 µg, 1 µg, 100 ng, 10 ng |
| Zymosan | TLR2/Dectin 1 | 10 µg, 1 µg, 100 ng, 10 ng |
| CLO75 | TLR8 | 10 µg, 1 µg, 100 ng, 10 ng |
| MDP | NOD2 | 10 µg, 1 µg, 100 ng, 10 ng |
| ODN2216 | TLR9 | 5 µg, 500 ng, 50 ng, 5 ng |
| E. coli O111:B4 LPS | TLR4 | 1 ng, 100 pg, 10 pg, 1 pg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holley, C.K.; Cedrone, E.; Donohue, D.; Neun, B.W.; Verthelyi, D.; Pang, E.S.; Dobrovolskaia, M.A. An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide. Molecules 2021, 26, 7461. https://doi.org/10.3390/molecules26247461
Holley CK, Cedrone E, Donohue D, Neun BW, Verthelyi D, Pang ES, Dobrovolskaia MA. An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide. Molecules. 2021; 26(24):7461. https://doi.org/10.3390/molecules26247461
Chicago/Turabian StyleHolley, Claire K., Edward Cedrone, Duncan Donohue, Barry W. Neun, Daniela Verthelyi, Eric S. Pang, and Marina A. Dobrovolskaia. 2021. "An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide" Molecules 26, no. 24: 7461. https://doi.org/10.3390/molecules26247461
APA StyleHolley, C. K., Cedrone, E., Donohue, D., Neun, B. W., Verthelyi, D., Pang, E. S., & Dobrovolskaia, M. A. (2021). An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide. Molecules, 26(24), 7461. https://doi.org/10.3390/molecules26247461







