Mipsagargin: The Beginning—Not the End—of Thapsigargin Prodrug-Based Cancer Therapeutics
Abstract
:1. Introduction
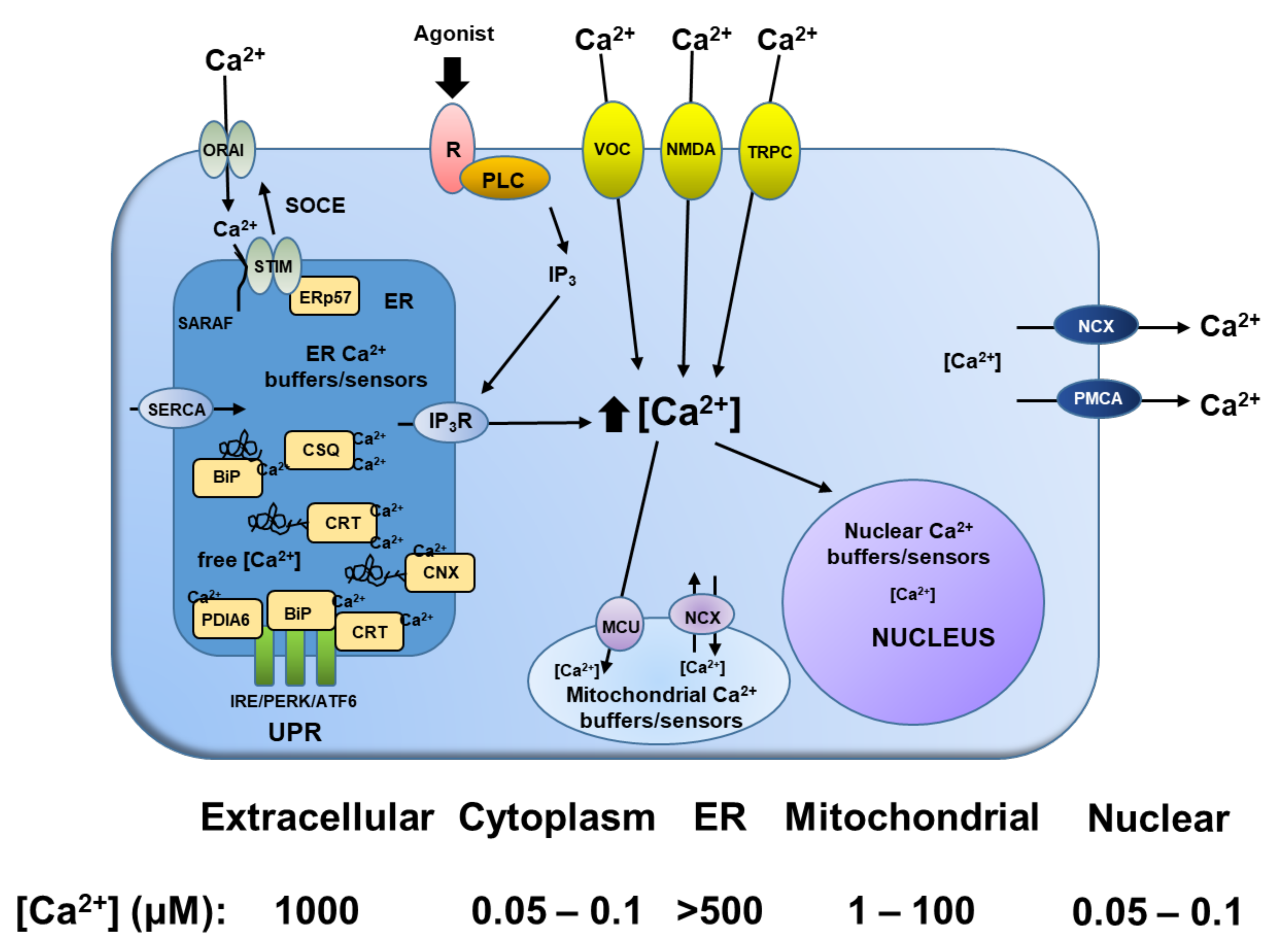
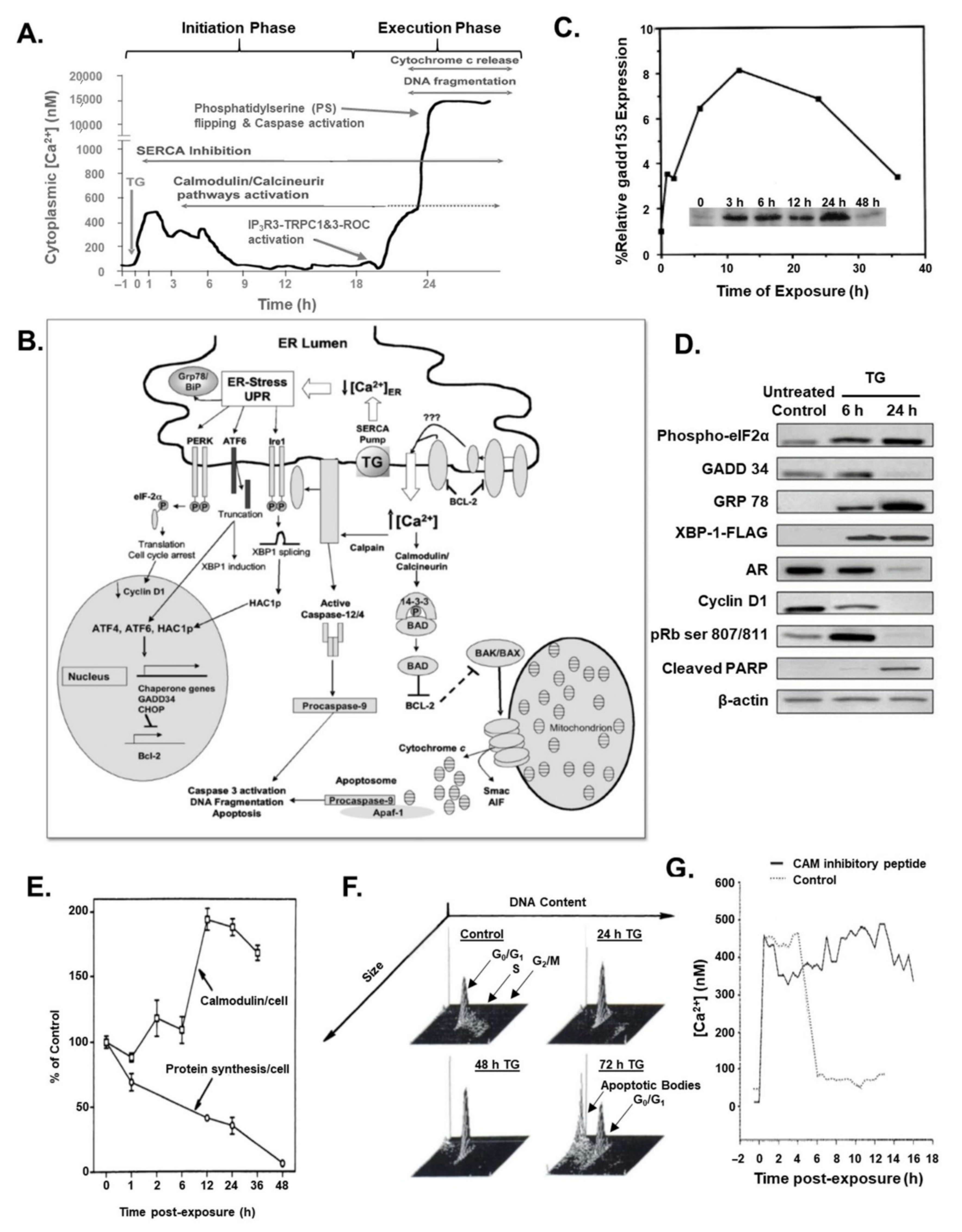
2. Role of Calcium in the Programmed Cell Death of Normal and Malignant Prostatic Epithelial Cells
3. Søren Brøgger Christensen and Thapsigargin Early Studies
4. Thapsigargin Drug Development
5. Rationale for Development of Protease-Activated Thapsigargin Prodrug for Prostate Cancer Therapy
6. Søren Brøgger Christensen and Identification of 12-ADT
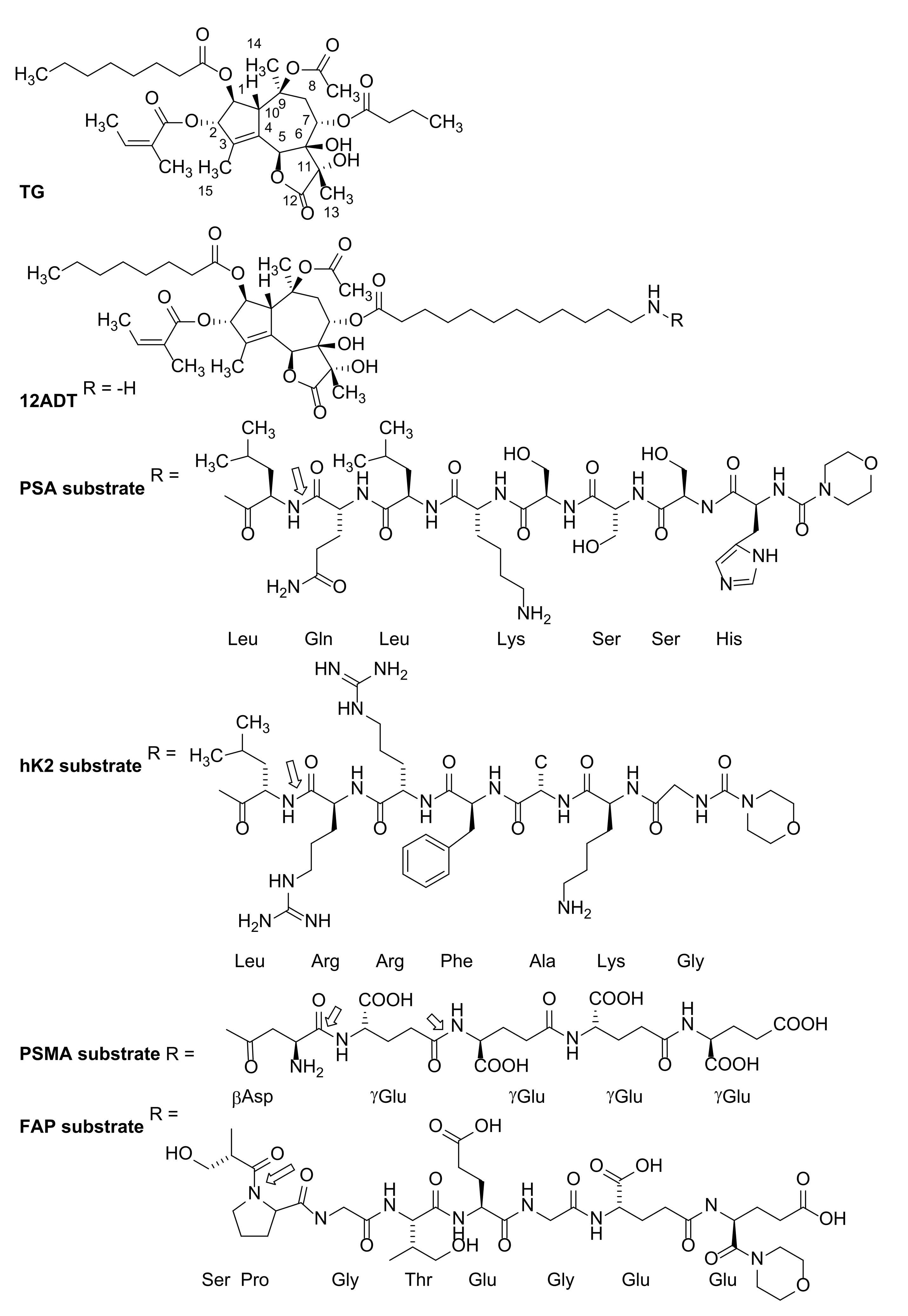
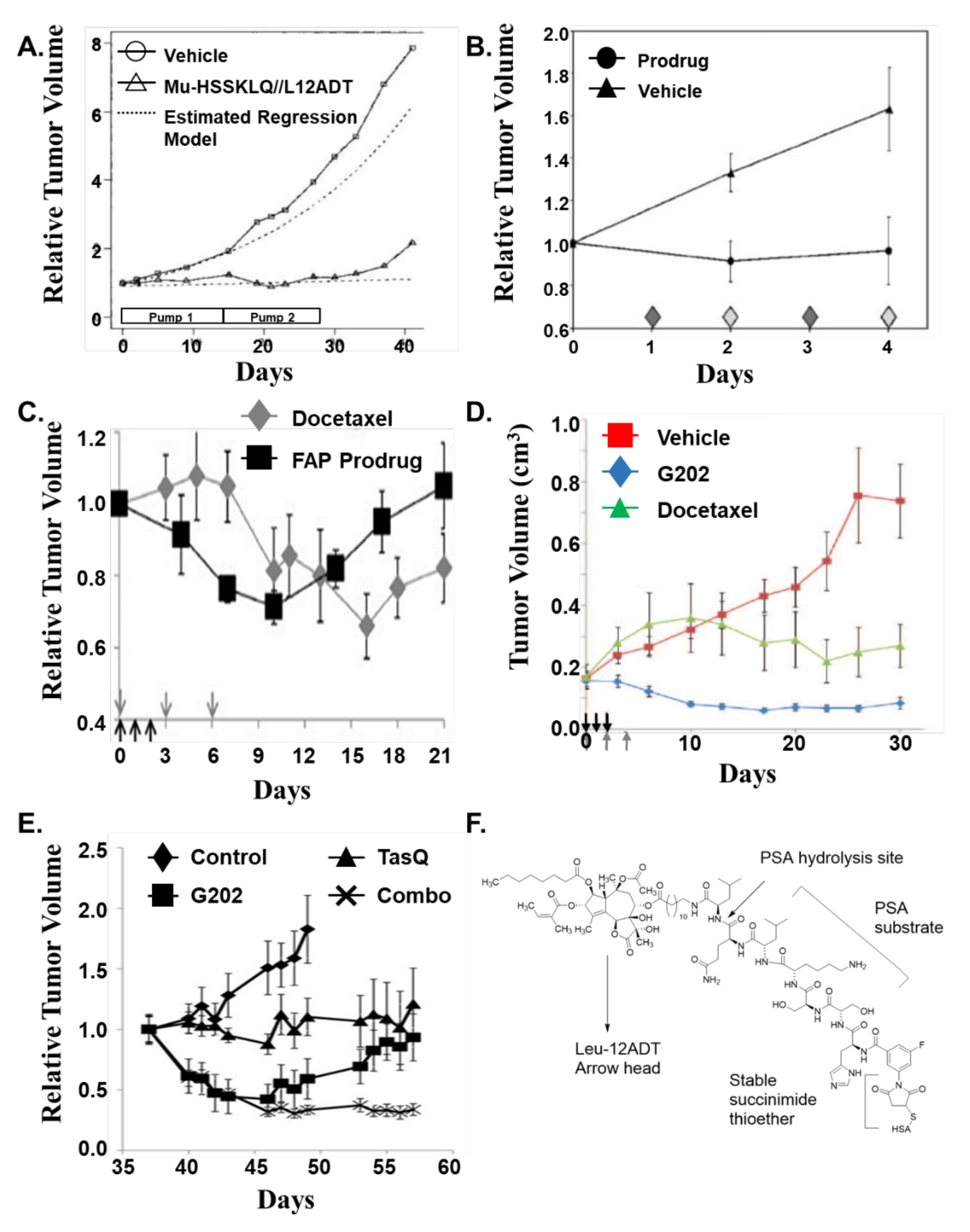
7. First-Generation Thapsigargin Prodrugs
8. Second-Generation Thapsigargin Prodrugs
9. Mipsagargin (G202) as a PSMA-Activated Prodrug for Solid Malignancies
10. Future Approaches
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Bono, J.S.; Smith, M.R.; Saad, F.; Rathkopf, D.E.; Mulders, P.F.A.; Small, E.J.; Shore, N.D.; Fizazi, K.; De Porre, P.; Kheoh, T.; et al. Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur. Urol. 2017, 71, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, M.; Nocera, L.; Collà Ruvolo, C.; Würnschimmel, C.; Tian, Z.; Shariat, S.F.; Saad, F.; Tilki, D.; Graefen, M.; Kluth, L.A.; et al. Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: A systematic review and network meta-analysis. Prostate Cancer Prostatic Dis. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Lucarelli, G.; Crocetto, F.; Dolce, P.; Verde, A.; La Civita, E.; Zappavigna, S.; de Cobelli, O.; Di Lorenzo, G.; Facchini, B.A.; et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Crit. Rev. Oncol. Hematol. 2021, 157, 103198. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F.; et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1250–1264. [Google Scholar] [CrossRef]
- Isaacs, J.T. Antagonistic effect of androgen on prostatic cell death. Prostate 1984, 5, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, N.; Isaacs, J.T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988, 122, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; De Marzo, A.M.; Isaacs, J.T. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J. Clin. Endocrinol. Metab. 2003, 88, 2972–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyprianou, N.; English, H.F.; Isaacs, J.T. Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration-induced prostatic cell death. Prostate 1988, 13, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, N.; Isaacs, J.T. Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol. Endocrinol. 1989, 3, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martikainen, P.; Kyprianou, N.; Isaacs, J.T. Effect of transforming growth factor-β on proliferation and death of rat prostatic cells. Endocrinology 1990, 127, 2963–2968. [Google Scholar] [CrossRef]
- Martikainen, P.; Isaacs, J. Role of calcium in the programmed death of rat prostatic glandular cells. Prostate 1990, 17, 175–187. [Google Scholar] [CrossRef]
- Kurita, T.; Wang, Y.Z.; Donjacour, A.A.; Zhao, C.; Lydon, J.P.; O’Malley, B.W.; Isaacs, J.T.; Dahiya, R.; Cunha, G.R. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ. 2001, 8, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Furuya, Y.; Isaacs, J.T. Differential gene regulation during programmed death (apoptosis) versus proliferation of prostatic glandular cells induced by androgen manipulation. Endocrinology 1993, 133, 2660–2666. [Google Scholar] [CrossRef]
- Furuya, Y.; Isaacs, J.T.; Shimazaki, J. Induction of programmed death/apoptosis androgen-dependent mouse mammary tumor cell line (Shionogi Carcinoma 115) by androgen withdrawal. Jpn. J. Cancer Res. 1995, 86, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Tombal, B.; Denmeade, S.R.; Gillis, J.M.; Isaacs, J.T. A supramicromolar elevation of intracellular free calcium ([Ca2+]i) is consistently required to induce the execution phase of apoptosis. Cell Death Differ. 2002, 9, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Isaacs, J.T. Development of an androgen receptor-null model for identifying the initiation site for androgen stimulation of proliferation and suppression of programmed (apoptotic) death of PC-82 human prostate cancer cells. Cancer Res. 1998, 58, 3299–3306. [Google Scholar] [PubMed]
- Gao, J.; Arnold, J.T.; Isaacs, J.T. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001, 61, 5038–5044. [Google Scholar] [PubMed]
- Vander Griend, D.J.; D’Antonio, J.; Gurel, B.; Antony, L.; Demarzo, A.M.; Isaacs, J.T. Cell-autonomous intracellular androgen receptor signaling drives the growth of human prostate cancer initiating cells. Prostate 2010, 70, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Kyprianou, N.; English, H.F.; Isaacs, J.T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990, 50, 3748–3753. [Google Scholar] [PubMed]
- Isaacs, J.T.; Wake, N.; Coffey, D.S.; Sandberg, A.A. Genetic instability coupled to clonal selection as a mechanism for tumor progression in the Dunning R-3327 rat prostatic adenocarcinoma system. Cancer Res. 1982, 42, 2353–2371. [Google Scholar] [PubMed]
- Isaacs, J.T.; Isaacs, W.B. Androgen receptor outwits prostate cancer drugs. Nat. Med. 2004, 10, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.; Kyprianou, N.; Tucker, R.W.; Isaacs, J.T. Programmed death of nonproliferating androgen-independent prostatic cancer cells. Cancer Res. 1991, 51, 4693–4700. [Google Scholar]
- Rasmussen, U.; Christensen, B.; Sandberg, F. Thapsigargin and thapsigargicin, two new histamine liberators from Thapsia garganica. Acta Pharm. Suec. 1978, 15, 133–140. [Google Scholar] [PubMed]
- Christensen, S.B.; Simonsen, H.T.; Engedal, N.; Nissen, P.; Møller, J.V.; Denmeade, S.R.; Isaacs, J.T. From Plant to Patient: Thapsigargin, a Tool for Understanding Natural Product Chemistry, Total Syntheses, Biosynthesis, Taxonomy, ATPases, Cell Death, and Drug Development. Prog. Chem. Org. Nat. Prod. 2021, 115, 59–114. [Google Scholar] [PubMed]
- Danese, A.; Leo, S.; Rimessi, A.; Wieckowski, M.R.; Fiorica, F.; Giorgi, C.; Pinton, P. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119061. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.W.; Owen, V.J.; Lamb, G.D.; Stephenson, D.G. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J. Physiol. 1995, 482, 123–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thastrup, O.; Cullen, P.J.; Drøthak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, M.J.; Garcia-Rodriguez, C.; Grinstein, S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of Thapsigargin, 2, 5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J. Biol. Chem. 1991, 266, 20856–20862. [Google Scholar] [CrossRef]
- Hogan, P.G.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, T.K.; Bian, J.H.; Short, A.D.; Rybak, S.L.; Gill, D.L. Persistent intracellular calcium pool depletion by Thapsigargin and its influence on cell growth. J. Biol. Chem. 1991, 266, 24690–24697. [Google Scholar] [CrossRef]
- Furuya, Y.; Lundmo, P.; Short, A.D.; Gill, D.L.; Isaacs, J.T. The role of calcium, pH, and cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by Thapsigargin. Cancer Res. 1994, 54, 6167–6175. [Google Scholar] [PubMed]
- Furuya, Y.; Isaacs, J.T. Proliferation-dependent vs. independent programmed cell death of prostatic cancer cells involves distinct gene regulation. Prostate 1994, 25, 301–309. [Google Scholar] [CrossRef]
- Tombal, B.; Denmeade, S.R.; Isaacs, J.T. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium 1999, 25, 19–28. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Isaacs, J.T. The SERCA pump as a therapeutic target: Making a “smart bomb” for prostate cancer. Cancer Biol. Ther. 2005, 4, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.S.; Denmeade, S.R.; Cisek, L.; Isaacs, J.T. Mechanism and role of growth arrest in programmed (apoptotic) death of prostatic cancer cells induced by Thapsigargin. Prostate 1997, 33, 201–207. [Google Scholar] [CrossRef]
- Tombal, B.; Weeraratna, A.T.; Denmeade, S.R.; Isaacs, J.T. Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate 2000, 43, 303–317. [Google Scholar] [CrossRef]
- Crouch, T.H.; Klee, C.B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry 1980, 19, 3692–3698. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, D.; Ducret, T.; Tajeddine, N.; Gala, J.L.; Tombal, B.; Gailly, P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium 2006, 39, 401–415. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Jakobsen, C.M.; Janssen, S.; Khan, S.R.; Garrett, E.S.; Lilja, H.; Christensen, S.B.; Isaacs, J.T. Prostate-specific antigen-activated Thapsigargin prodrug as targeted therapy for prostate cancer. J. Natl. Cancer Inst. 2003, 95, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hom, J.R.; Gewandter, J.S.; Michael, L.; Sheu, S.S.; Yoon, Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J. Cell. Physiol. 2007, 212, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Mhaka, A.M.; Rosen, D.M.; Brennen, W.N.; Dalrymple, S.; Dach, I.; Olesen, C.; Gurel, B.; Demarzo, A.M.; Wilding, G.; et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci. Transl. Med. 2012, 4, 140ra86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Soloski, M.J.; Sharp, A.H.; Schilling, G.; Sabatini, D.M.; Li, S.H.; Ross, C.A.; Snyder, S.H. Lymphocyte apoptosis: Mediation by increased type 3 inositol 1, 4, 5-trisphosphate receptor. Science 1996, 273, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.R.; Lu, W.W.; Lai, J.Z.; Tsai, F.L.; Wu, S.H.; Lin, C.W.; Kung, S.H. Calcium flux and calpain mediated activation of the apoptosis inducing factor contribute to enterovirus 71 induced apoptosis. J. Gen. Virol. 2013, 94 Pt 7, 1477–1485. [Google Scholar] [CrossRef] [Green Version]
- Chukai, Y.; Iwamoto, T.; Itoh, K.; Tomita, H.; Ozaki, T. Characterization of mitochondrial calpain-5. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118989. [Google Scholar] [CrossRef]
- Lilja, H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J. Clin. Investig. 1985, 76, 1899–1903. [Google Scholar] [CrossRef]
- Christensson, A.; Laurell, C.-B.; Lijia, H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine protease inhibitors. Eur. J. Biochem. 1990, 194, 755–765. [Google Scholar] [CrossRef]
- Lilja, H.; Christensson, A.; Dahlén, U.; Matikainen, M.T.; Nilsson, O.; Pettersson, K.; Lövgren, T. Prostate specific antigen in human serum occurs predominantly in complex with α1-anti-chymotrypsin. Clin. Chem. 1991, 37, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Lou, W.; Lovgren, J.; Malm, J.; Lilja, H.; Isaacs, J.T. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997, 57, 4924–4930. [Google Scholar] [PubMed]
- Singh, P.; LeBeau, A.M.; Lilja, H.; Denmeade, S.R.; Isaacs, J.T. Molecular insights into substrate specificity of prostate specific antigen through structural modeling. Proteins 2009, 77, 984–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denmeade, S.R.; Nagy, A.; Gao, J.; Lilja, H.; Schally, A.V.; Isaacs, J.T. Enzymatic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Res. 1998, 58, 2537–2540. [Google Scholar] [PubMed]
- Khan, S.R.; Denmeade, S.R. In vivo activity of a PSA activated doxorubicin prodrug prodrug against PSA-producing human prostate cancer xenografts. Prostate 2000, 45, 80–83. [Google Scholar] [CrossRef]
- DiPaola, R.S.; Rinehart, J.; Nemunaitis, J.; Ebbinghaus, S.; Rubin, E.; Capanna, T.; Ciardella, M.; Doyle-Lindrud, S.; Goodwin, S.; Fontaine, M.; et al. Characterization of a novel prostate-specific antigen-activated peptide-doxorubicin conjugate in patients with prostate cancer. J. Clin. Oncol. 2002, 20, 1874–1879. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Sokoll, L.J.; Chan, D.W.; Khan, S.R.; Isaacs, J.T. Concentration of enzymatically active prostate-specific antigen (PSA) in the extracellular fluid of primary human prostate cancers and human prostatic cancer xenograft models. Prostate 2001, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Isaacs, J.T. Engineering enzymatically activated “molecular grenades” for cancer. Oncotarget 2012, 3, 666–667. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.B.; Andersen, A.; Kromann, H.; Treiman, M.; Tombal, B.; Denmeade, S.R.; Isaacs, J.T. Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg. Med. Chem. 1999, 7, 1273–1280. [Google Scholar] [CrossRef]
- Jakobsen, C.M.; Denmeade, S.R.; Isaacs, J.T.; Gady, A.; Olsen, C.E.; Christensen, S.B. Design, synthesis, and pharmacological evaluation of Thapsigargin analogues for targeting apoptosis to prostatic cancer cells. J. Med. Chem. 2001, 44, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mhaka, A.M.; Christensen, S.B.; Gray, J.J.; Denmeade, S.R.; Isaacs, J.T. Applying linear interaction energy method for rational design of noncompetitive allosteric inhibitors of the sarco- and endoplasmic reticulum calcium-ATPase. J. Med. Chem. 2005, 48, 3005–3014. [Google Scholar] [CrossRef]
- Søhoel, H.; Liljefors, T.; Ley, S.V.; Oliver, S.F.; Antonello, A.; Smith, M.D.; Olsen, C.E.; Isaacs, J.T.; Christensen, S.B. Total synthesis of two novel subpicomolar sarco/endoplasmatic reticulum Ca2+-ATPase inhibitors designed by an analysis of the binding site of Thapsigargin. J. Med. Chem. 2005, 48, 7005–7011. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Rosen, D.M.; Ricklis, R.M.; Dionne, C.A.; Lilja, H.; Christensen, S.B.; Isaacs, J.T.; Denmeade, S.R. Pharmacokinetics, biodistribution, and antitumor efficacy of a human glandular kallikrein 2 (hK2)-activated Thapsigargin prodrug. Prostate 2006, 66, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Søhoel, H.; Jensen, A.M.; Møller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Olsen, C.E.; Christensen, S.B. Natural products as starting materials for development of second-generation SERCA inhibitors targeted towards prostate cancer cells. Bioorg. Med. Chem. 2006, 14, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Skytte, D.M.; Denmeade, S.R.; Dionne, C.; Møller, J.V.; Nissen, P.; Isaacs, J.T. A Trojan horse in drug development: Targeting of Thapsigargins towards prostate cancer cells. Anticancer Agents Med. Chem. 2009, 9, 276–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Griend, D.J.; Antony, L.; Dalrymple, S.L.; Xu, Y.; Christensen, S.B.; Denmeade, S.R.; Isaacs, J.T. Amino acid containing Thapsigargin analogues deplete androgen receptor protein via synthesis inhibition and induce the death of prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, N.T.; Paulsen, E.S.; Sehgal, P.; Møller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Dionne, C.A.; Christensen, S.B. Targeting Thapsigargin towards tumors. Steroids 2015, 97, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 angstrom resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Rosen, D.M.; Wang, H.; Isaacs, J.T.; Denmeade, S.R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl. Cancer Inst. 2012, 104, 1320–1334. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, J.T.; Antony, L.; Dalrymple, S.L.; Brennen, W.N.; Gerber, S.; Hammers, H.; Wissing, M.; Kachhap, S.; Luo, J.; Xing, L.; et al. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013, 73, 1386–1399. [Google Scholar] [CrossRef] [Green Version]
- Denmeade, S.R.; Lövgren, J.; Khan, S.R.; Lilja, H.; Isaacs, J.T. Activation of latent protease function of pro-hK2, but not pro-PSA, involves autoprocessing. Prostate 2001, 48, 122–126. [Google Scholar] [CrossRef]
- Janssen, S.; Jakobsen, C.M.; Rosen, D.M.; Ricklis, R.M.; Reineke, U.; Christensen, S.B.; Lilja, H.; Denmeade, S.R. Screening a combinatorial peptide library to develop a human glandular kallikrein 2-activated prodrug as targeted therapy for prostate cancer. Mol. Cancer Ther. 2004, 3, 1439–1450. [Google Scholar]
- Aggarwal, S.; Brennen, W.N.; Kole, T.P.; Schneider, E.; Topaloglu, O.; Yates, M.; Cotter, R.J.; Denmeade, S.R. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 2008, 47, 1076–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBeau, A.M.; Brennen, W.N.; Aggarwal, S.; Denmeade, S.R. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 2009, 8, 1378–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennen, W.N.; Rosen, D.M.; Chaux, A.; Netto, G.J.; Isaacs, J.T.; Denmeade, S.R. Pharmacokinetics and toxicology of a fibroblast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer. Prostate 2014, 74, 1308–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.T.; Suffoleto, B.P.; Berzin, T.M.; Qiao, C.H.; Lin, S.; Tong, W.P.; May, F.; Mukherjee, B.; Heston, W.D.W. Prostate-specific membrane antigen: A novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 1996, 2, 1445–1451. [Google Scholar]
- Wright, G.L., Jr.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Chang, S.S.; O’Keefe, D.S.; Bacich, D.J.; Reuter, V.E.; Heston, W.D.; Gaudin, P.B. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999, 5, 2674–2681. [Google Scholar]
- Bander, N.H.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 2005, 23, 4591–4601. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Sheehan, C.E.; Vallabhajosula, S.; Goldsmith, S.J.; Ross, J.S.; Bander, N.H. Vascular targeted therapy with anti–prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007, 25, 540–547. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel Thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef]
- Mahalingam, D.; Peguero, J.; Cen, P.; Arora, S.P.; Sarantopoulos, J.; Rowe, J.; Allgood, V.; Tubb, B.; Campos, L. A Phase, I.I.; Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers 2019, 11, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, T.; Drašar, P.; Rimpelová, S.; Christensen, S.B.; Khripach, V.A.; Jurášek, M. Large Scale Conversion of Trilobolide into the Payload of Mipsagargin: 8-O-(12-Aminododecanoyl)-8-O-DebutanoylThapsigargin. Biomolecules 2020, 10, 1640. [Google Scholar] [CrossRef]
- Dubois, C.; Kondratskyi, A.; Bidaux, G.; Noyer, L.; Vancauwenberghe, E.; Farfariello, V.; Toillon, R.A.; Roudbaraki, M.; Tierny, D.; Bonnal, J.L.; et al. Co-targeting Mitochondrial Ca2+ Homeostasis and Autophagy Enhances Cancer Cells’ Chemosensitivity. iScience 2020, 23, 101263. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, C.; Long, F.; Su, Z.; Jia, W.; Qin, M.; Huang, M.; Wu, W.; Suguro, R.; Liu, X.; et al. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc. Res. 2018, 114, 1016–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinboye, E.S.; Rogers, O.C.; Isaacs, J.T. 2-fluoro-5-maleimidobenzoic acid-linked albumin drug (MAD) delivery for selective systemic targeting of metastatic prostate cancer. Prostate 2018, 78, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Akinboye, E.S.; Brennen, W.N.; Denmeade, S.R.; Isaacs, J.T. Albumin-linked prostate-specific antigen-activated Thapsigargin- and niclosamide-based molecular grenades targeting the microenvironment in metastatic castration-resistant prostate cancer. Asian J. Urol. 2019, 6, 99–108. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaacs, J.T.; Brennen, W.N.; Christensen, S.B.; Denmeade, S.R. Mipsagargin: The Beginning—Not the End—of Thapsigargin Prodrug-Based Cancer Therapeutics. Molecules 2021, 26, 7469. https://doi.org/10.3390/molecules26247469
Isaacs JT, Brennen WN, Christensen SB, Denmeade SR. Mipsagargin: The Beginning—Not the End—of Thapsigargin Prodrug-Based Cancer Therapeutics. Molecules. 2021; 26(24):7469. https://doi.org/10.3390/molecules26247469
Chicago/Turabian StyleIsaacs, John T., William Nathaniel Brennen, Søren Brøgger Christensen, and Samuel R. Denmeade. 2021. "Mipsagargin: The Beginning—Not the End—of Thapsigargin Prodrug-Based Cancer Therapeutics" Molecules 26, no. 24: 7469. https://doi.org/10.3390/molecules26247469
APA StyleIsaacs, J. T., Brennen, W. N., Christensen, S. B., & Denmeade, S. R. (2021). Mipsagargin: The Beginning—Not the End—of Thapsigargin Prodrug-Based Cancer Therapeutics. Molecules, 26(24), 7469. https://doi.org/10.3390/molecules26247469





