Evaluating the Potential for Smoke from Stubble Burning to Taint Grapes and Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. Use of Excised Grape Bunches to Monitor Smoke Exposure
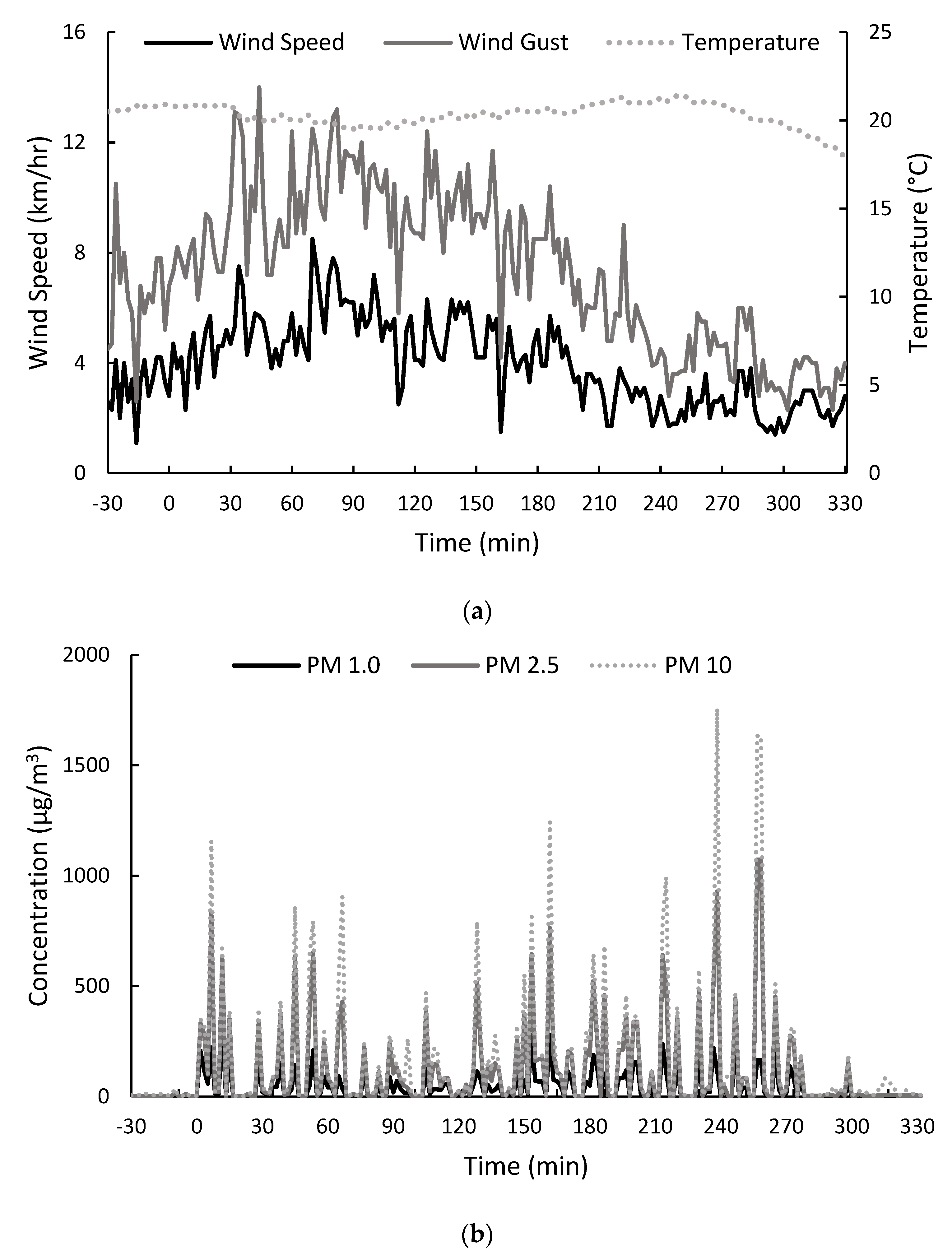
2.1.1. Environmental Conditions before, during and after the Preliminary Field Trial
2.1.2. Density and Duration of Smoke Exposure-Affected Grape Volatile Phenols
2.1.3. Density and Duration of Smoke Exposure Affect Wine Volatile Phenols
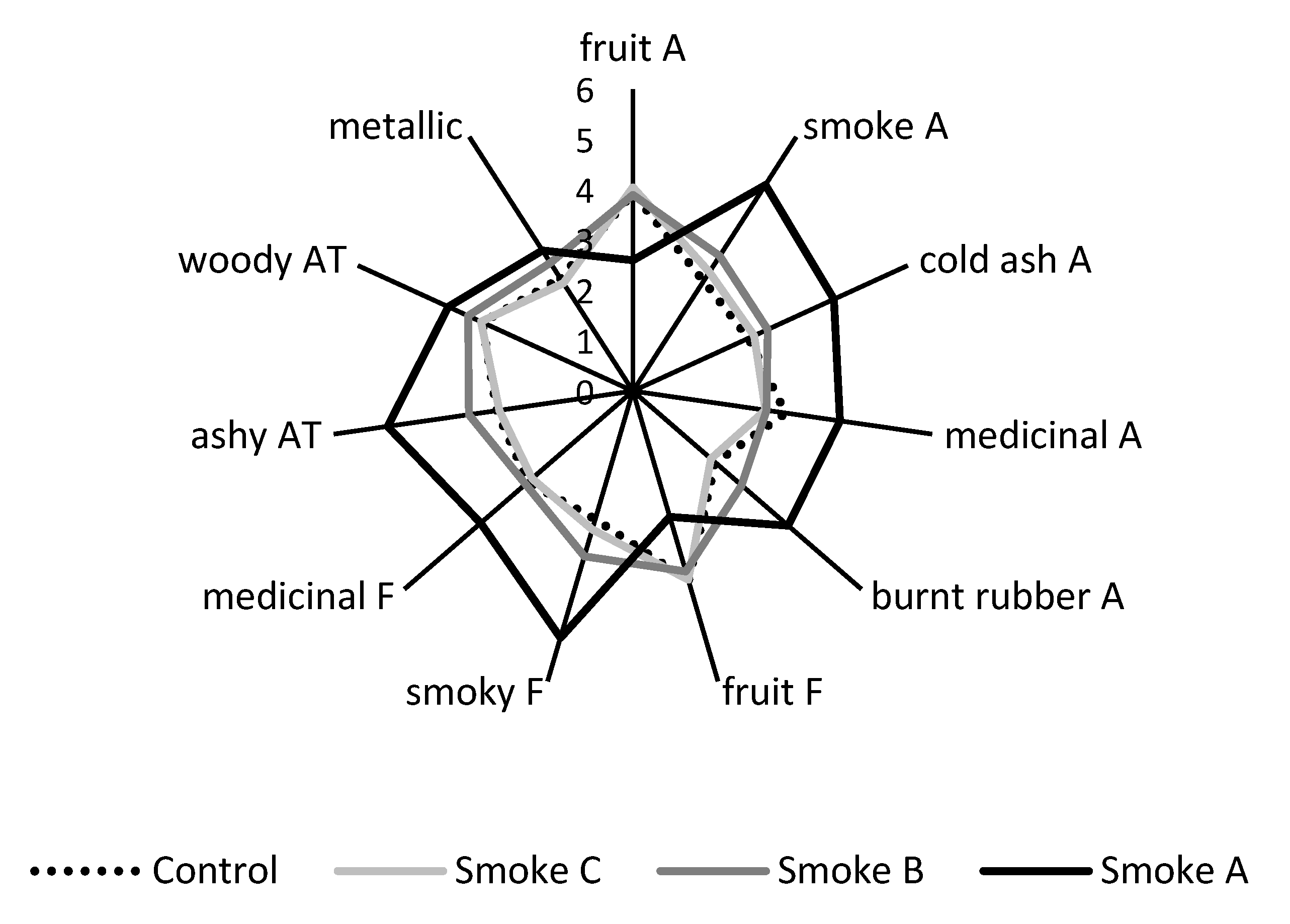
2.1.4. Density and Duration of Smoke Exposure Affect Wine Sensory Profiles
2.2. Evaluating the Potential for Smoke from Pea Stubble Burning to Taint Grapes and Wine
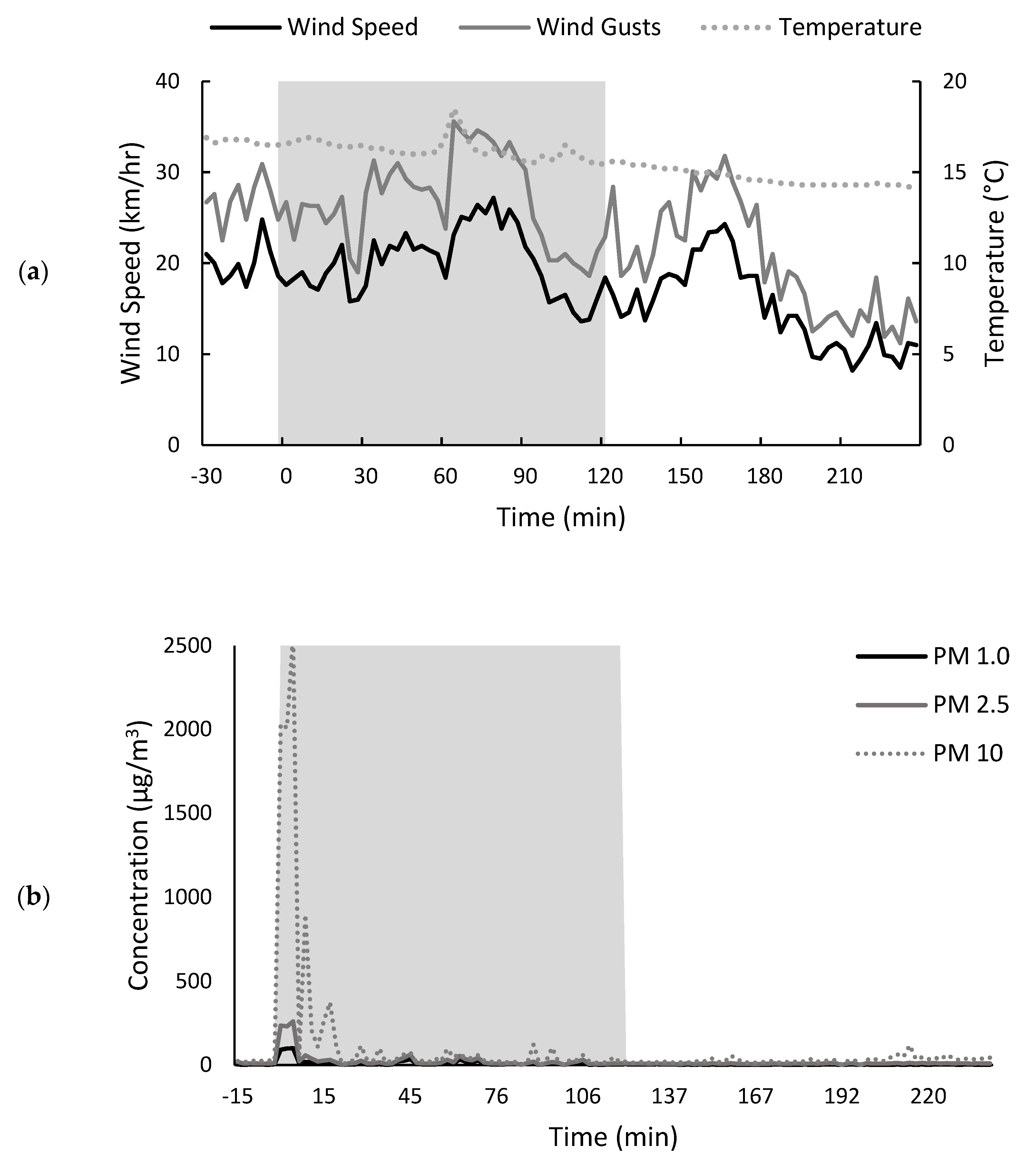
2.2.1. Environmental Conditions before, during and after the Stubble Burn Trial
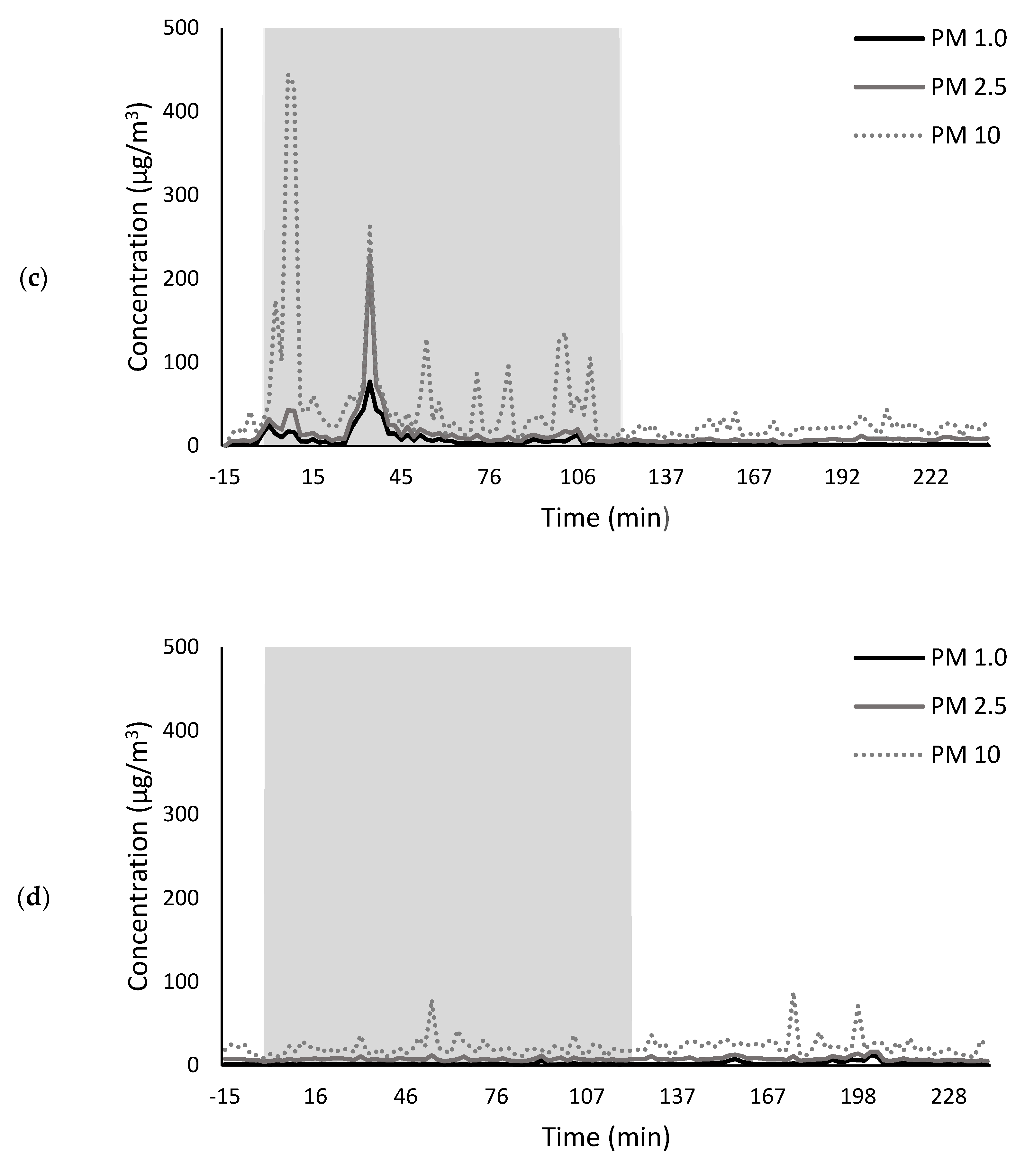
2.2.2. Compositional Consequences of Fruit Exposure to Smoke from Pea Stubble Burn
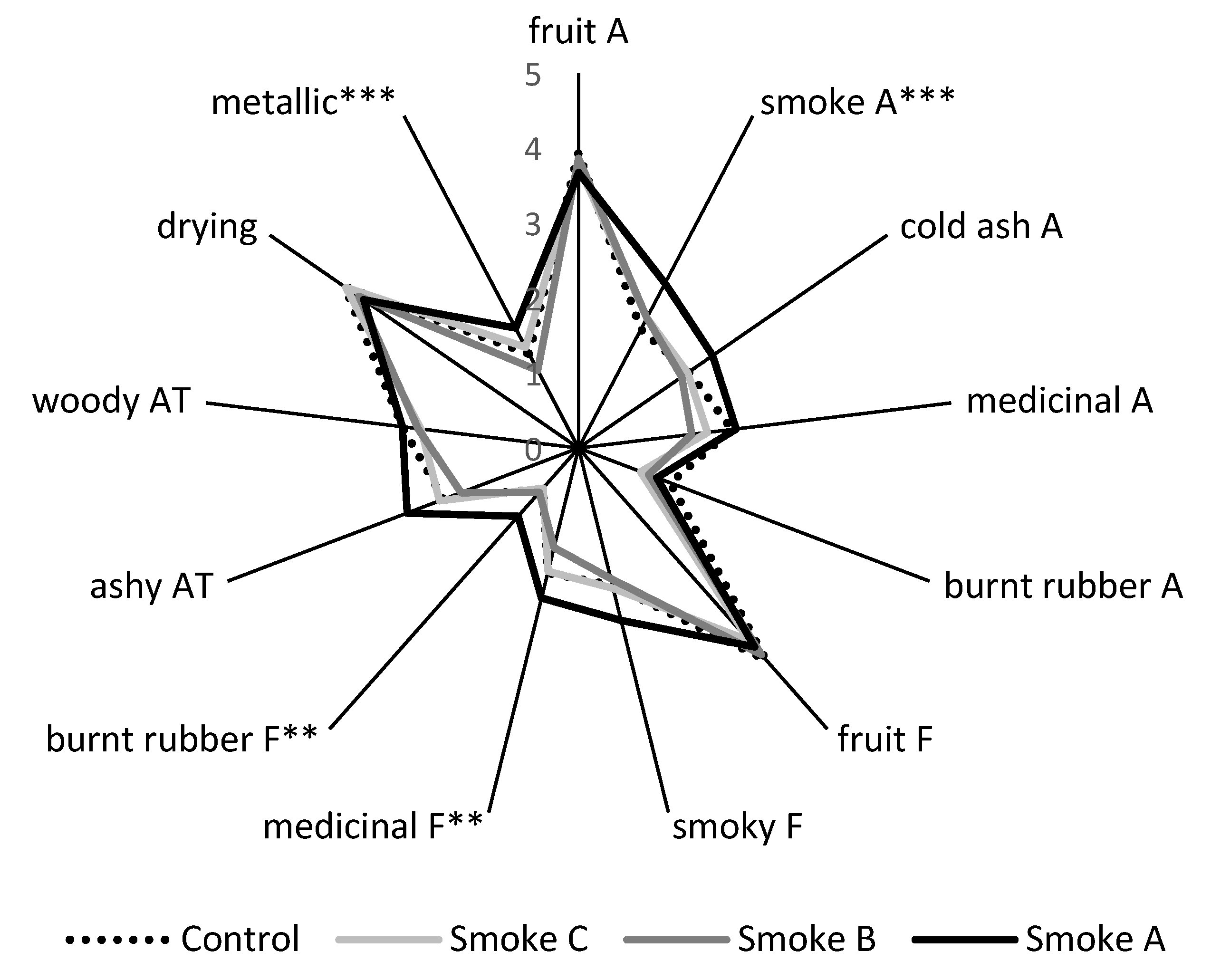
2.2.3. Sensory Consequences of Fruit Exposure to Smoke from Pea Stubble Burn
3. Materials and Methods
3.1. Field Trials
3.1.1. Preliminary Field Trial: Exposure of Shiraz Grapes to Barley Straw Smoke
3.1.2. Stubble Burn Trial: Exposure of Cabernet Sauvignon Grapes to Pea Stubble Smoke
3.2. Winemaking
3.3. Chemical Analysis of Grapes and Wine
3.4. Sensory Analysis of Wine
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Aust. J. Grape Wine Res. 2011, 17, S5–S12. [Google Scholar] [CrossRef]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.D.; Wilkinson, K.L. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Physiol. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A. The effect of phenol composition on the sensory profile of smoke affected wines. Molecules 2015, 20, 9536–9549. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Dungey, K.A.; Baldock, G.A.; Kennison, K.R.; Wilkinson, K.L. Identification of a β-D-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal. Chim. Acta 2010, 660, 143–148. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef]
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography-tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Noestheden, M.; Dennis, E.G.; Romero-Montalvo, E.; DiLabio, G.A.; Zandberg, W.F. Detailed characterization of glycosylated sensory-active volatile phenols in smoke-exposed grapes and wine. Food Chem. 2018, 259, 147–156. [Google Scholar] [CrossRef] [PubMed]
- van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef]
- Caffrey, A.; Lerno, L.; Rumbaugh, A.; Girardello, R.; Zweigenbaum, J.; Oberholster, A.; Ebeler, S.E. Changes in smoke-taint volatile-phenol glycosides in wildfire smoke-exposed Cabernet Sauvignon grapes throughout winemaking. Am. J. Enol. Vitic. 2019, 70, 373–381. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and glycosylation of smoke-derived volatile phenols by Cabernet Sauvignon grapes and their subsequent fate during winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Ristic, R.; Pinchbeck, K.A.; Fudge, A.L.; Singh, D.P.; Pitt, K.M.; Downey, M.O.; Baldock, G.A.; Hayasaka, Y.; Parker, M.; et al. Comparison of methods for the analysis of smoke related phenols and their conjugates in grapes and wine. Aust. J. Grape Wine Res. 2011, 17, S22–S28. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of an HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2013, 61, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Noestheden, M.; Thiessen, K.; Dennis, E.G.; Zandberg, W.F. Quantitating organoleptic volatile phenols in smoke-exposed Vitis vinifera berries. J. Agric. Food Chem. 2017, 65, 8418–8425. [Google Scholar] [CrossRef]
- Bell, T.L.; Stephens, S.L.; Moritz, M.A. Short-term physiological effects of smoke on grapevine leaves. Int. J. Wildland Fire 2013, 22, 933–946. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Szeto, C.; Wilkinson, K.L.; Torrico, D.D.; Pang, A.; De Bei, R.; Fuentes, S. Classification of smoke contaminated Cabernet Sauvignon berries and leaves based on chemical fingerprinting and machine learning algorithms. Sensors 2020, 20, 5099. [Google Scholar] [CrossRef]
- Ristic, R.; Boss, P.K.; Wilkinson, K.L. Influence of fruit maturity at harvest on the intensity of smoke taint in wine. Molecules 2015, 20, 8913–8927. [Google Scholar] [CrossRef]
- Ristic, R.; Pinchbeck, K.A.; Fudge, A.L.; Hayasaka, Y.; Wilkinson, K.L. Effect of leaf removal and grapevine smoke exposure on colour, chemical composition and sensory properties of Chardonnay wines. Aust. J. Grape Wine Res. 2013, 19, 230–237. [Google Scholar] [CrossRef]
- Favell, J.W.; Noestheden, M.; Lyon, S.M.; Zandberg, W.F. Development and evaluation of a vineyard-based strategy to mitigate smoke-taint in wine grapes. J. Agric. Food Chem. 2019, 67, 14137–14142. [Google Scholar] [CrossRef]
- Culbert, J.C.; Krstic, M.; Herderich, M. Development and utilization of a model system to evaluate the potential of surface coatings for protecting grapes from volatile phenols implicated in smoke taint. Molecules 2021, 26, 5197. [Google Scholar] [CrossRef] [PubMed]
- Fudge, A.L.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Culbert, J.A.; Jiang, W.; Eleanor, B.; Likos, D.; Francis, I.L.; Krstic, M.P.; Herderich, M.J. Compositional changes in smoke-affected grape juice as a consequence of activated carbon treatment and the impact on phenolic compounds and smoke flavor in wine. J. Agric. Food Chem. 2021, 69, 10246–10259. [Google Scholar] [CrossRef]
- Modesti, M.; Szeto, C.; Ristic, R.; Jiang, W.; Culbert, J.; Bindon, K.; Catelli, C.; Mencarelli, F.; Tonutti, P.; Wilkinson, K. Potential mitigation of smoke taint in wines by post-harvest ozone treatment of grapes. Molecules 2021, 26, 1798. [Google Scholar] [CrossRef]
- Scott, B.J.; Eberback, P.L.; Evans, J.; Wade, L.J.E.H. Graham Centre Monograph No. 1: Stubble Retention in Cropping Systems in Southern Australia: Benefits and Challenges; Clayton, E.H., Burns, H.M., Eds.; Industry and Investment: Orange, NSW, Australia, 2010; pp. 1–105. Available online: https://www.csu.edu.au/research/grahamcentre/publications/monograph/stubble-retention-in-cropping-systems-in-southern-australia-benefits-and-challenges (accessed on 4 November 2021).
- Local Government Air Quality Toolkit. Module 3: Guidelines for Managing Air Pollution. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjyoNO3rP3zAhUDfH0KHV14B34QFnoECDYQAQ&url=http%3A%2F%2Fwww.epa.nsw.gov.au%2Fresources%2Fair%2Fmod3p3agstubble07268.pdf&usg=AOvVaw1Nqt9-vOej-zrowFhHPguY (accessed on 4 November 2021).
- Abdurrahman, M.I.; Chaki, S.; Saini, G. Stubble burning: Effects of health and environment, regulations and management practices. Environ. Adv. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Walsh, M.; Newman, P. Burning narrow windrows for weed seed destruction. Field Crops Res. 2007, 104, 24–30. [Google Scholar] [CrossRef]
- South Australian Country Fire Service Broad Acre Burning Code of Practice. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwinlamqyv_zAhUmGKYKHfmQCYIQFnoECAkQAQ&url=https%3A%2F%2Fwww.cfs.sa.gov.au%2Fpublic%2Fdownload%2F%3Fid%3D104046&usg=AOvVaw2TJ5ERb_b6U721Hcr7jME0 (accessed on 5 November 2021).
- Keywood, M.D.; Ayers, G.P.; Gras, J.L.; Gillett, R.W.; Cohen, D.D. Size distribution and sources of aerosol in Launceston, Australia, during winter 1997. J. Air Waste Manag. Assoc. 2000, 50, 418–427. [Google Scholar] [CrossRef][Green Version]
- Sefton, M.A. Hydrolytically-released volatile secondary metabolites from a juice sample of Vitis vinifera grape cvs Merlot and Cabernet Sauvignon. Aust. J. Grape Wine Res. 1988, 4, 30–38. [Google Scholar] [CrossRef]
- Wirth, J.; Guo, W.; Baumes, R.; Günata, Z. Volatile compounds released by enzymatic hydrolysis of glycoconjugates of leaves and grape berries from Vitis vinifera Muscat of Alexandria and Shiraz cultivars. J. Agric. Food Chem. 2001, 49, 2917–2923. [Google Scholar] [CrossRef]
- Culbert, J.A.; Jiang, W.; Ristic, R.; Puglisi, C.J.; Nixon, E.C.; Shi, H.; Wilkinson, K.L. Glycosylation of volatile phenols in grapes following pre-harvest (on-vine) vs. post-harvest (off-vine) exposure to smoke. Molecules 2021, 26, 5277. [Google Scholar] [CrossRef]
- Favell, J.W.; Fordwour, O.B.; Morgan, S.C.; Zigg, I.; Zandberg, W. Large-scale reassessment of in-vineyard smoke-taint grapevine protection strategies and the development of predictive off-vine models. Molecules 2021, 26, 4311. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Influence of vine training and sunlight exposure on the 3-alkyl-2-methoxypyrazine content in musts and wines from Vitis vinifera variety Cabernet Sauvignon. J. Agric. Food Chem. 2004, 52, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Pollnitz, A.P.; Pardon, K.H.; Sykes, M.; Sefton, M.A. The effects of sample preparation and gas chromatograph injection techniques on the accuracy of measuring guaiacol, 4-methylguaiacol and other volatile oak compounds in oak extracts by stable isotope dilution analyses. J. Agric. Food Chem. 2004, 52, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a rating-based variant of check-all-that-apply questions: Rate-all-that-apply (RATA). Food Qual. Pref. 2014, 36, 87–95. [Google Scholar] [CrossRef]

| Treatment | Guaiacol | 4-Methyl Guaiacol | o-Cresol | m-Cresol | p-Cresol | Syringol | 4-Methyl Syringol | |
|---|---|---|---|---|---|---|---|---|
| Grapes | Control | nd | nd | nd | nd | nd | nd | nd |
| Smoke A | 2 | nd | 1 | 1 | nd | nd | nd | |
| Smoke B | 2 | nd | nd | nd | nd | nd | nd | |
| Smoke C | 1 | nd | nd | nd | nd | nd | nd | |
| Wine | Control | 2 | nd | nd | nd | nd | 2 | nd |
| Smoke A | 2 | nd | 1 | 1 | 1 | 3 | nd | |
| Smoke B | 2 | nd | nd | nd | 1 | 3 | nd | |
| Smoke C | 2 | nd | nd | nd | 1 | 3 | nd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, K.; Ristic, R.; McNamara, I.; Loveys, B.; Jiang, W.; Krstic, M. Evaluating the Potential for Smoke from Stubble Burning to Taint Grapes and Wine. Molecules 2021, 26, 7540. https://doi.org/10.3390/molecules26247540
Wilkinson K, Ristic R, McNamara I, Loveys B, Jiang W, Krstic M. Evaluating the Potential for Smoke from Stubble Burning to Taint Grapes and Wine. Molecules. 2021; 26(24):7540. https://doi.org/10.3390/molecules26247540
Chicago/Turabian StyleWilkinson, Kerry, Renata Ristic, Imogen McNamara, Beth Loveys, WenWen Jiang, and Mark Krstic. 2021. "Evaluating the Potential for Smoke from Stubble Burning to Taint Grapes and Wine" Molecules 26, no. 24: 7540. https://doi.org/10.3390/molecules26247540
APA StyleWilkinson, K., Ristic, R., McNamara, I., Loveys, B., Jiang, W., & Krstic, M. (2021). Evaluating the Potential for Smoke from Stubble Burning to Taint Grapes and Wine. Molecules, 26(24), 7540. https://doi.org/10.3390/molecules26247540







