Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology
Abstract
1. Introduction
2. Results
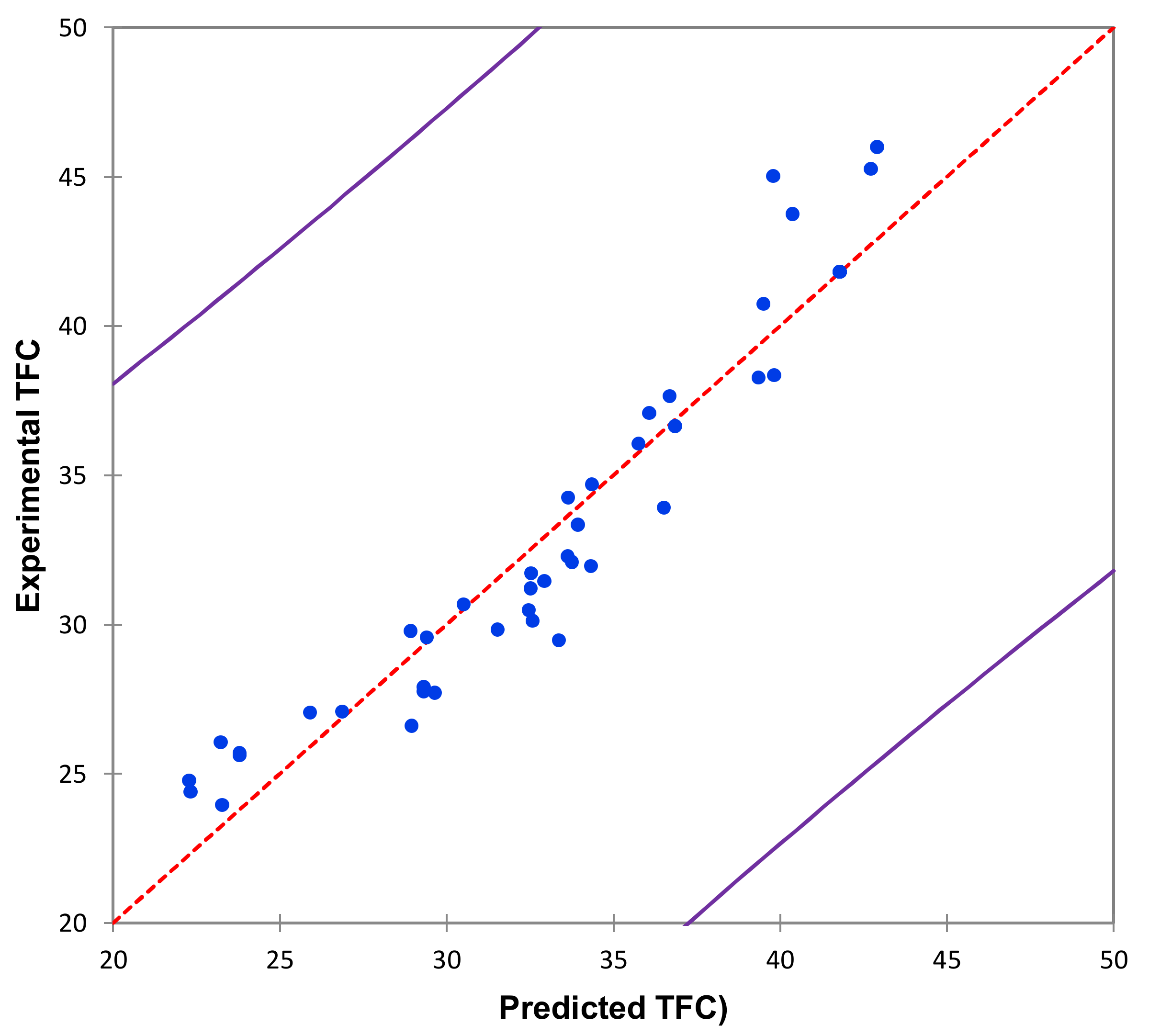
2.1. Extraction Optimization Using the Box–Behnken Design (BBD)
2.2. Characterization of Antioxidant and Anti-Aging Potential
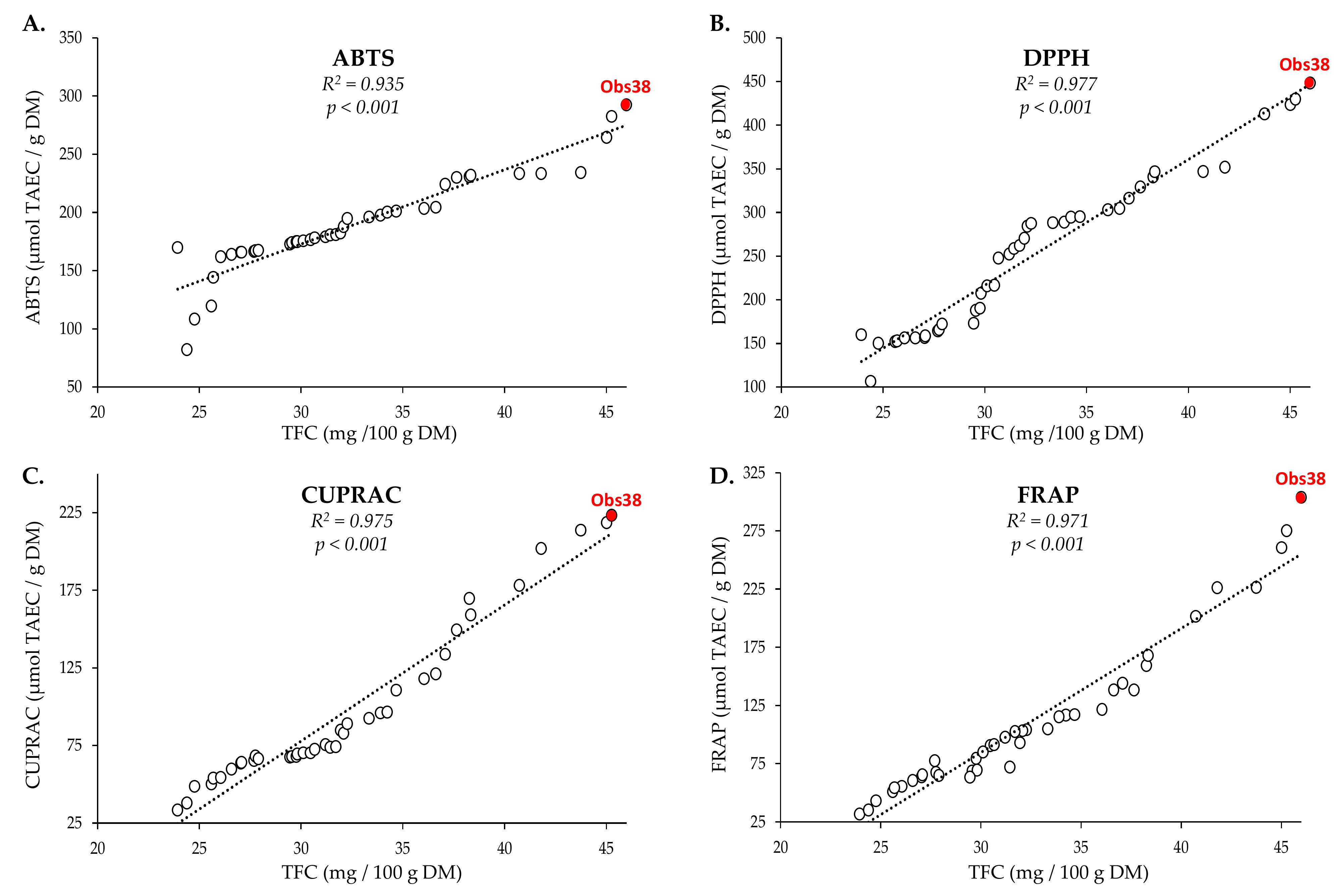
2.2.1. Correlation between TFC and In Vitro Antioxidant Capacity
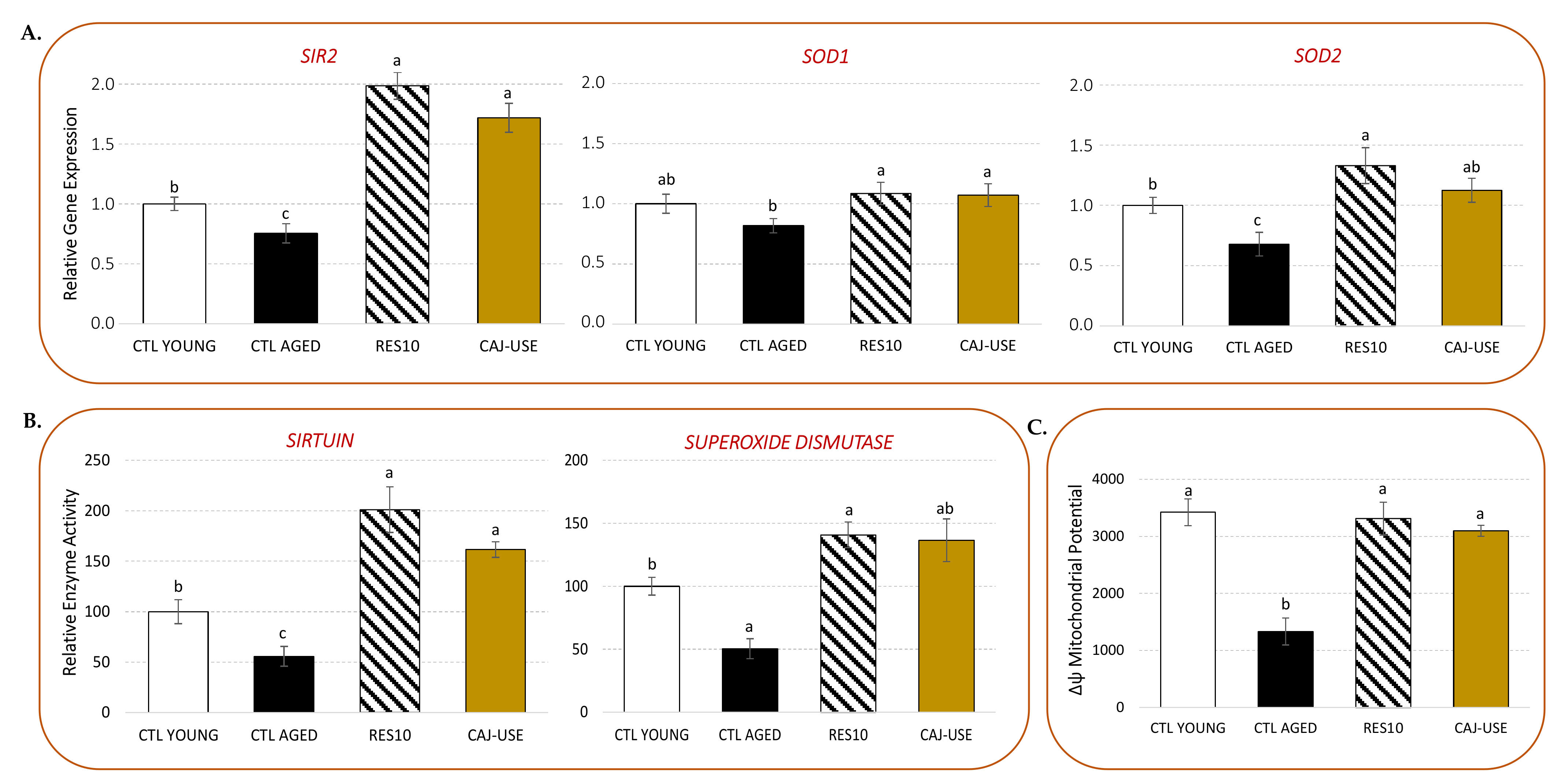
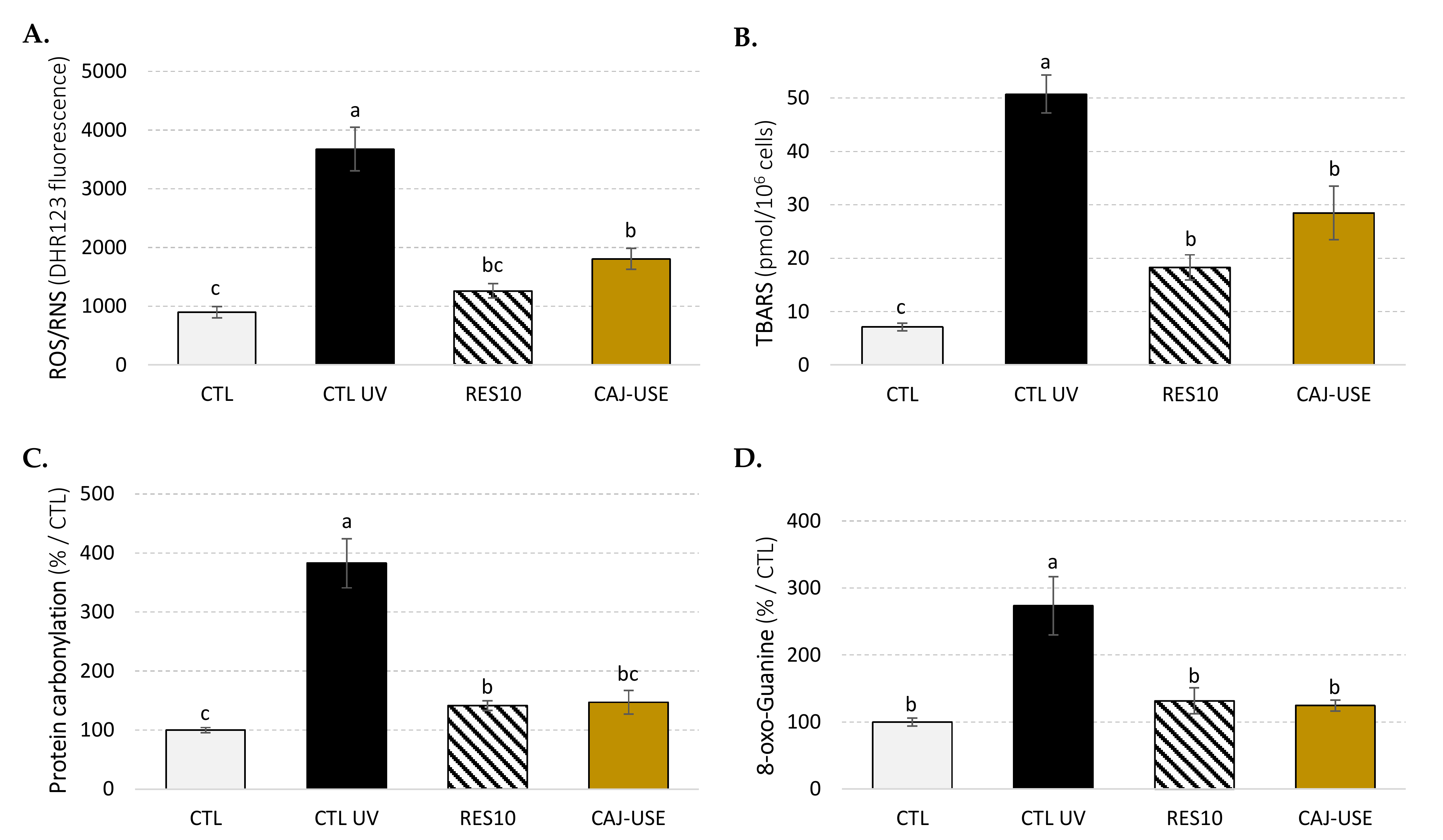
2.2.2. Cellular Antioxidant and Anti-Aging Potential
2.3. HPLC-UV-DAD Analysis and Comparison with the Conventional Method
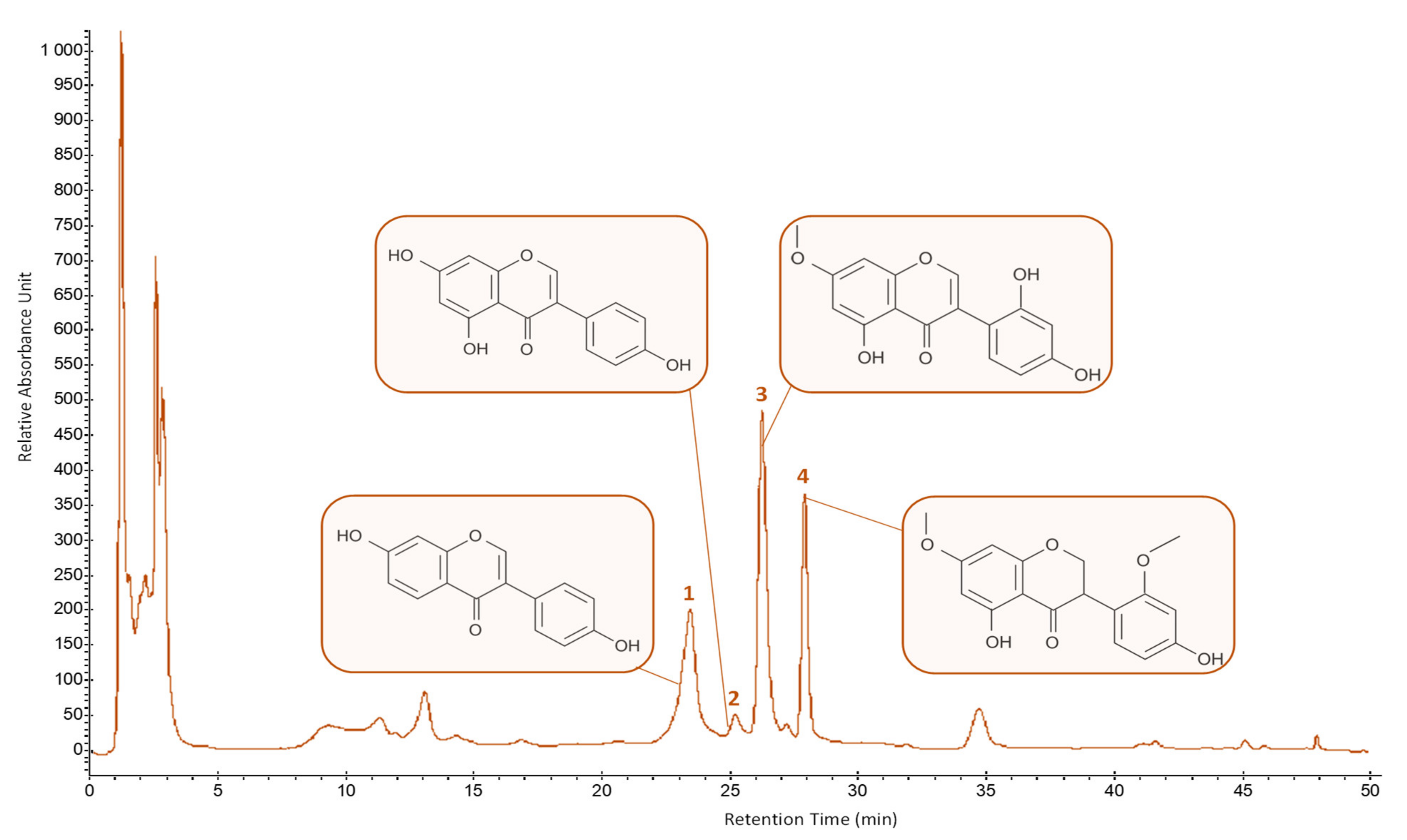
2.3.1. HPLC-UV-DAD Analysis
2.3.2. Comparison with the Conventional Extraction Method
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials
4.3. Ultrasound-Assisted Extraction Method Development
4.4. Determination of the Total Flavonoid Content
4.5. HPLC-UV-DAD Analysis
4.6. In Vitro Antioxidant Activities
4.7. Yeast Culture Conditions
4.8. Cellular Antioxidant Assay
4.9. Mitochondria Membrane Potential Evaluation
4.10. Gene Expression by RT-qPCR Analysis
4.11. Enzymatic SIRT1/SIR2 and Total SOD Activity Determinations
4.12. Cellular Oxidative Stress Products
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tungmunnithum, D.; Hano, C. Cosmetic potential of Cajanus cajan (L.) millsp: Botanical data, traditional uses, phytochemistry and biological activities. Cosmetics 2020, 7, 84. [Google Scholar] [CrossRef]
- Ahsan, R.; Islam, M. In vitro antibacterial screening and toxicological study of some useful plants (Cajanus cajan). Eur. J. Sci. Res. 2009, 41, 227–232. [Google Scholar]
- Ambasta, S.P. The Useful Plants of India; National Institute of Science Communication: New Delhi, India, 2004; pp. 94–95. [Google Scholar]
- Kiwia, A.; Kimani, D.; Harawa, R.; Jama, B.; Sileshi, G.W. Sustainable Intensification with Cereal-Legume Intercropping in Eastern and Southern Africa. Sustainability 2019, 11, 2891. [Google Scholar] [CrossRef]
- Owens, J.D.; Astuti, M.K. Tempe and related products. In Indigenous Fermented Foods of Southeast Asia; Owens, J.D., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Fuller, D.Q.; Murphy, C.; Kingwell-Banham, E.; Castillo, C.C.; Naik, S. Cajanus cajan (L.) Millsp. origins and domestication: The South and Southeast Asian archaeobotanical evidence. Genet. Resour. Crop. Evol. 2019, 66, 1175–1188. [Google Scholar] [CrossRef]
- Upadhyay, B.; Parveen; Dhaker, A.K.; Kumar, A. Ethnomedicinal and ethnopharmaco—Statistical studies of Eastern Rajasthan, India. J. Ethnopharmacol 2010, 129, 64–86. [Google Scholar] [CrossRef]
- Darbyshire, I.; Kordofani, M.; Farag, I.; Candiga, R.; Pickering, H. The Plants of Sudan and South. Sudan; Kew Publishing, Royal Botanic Gardens, Kew: Richmond, UK, 2015; ISBN 9781842465172. [Google Scholar]
- Plants of the World. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:1152177-2 (accessed on 25 October 2021).
- Wu, Z.; Raven, P.H. Cajanus. In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2010; pp. 230–232. [Google Scholar]
- Ganesan, S. Traditional oral care medicinal plants survey of Tamil nadu. Nat. Prod. Rad. 2008, 7, 166–172. [Google Scholar]
- Al-Saeedi, A.H.; Amzad Hossain, M. Total phenols, total flavonoids contents and free radical scavenging activity of seeds crude extracts of pigeonpea traditionally used in Oman for the treatment of several chronic diseases. Asian Pac. J. Trop. Dis. 2015, 5, 316–321. [Google Scholar] [CrossRef]
- Hassan, E.M.; Matloub, A.; Aboutabl, M.E.; Ibrahim, N.A.; Mohamed, S. Assessment of anti-inflammatory, antinociceptive, immunomodulatory, and antioxidant activities of Cajanus cajan L. seeds cultivated in Egypt and its phytochemical composition. Pharm. Biol. 2015, 54, 1380–1391. [Google Scholar] [CrossRef]
- Uchegbu, N.N.; Ishiwu, C.N. Germinated Pigeon Pea (Cajanus cajan): A novel diet for lowering oxidative stress and hyperglycemia. Food Sci. Nutr. 2016, 4, 772–777. [Google Scholar] [CrossRef]
- Sarkar, R.; Mandal, N. Hydroalcoholic extracts of Indian medicinal plants can help in amelioration from oxidative stress through antioxidant properties. J. Complement. Integr. Med. 2012, 9, 1553–1583. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Jin, S.; Luo, M.; Pan, Y.-Z.; Li, T.-T.; Qi, X.-L.; Efferth, T.; Fu, Y.-J.; Zu, Y.-G. Variation in Contentsof Main Active Components and Antioxidant Activity in Leaves of Dierent Pigeon Pea Cultivars during Growth. J. Agric. Food Chem. 2013, 61, 10002–10009. [Google Scholar] [CrossRef]
- Mahitha, B.; Archana, P.; Ebrahimzadeh, M.H.; Srikanth, K.; Rajinikanth, M.; Ramaswamy, N. In vitro Antioxidant and Pharmacognostic Studies of Leaf Extracts of Cajanus cajan (L.) Millsp. Indian J. Pharm. Sci. 2015, 77, 170. [Google Scholar]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Vo, T.-L.T.; Yang, N.-C.; Yang, S.-E.; Chen, C.-L.; Wu, C.-H.; Song, T.-Y. Effects of Cajanus cajan (L.) millsp. roots extracts on the antioxidant and anti-inflammatory activities. Chin. J. Physiol. 2020, 63, 137. [Google Scholar]
- Zheng, X.; Wang, X.; Lan, Y.; Shi, J.; Jun, S.; Liu, C. Application of response surface methodology to optimize microwave-assisted extraction of silymarin from milk thistle seeds. Sep. Purif. Technol 2009, 70, 34–40. [Google Scholar] [CrossRef]
- Fliniaux, O.; Corbin, C.; Ramsay, A.; Renouard, S.; Beejmohun, V.; Doussot, J.; Falguières, A.; Ferroud, C.; Lamblin, F.; Lainé, E. Microwave-Assisted Extraction of Herbacetin Diglucoside from Flax (Linum usitatissimum L.) Seed Cakes and Its Quantification using an RP-HPLC-UV System. Molecules 2014, 19, 3025–3037. [Google Scholar] [CrossRef]
- Benthin, B.; Danz, H.; Hamburger, M. Pressurized liquid extraction of medicinal plants. J. Chromatogr. A 1999, 837, 211–219. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Ozkur, M.K.; Bozkurt, M.S.; Balabanli, B.; Aricioglu, A.; Ilter, N.; Gurer, M.A.; Inaloz, H.S. The effect of EGb 761 on lipid peroxide levels and superoxide dismutase activity in sunburn. Photodermatol. Photoimmunol. Photomed. 2002, 18, 117–120. [Google Scholar] [CrossRef]
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, S.; Nishiba, Y.; Terahara, N.; Suda, A.I. Involvement of Anthocyanins and Other Phenolic Compounds in Radical Scavenging Activity of Purple-Fleshed Sweet Potato Cultivars. Food Chem. Toxicol. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- Okpuzor, J.; Ogbunugafor, H.; Kareem, G.K.; Igwo-Ezikpe, M.N. In vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res. J. Phytochem. 2009, 3, 68–76. [Google Scholar] [CrossRef]
- Nagja, T.; Vimal, K.; Sanjeev, A. Myristica Fragrans: A comprehensive review. Int. J. Pharm. Pharm. Sci. 2016, 8, 27–30. [Google Scholar]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lain, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef]
- Giordano, M.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Stojković, D.; Soković, M.; Tavares, D.; Cánepa, A.L.; Ferreira, I.C.F.R.; Caleja, C.; et al. Ultrasound-Assisted Extraction of Flavonoids from Kiwi Peel: Process Optimization and Bioactivity Assessment. Appl. Sci. 2021, 11, 6416. [Google Scholar] [CrossRef]
- Lee, M.; Lin, C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 248, 248–263. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. With Distinct in Vitro Antioxidant Activities and in Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kaihara, A.; Yoshii, K.; Tsumura, Y.; Ishimitsu, S.; Tonogai, Y. Content and composition of isoflavonoids in mature or immature beans and bean sprouts consumed in Japan. J. Health Sci. 2001, 47, 394–406. [Google Scholar] [CrossRef][Green Version]
- Drouet, S.; Doussot, J.; Garros, L.; Mathiron, D.; Bassard, S.; Favre-Réguillon, A.; Molinié, R.; Lainé, É.; Hano, C. Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media. Molecules 2018, 23, 2594. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef]
- Hostettmann, K.; Hostettmann, M. Isolation Techniques for Flavonoids. In The Flavonoids; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Lopez, T.; Corbin, C.; Falguieres, A.; Doussot, J.; Montguillon, J.; Hagège, D.; Hano, C.; Lainé, É. Secondary metabolite accumulation, antibacterial and antioxidant properties of in vitro propagated Clidemia hirta L. extracts are influenced by the basal culture medium. Comptes Rendus Chim. 2016, 19, 1071–1076. [Google Scholar] [CrossRef]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications, L’ortie (Urtica dioica L.), une source de produits antioxidants et phytochimiques anti-âge pour des applications en cosmétique. Comptes Rendus Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Renouard, S.; Corbin, C.; Colas, C.; Fidel, T.; Lopez, T.; Leclerc, E.A.; Hendrawati, O.; Falguières, A.; Doussot, J.; Ferroud, C.; et al. Aerial parts of Callitris species as a rich source of deoxypodophyllotoxin. Ind. Crops Prod. 2015, 63, 53–57. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0198502346. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; de Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (Linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef]
- Drouet, S.; Abbasi, B.H.; Falguières, A.; Ahmad, W.; Sumaira; Ferroud, C.; Doussot, J.; Vanier, J.R.; Lainé, E.; Hano, C. Single Laboratory Validation of a Quantitative Core Shell-Based LC Separation for the Evaluation of Silymarin Variability and Associated Antioxidant Activity of Pakistani Ecotypes of Milk Thistle (Silybum Marianum L.). Molecules 2018, 23, 904. [Google Scholar]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Steels, E.L.; Learmonth, R.P.; Watson, K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994, 140, 569–576. [Google Scholar] [CrossRef]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-Kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G.; Catherine, A.R.-E.; Nicholas, J.M.; George, P. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lacza, Z.; Pankotai, E.; Csordás, A.; Gero, D.; Kiss, L.; Horváth, E.M.; Kollai, M.; Busija, D.W.; Szabó, C. Mitochondrial NO and reactive nitrogen species production: Does mtNOS exist? Nitric Oxide 2006, 14, 162–168. [Google Scholar] [CrossRef]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging 2013, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010, 285, 8375–8382. [Google Scholar] [CrossRef] [PubMed]
- Daitoku, H.; Hatta, M.; Matsuzaki, H.; Aratani, S.; Ohshima, T.; Miyagishi, M.; Nakajima, T.; Fukamizu, A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10042–10047. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life 2020, 10, 80. [Google Scholar] [CrossRef]
- Sun, K.; Cao, S.; Pei, L.; Matsuura, A.; Xiang, L.; Qi, J. A steroidal saponin from Ophiopogon japonicus extends the lifespan of yeast via the pathway involved in SOD and UTH1. Int. J. Mol. Sci. 2013, 14, 4461–4475. [Google Scholar] [CrossRef]
- Anna Malinowska, M.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety Assessment of Butylated Hydroxyanisole and Butylated Hydroxytoluene as Antioxidant Food Additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]

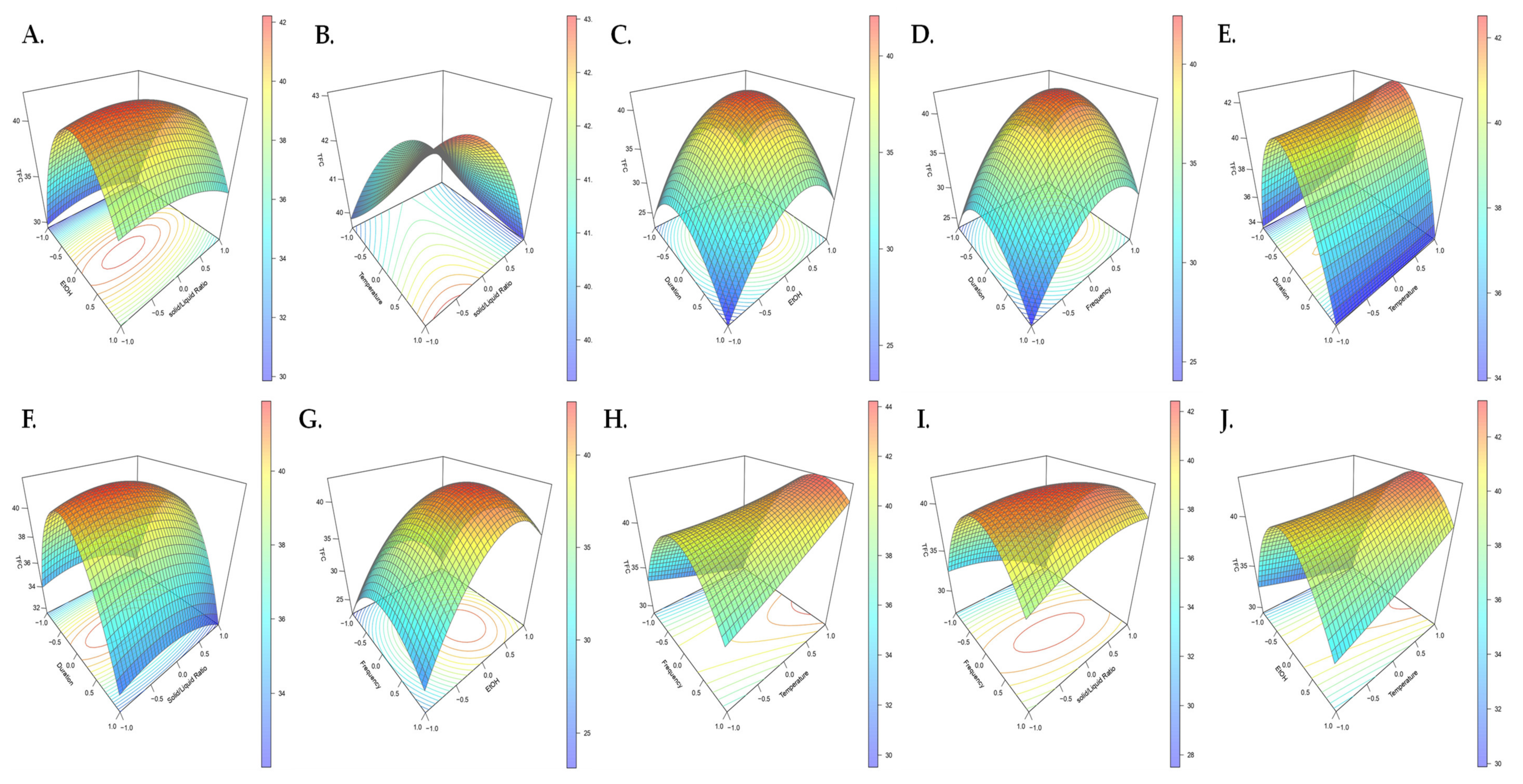
| Independent Variable | Code Unit | Coded Variable Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Extraction duration (min) | X1 | 20 | 40 | 60 |
| US frequency (kHz) | X2 | 0 | 22.5 | 45 |
| Ethanol concentration (% v/v) 1 | X3 | 50 | 75 | 100 |
| Extraction temperature (°C) | X4 | 30 | 45 | 60 |
| Liquid/solid ratio (mg/mL) 2 | X5 | 1 | 5.5 | 10 |
| Run ID | Run Order | X1 | X2 | X3 | X4 | X5 | Experimental TFC (mg/100 g DM) | Predicted TFC (mg/100 g DM) |
|---|---|---|---|---|---|---|---|---|
| Obs1 | 21 | −1 | −1 | 0 | 0 | 0 | 23.94 ± 1.35 | 23.28 |
| Obs2 | 15 | +1 | −1 | 0 | 0 | 0 | 26.05 ± 2.68 | 23.23 |
| Obs3 | 1 | −1 | +1 | 0 | 0 | 0 | 30.46 ± 1.71 | 32.47 |
| Obs4 | 16 | +1 | +1 | 0 | 0 | 0 | 30.66 ± 0.37 | 30.51 |
| Obs5 | 33 | −1 | 0 | −1 | 0 | 0 | 25.60 ± 1.62 | 23.81 |
| Obs6 | 28 | +1 | 0 | −1 | 0 | 0 | 24.76 ± 2.31 | 22.28 |
| Obs7 | 27 | −1 | 0 | +1 | 0 | 0 | 29.56 ± 0.03 | 29.41 |
| Obs8 | 17 | +1 | 0 | +1 | 0 | 0 | 29.76 ± 0.67 | 28.93 |
| Obs9 | 34 | −1 | 0 | 0 | −1 | 0 | 34.24 ± 0.12 | 33.65 |
| Obs10 | 9 | +1 | 0 | 0 | −1 | 0 | 32.27 ± 0.94 | 33.64 |
| Obs11 | 20 | −1 | 0 | 0 | +1 | 0 | 36.04 ± 0.50 | 35.76 |
| Obs12 | 13 | +1 | 0 | 0 | +1 | 0 | 32.08 ± 1.32 | 33.76 |
| Obs13 | 4 | −1 | 0 | 0 | 0 | −1 | 33.33 ± 0.25 | 33.95 |
| Obs14 | 24 | +1 | 0 | 0 | 0 | −1 | 31.44 ± 1.46 | 32.94 |
| Obs15 | 3 | −1 | 0 | 0 | 0 | +1 | 31.70 ± 0.36 | 32.54 |
| Obs16 | 8 | +1 | 0 | 0 | 0 | +1 | 29.81 ± 1.57 | 31.54 |
| Obs17 | 29 | 0 | −1 | −1 | 0 | 0 | 24.39 ± 2.42 | 22.33 |
| Obs18 | 10 | 0 | +1 | −1 | 0 | 0 | 27.04 ± 1.10 | 25.92 |
| Obs19 | 11 | 0 | −1 | +1 | 0 | 0 | 25.69 ± 2.24 | 23.80 |
| Obs20 | 37 | 0 | +1 | +1 | 0 | 0 | 37.64 ± 0.92 | 36.69 |
| Obs21 | 30 | 0 | −1 | 0 | −1 | 0 | 29.46 ± 3.63 | 33.37 |
| Obs22 | 2 | 0 | +1 | 0 | −1 | 0 | 37.07 ± 0.85 | 36.08 |
| Obs23 | 36 | 0 | −1 | 0 | +1 | 0 | 26.59 ± 1.87 | 28.96 |
| Obs24 | 19 | 0 | +1 | 0 | +1 | 0 | 45.26 ± 2.61 | 42.72 |
| Obs25 | 22 | 0 | −1 | 0 | 0 | −1 | 31.20 ± 2.70 | 32.53 |
| Obs26 | 35 | 0 | +1 | 0 | 0 | −1 | 33.90 ± 2.81 | 36.52 |
| Obs27 | 40 | 0 | −1 | 0 | 0 | +1 | 27.07 ± 0.48 | 26.87 |
| Obs28 | 26 | 0 | +1 | 0 | 0 | +1 | 38.26 ± 0.60 | 39.36 |
| Obs29 | 25 | 0 | 0 | −1 | −1 | 0 | 30.11 ± 2.53 | 32.59 |
| Obs30 | 12 | 0 | 0 | +1 | −1 | 0 | 31.94 ± 2.44 | 34.33 |
| Obs31 | 7 | 0 | 0 | −1 | +1 | 0 | 27.75 ± 1.70 | 29.32 |
| Obs32 | 41 | 0 | 0 | +1 | +1 | 0 | 38.24 ± 1.61 | 39.83 |
| Obs33 | 5 | 0 | 0 | −1 | 0 | −1 | 27.70 ± 1.68 | 29.66 |
| Obs34 | 31 | 0 | 0 | +1 | 0 | −1 | 36.62 ± 0.05 | 36.86 |
| Obs35 | 39 | 0 | 0 | −1 | 0 | +1 | 27.90 ± 1.53 | 29.33 |
| Obs36 | 18 | 0 | 0 | +1 | 0 | +1 | 34.67 ± 0.20 | 34.37 |
| Obs37 | 23 | 0 | 0 | 0 | −1 | −1 | 45.01 ± 5.53 | 39.80 |
| Obs38 | 6 | 0 | 0 | 0 | +1 | −1 | 45.98 ± 3.32 | 42.91 |
| Obs39 | 38 | 0 | 0 | 0 | −1 | +1 | 43.75 ± 3.28 | 40.39 |
| Obs40 | 32 | 0 | 0 | 0 | +1 | +1 | 40.73 ± 1.07 | 39.51 |
| Obs41 * | 14 | 0 | 0 | 0 | 0 | 0 | 41.80 ± 1.15 * | 41.80 |
| Source | Sum of Square | df | Mean of Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2003.80 | 20 | 100.19 | 19.516 | <0.0001 |
| Lack of fit | 154.01 | 30 | 5.13 | - | - |
| Residual | 154.01 | 30 | 5.13 | - | - |
| Pure Error | 0.000 | 0 | - | - | - |
| Cor. Error | 2157.81 | 50 | - | - | - |
| R2 | 0.929 | ||||
| R2 adj | 0.881 | ||||
| CV % | 0.715 |
| Source | Value | SD | t | p > |t| |
|---|---|---|---|---|
| Constant | 41.797 | 0.683 | 61.181 | <0.0001 *** |
| X1 | −0.502 | 0.566 | −0.886 | 0.383 |
| X2 | 4.119 | 0.566 | 7.272 | <0.0001 *** |
| X3 | 3.062 | 0.566 | 5.405 | <0.0001 *** |
| X4 | 0.558 | 0.566 | 0.984 | 0.333 |
| X5 | −0.705 | 0.566 | −1.245 | 0.223 |
| X12 | −7.751 | 0.701 | −11.062 | <0.0001 *** |
| X22 | −6.671 | 0.701 | −9.521 | <0.0001 *** |
| X32 | −7.938 | 0.701 | −11.329 | <0.0001 *** |
| X42 | 0.159 | 0.701 | 0.227 | 0.822 |
| X52 | −1.303 | 0.701 | −1.860 | 0.073 |
| X1X2 | −0.478 | 1.133 | −0.422 | 0.676 |
| X1X3 | 0.260 | 1.133 | 0.229 | 0.820 |
| X1X4 | −0.498 | 1.133 | −0.440 | 0.663 |
| X1X5 | 0.001 | 1.133 | 0.001 | 0.999 |
| X2X3 | 2.325 | 1.133 | 2.052 | 0.049 * |
| X2X4 | 2.763 | 1.133 | 2.439 | 0.021 * |
| X2X5 | 2.124 | 1.133 | 1.875 | 0.071 |
| X3X4 | 2.190 | 1.133 | 1.933 | 0.063 |
| X3X5 | −0.539 | 1.133 | −0.475 | 0.638 |
| X4X5 | −0.998 | 1.133 | −0.881 | 0.385 |
| TFC (mg/100 g DM) | Daidzein (mg/100 g DM) | Genistein (mg/100 g DM) | Cajanin (mg/100 g DM) | Cajanol (mg/100 g DM) | DPPH (µmol TEAC/g DM) | |
|---|---|---|---|---|---|---|
| Optimized USAE | 48.96 ± 0.54 a | 9.03 ± 0.14 a | 0.78 ± 0.02 a | 18.11 ± 0.27 a | 11.64 ± 0.17 a | 514.68 ± 7.88 a |
| Conventional HRE | 28.77 ± 4.01 b | 5.12 ± 0.41 b | 0.32 ± 0.07 b | 11.24 ± 0.33 b | 6.31 ± 0.48 b | 172.52 ± 11.47 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology. Molecules 2021, 26, 7557. https://doi.org/10.3390/molecules26247557
Tungmunnithum D, Drouet S, Lorenzo JM, Hano C. Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology. Molecules. 2021; 26(24):7557. https://doi.org/10.3390/molecules26247557
Chicago/Turabian StyleTungmunnithum, Duangjai, Samantha Drouet, Jose Manuel Lorenzo, and Christophe Hano. 2021. "Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology" Molecules 26, no. 24: 7557. https://doi.org/10.3390/molecules26247557
APA StyleTungmunnithum, D., Drouet, S., Lorenzo, J. M., & Hano, C. (2021). Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology. Molecules, 26(24), 7557. https://doi.org/10.3390/molecules26247557









