Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Separation and Identification
2.2. Extraction Optimization
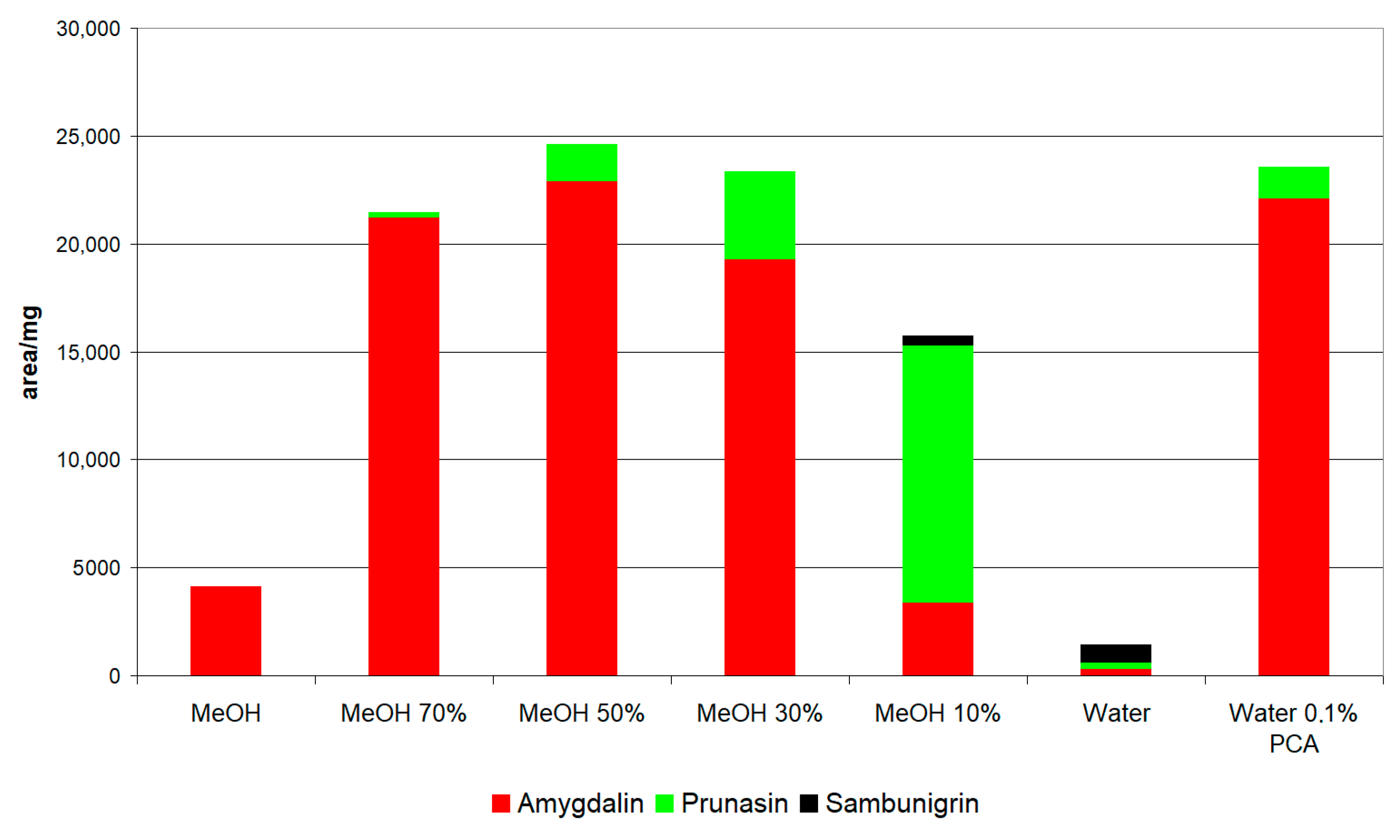
2.2.1. Preselection of Extraction Conditions
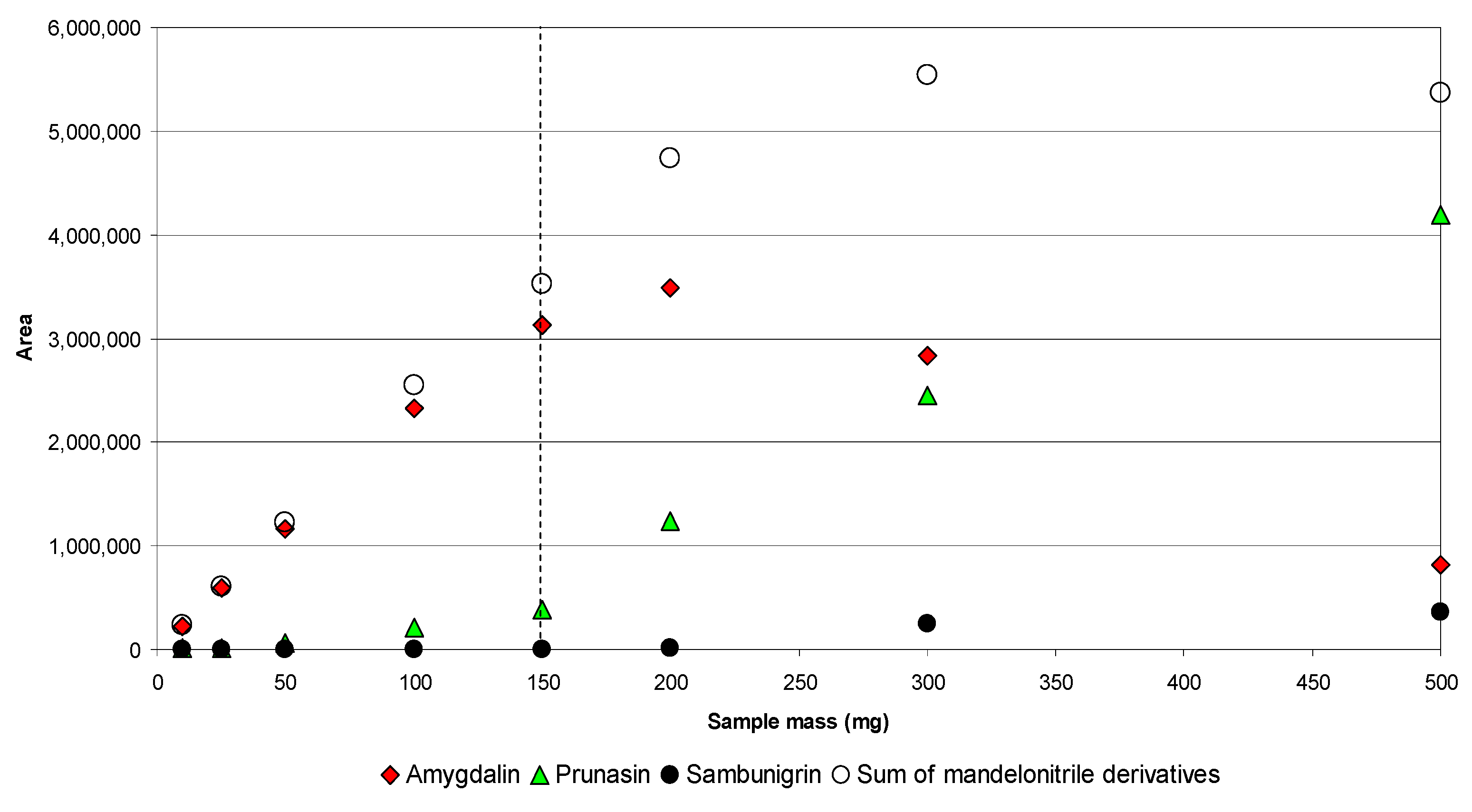
2.2.2. Optimization of the Extraction Condition by UAE
2.3. Validation Procedure
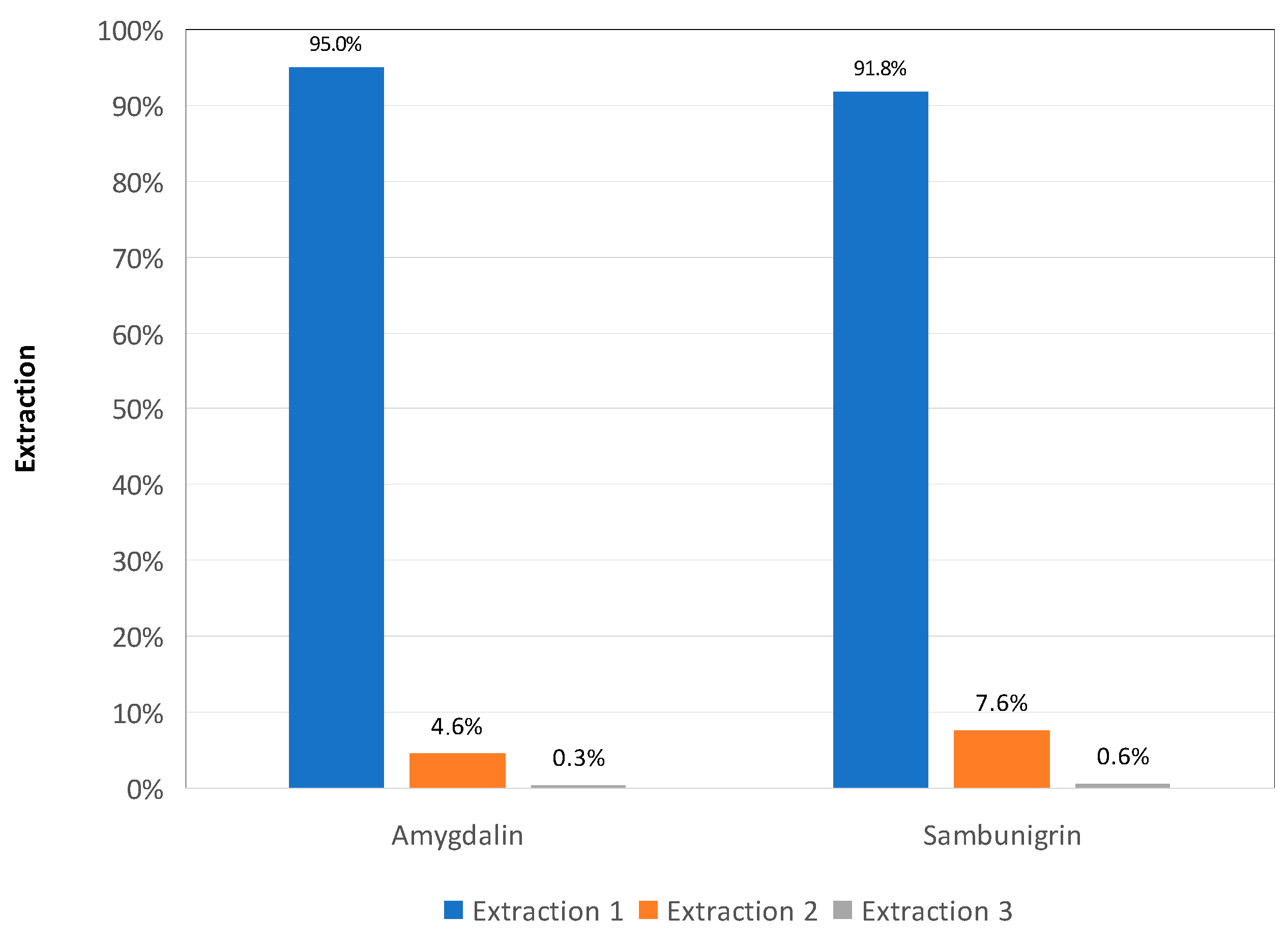
2.3.1. Precision, Accuracy, and Extract Stability
2.3.2. Calibration Curves, LD, and LQ
2.4. Cyanogenic Glycosides Contents in the Analyzed Samples
2.4.1. Rosacea
2.4.2. Sambucus nigra
3. Materials and Methods
3.1. Sample Material and Preparation of the Extracts
3.2. Reagents and Standards
3.3. Chromatographic Separation and Identification
3.4. Experimental Design and Extraction Optimization
3.5. UAE Conditions
3.6. Validation Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cuny, M.; La Forgia, D.; Desurmont, G.A.; Glauser, G.; Benrey, B. Role of cyanogenic glycosides in the seeds of wild lima bean, Phaseolus lunatus: Defense, plant nutrition or both? Planta 2019, 250, 1281–1292. [Google Scholar] [CrossRef]
- Del Cueto, J.; Ionescu, I.A.; Pičmanová, M.; Gericke, O.; Motawia, M.S.; Olsen, C.E.; Campoy, J.A.; Dicenta, F.; Møller, B.L.; Sánchez-Pérez, R. Cyanogenic Glucosides and Derivatives in Almond and Sweet Cherry Flower Buds from Dormancy to Flowering. Front. Plant Sci. 2017, 8, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibamoto, T.; Bjeldanes, L.F. Introduction to Food Toxicology, 2nd ed.; Academic Press: New York, NY, USA, 2009; pp. 134–138. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Woodrow, I.E. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. J. Chem. Ecol. 2002, 28, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA J. 2016, 14, 4424. [Google Scholar]
- Regulation (EC) No. 1881/2006 of the European Parliament and the Council of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; OJ L 364; European Union: Maastricht, The Netherlands, 2006; pp. 5–24.
- Regulation (EC) No. 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certainfood Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No. 160/1, Regulations (EC) No. 2232/96 and (EC) No. 110/2008 and Directive 2000/13/EC; OJ L 354; European Union: Maastricht, The Netherlands, 2008; pp. 34–50.
- Regulation (EC) No. 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC)No. 1576/89; OJ L 39; European Union: Maastricht, The Netherlands, 2008; p. 16.
- The Stationery Office. Available online: https://www.legislation.gov.uk/uksi/2012/1916/pdfs/uksi_20121916_en.pdf (accessed on 30 November 2021).
- Bundesministerium de Justiz und für Verbraucherschutz. Available online: http://www.gesetze-im-internet.de/amg_1976/BJNR024480976.html (accessed on 30 November 2021).
- Gazzete. Available online: http://www.foodstandards.gov.au/code/changes/gazette/Documents/GazetteNo159WebVersion.pdf (accessed on 30 November 2021).
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Ballhom, D.J. Cyanogenic glycosides in nuts and sedds. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.D., Eds.; Acedemic Press: San Diego, CA, USA, 2011; pp. 129–136. [Google Scholar]
- Castaño, J.A.G.; Salcedo, J.C.A.; Gónzalez, O.E.C. Solid-to-liquid extraction and HPLC/UV determination of amygdalin of seeds of apple (Malus pumila Mill): Comparison between traditional-solvent and microwave methodologies. Acta Agron. 2018, 67, 381–388. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially- available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Harmful (cyanogenic glycoside) and beneficial (phenolic) compounds in different Sambucus species. J. Berry Res. 2019, 9, 395–409. [Google Scholar] [CrossRef]
- Haque, M.R.; Bradbury, J.H. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. Food Chem. 2002, 77, 107–114. [Google Scholar] [CrossRef]
- Cho, H.-J.; Do, B.-K.; Shim, S.-M.; Kwon, H.; Lee, D.-H.; Nah, A.-H.; Choi, Y.-J.; Lee, S.-Y. Determination of Cyanogenic Compounds in Edible Plants by Ion Chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef]
- Berenguer-Navarro, V.; Giner-Galván, R.M.; Grané-Teruel, N.; Arrazola-Paternina, G. Chromatographic determination of cyanoglycosides prunasin and amygdalin in plant extracts using a porous graphitic carbon column. J. Agric. Food Chem. 2002, 50, 6960–6963. [Google Scholar] [CrossRef] [PubMed]
- Appenteng, M.; Krueger, R.; Johnson, M.; Ingold, H.; Bell, R.; Thomas, A.; Greenlief, C. Cyanogenic Glycoside Analysis in American Elderberry. Molecules 2021, 26, 1384. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Cui, B.; Zhu, H.; Phommakoun, B.; Zhang, D.; Li, Y.; Zhao, F.; Zhao, Z. Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development. Foods 2021, 10, 397. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J. Food Compos. Anal. 2019, 85, 103334. [Google Scholar] [CrossRef]
- Wahab, M.F.; Breitbach, Z.S.; Armstrong, D.W.; Strattan, R.; Berthod, A. Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer. J. Agric. Food Chem. 2015, 63, 8966–8973. [Google Scholar] [CrossRef]
- Yu, H.-L.; Xu, J.-H.; Lu, W.-Y.; Lin, G.-Q. Identification, purification and characterization of β-glucosidase from apple seed as a novel catalyst for synthesis of O-glucosides. Enzym. Microb. Technol. 2007, 40, 354–361. [Google Scholar] [CrossRef]
- Miller, J.N. Basic Statistical Methods for Analytical Chemistry Part 2. Calibration and Regression Methods. A Review. Analyst 1991, 116, 3–14. [Google Scholar] [CrossRef]
- Akinci Yildirim, F.; Atilla Askin, M. Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey. Afr. J. Biotechnol. 2010, 9, 6522–6524. [Google Scholar]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical Composition of Bitter and Sweet Apricot Kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Voldřich, M.; Kyzlink, V. Cyanogenesis in Canned Stone Fruits. J. Food Sci. 1992, 57, 161–162. [Google Scholar] [CrossRef]
- Lee, S.-H.; Oh, A.; Shin, S.-H.; Kim, H.-N.; Kang, W.-W.; Chung, S.-K. Amygdalin Contents in Peaches at Different Fruit Development Stages. Prev. Nutr. Food Sci. 2017, 22, 237–240. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Fuit seeds of the Rosaceae family: A waste, new life, or a danger to human health? J. Agric. Food Chem. 2017, 65, 10621–10629. [Google Scholar] [CrossRef]
- Opyd, P.M.; Jurgoński, A.; Juśkiewicz, J.; Milala, J.; Zduńczyk, Z.; Król, B. Nutritional and Health-Related Effects of a Diet Containing Apple Seed Meal in Rats: The Case of Amygdalin. Nutrients 2017, 9, 1091. [Google Scholar] [CrossRef] [Green Version]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Cyanogenic glycosides and phenolics in apple seeds and their changes during long term storage. Sci. Hortic. 2019, 255, 30–36. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Enhancement of the nutritional properties of apple pomace by fermentation with autochthonous yeasts. LWT Food Sci. Technol. 2017, 79, 27–33. [Google Scholar] [CrossRef]
- García, Y.D.; Valles, B.S.; Lobo, A.P. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Characterization of apple seeds and their oils from the cider-making industry. Eur. Food Res. Technol. 2018, 244, 1821–1827. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2016, 97, 2623–2632. [Google Scholar] [CrossRef]



| Run | Time (s) | Pulse (%) | Amplitude (%) | Amygdalin (Area/mg) | Sambunigrin (Area/mg) |
|---|---|---|---|---|---|
| 1 | −1 (20) | 0 (60) | −1 (20) | 21,640 | 27,098 |
| 2 | −1 (20) | 0 (60) | 1 (100) | 22,075 | 29,157 |
| 3 | 1 (60) | 0 (60) | −1 (20) | 21,495 | 27,692 |
| 4 | 1 (60) | 0 (60) | 1 (100) | 23,625 | 29,619 |
| 5 | 0 (40) | −1 (20) | −1 (20) | 20,551 | 28,134 |
| 6 | 0 (40) | −1 (20) | 1 (100) | 22,408 | 29,720 |
| 7 | 0 (40) | 1 (100) | −1 (20) | 22,193 | 29,577 |
| 8 | 0 (40) | 1 (100) | 1 (100) | 23,123, | 30,537 |
| 9 | −1 (10) | −1 (20) | 0 (60) | 21,025 | 29,194 |
| 10 | 1 (60) | −1 (20) | 0 (60) | 21,457 | 27,229 |
| 11 | −1 (20) | 1 (100) | 0 (60) | 22,909 | 28,600 |
| 12 | 1 (60) | 1 (100) | 0 (60) | 23,246 | 30,188 |
| 13 | 0 (40) | 0 (60) | 0 (60) | 23,138 | 29,981 |
| 14 | 0 (40) | 0 (60) | 0 (60) | 23,770 | 29,791 |
| 15 | 0 (40) | 0 (60) | 0 (60) | 23,549 | 29,505 |
| 16 | 0 (40) | 0 (60) | 0 (60) | 23,040 | 30,088 |
| 17 | 0 (40) | 0 (60) | 0 (60) | 22,723 | 30,575 |
| Amygdalin | Sambunigrin | |||
|---|---|---|---|---|
| Model | *** | *** | ||
| Intercept | 23,244.29 | 29,970.60 | ||
| A-Time | 668.91 | *** | 816.43 | *** |
| B-Pulse | 271.72 | n.s. | 84.87 | n.s. |
| C-Amplitude | 753.95 | *** | 578.12 | *** |
| AB | 423.64 | * | - | - |
| BC | - | - | 888.15 | *** |
| A2 | −562.82 | * | −455.76 | * |
| B2 | −472.28 | * | −1145.16 | *** |
| C2 | −612.19 | ** | ||
| Lack of Fit | n.s. | n.s. | ||
| R-Squared | 0.921 | 0.935 | ||
| Adjusted R2 | 0.859 | 0.895 | ||
| Predicted R2 | 0.773 | 0.805 | ||
| RSD | 1.61 | 1.21 | ||
| Amygdalin (Area/mg) | Sambunigrin (Area/mg) | |
|---|---|---|
| Prediction | 23,770 | 30,664 |
| 95% CI low | 23,376 | 30,299 |
| 95% CI high | 24,165 | 31,028 |
| 95% PI low | 22,860 | 29,494 |
| 95% PI high | 24,681 | 31,533 |
| Operator 1 (n = 3) | 23,193 | 31,185 |
| Repeatability, r (RSD) | 1.0 | 4.1 |
| Operator 2 (n = 3) | 23,062 | 30,205 |
| Repeatability, r (RSD) | 2.0 | 1.9 |
| Reproducibility, R (RSD) | 0.4 | 2.3 |
| Compound | Calibration Curve | Instrumental | Method | |||||
|---|---|---|---|---|---|---|---|---|
| Linear Range (mg/L) | Slope | Intercept | Correlation Coefficient | LOD (10−3 mg/L) | LOQ (10−3 mg/L) | LOD (10−3 mg/g) | LOQ (10−3 mg/g) | |
| Amygdalin (n = 11) | 0.24–480 | 16224 | −8999 | 0.9999 | 6.5 | 21.7 | 0.7 | 2.2 |
| Prunasin (n = 11) | 0.19–296 | 20007 | −7906 | 0.9999 | 16.1 | 55.7 | 1.6 | 5.6 |
| Sambunigrin (n = 10) | 0.20–390 | 22257 | −2506 | 0.9998 | 28.8 | 96.0 | 2.9 | 9.6 |
| Amygdalin | Prunasin | Sambunigrin | |
|---|---|---|---|
| Rosaceae Kernels | |||
| Cherry-1 (P. avium) | 12.6 | 1.8 | n.d. |
| Cherry-2 (P. avium) | 11.8 | 2.2 | n.d. |
| Cherry cv picota (P. avium) | 8.2 | 1.1 | n.d. |
| Apricot (P. armeniaca) | 45.0 | 1.5 | n.d. |
| Peach (P. persica) | 43.3 | 0.6 | n.d. |
| Flat peach (P. persica var. platycarpa) | 23.7 | 2.0 | n.d. |
| Nectarine (P. persica var. nucipersica) | 32.7 | 1.3 | n.d. |
| Plum cv Reina Claudia verde (P. domestica) | 57.3 | 0.9 | n.d. |
| Prune kernel (P. domestica) | 3.6 | 0.5 | 0.1 |
| Apple seed | |||
| Golden delicious | 13.6 | 0.9 | 0.1 |
| Durona de Tresali | 14.9 | 0.3 | n.d. |
| Seed from apple pomace-1 | 13.4 | 0.3 | n.d. |
| Seed from apple pomace-2 | 13.8 | 0.3 | n.d. |
| Seed from apple pomace-3 | 15.0 | 0.5 | n.d. |
| Seed from apple pomace-4 | 18.6 | 0.8 | n.d. |
| Seed from apple pomace-5 | 16.4 | 1.1 | n.d. |
| Apple pomace flour | |||
| Apple pomace-1 | 0.1 | n.d. | n.d. |
| Apple pomace-2 | 0.1 | n.d. | n.d. |
| Sambucus nigra | |||
| Young leaf | 0.2 | 0.4 | 16.2 |
| Old leaf | 0.1 | 0.8 | 20.7 |
| Flower-1 | n.d. | n.d. | 0.6 |
| Flower-2 | n.d. | n.d. | 0.4 |
| Flower bud | n.d. | n.d. | 0.3 |
| Stalk | n.d. | n.d. | 1.7 |
| Fruit-1 (elderberry) | n.d. | n.d. | 0.4 |
| Fruit-2 (elderberry) | n.d. | n.d. | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Madrera, R.; Suárez Valles, B. Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families. Molecules 2021, 26, 7563. https://doi.org/10.3390/molecules26247563
Rodríguez Madrera R, Suárez Valles B. Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families. Molecules. 2021; 26(24):7563. https://doi.org/10.3390/molecules26247563
Chicago/Turabian StyleRodríguez Madrera, Roberto, and Belén Suárez Valles. 2021. "Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families" Molecules 26, no. 24: 7563. https://doi.org/10.3390/molecules26247563
APA StyleRodríguez Madrera, R., & Suárez Valles, B. (2021). Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families. Molecules, 26(24), 7563. https://doi.org/10.3390/molecules26247563






