Pressure-Induced Polymerization: Addition and Condensation Reactions
Abstract
:1. Introduction
2. Results
2.1. PIP: Addition Reactions
2.1.1. Alkyne and Acetylides
2.1.2. Aromatic Compounds
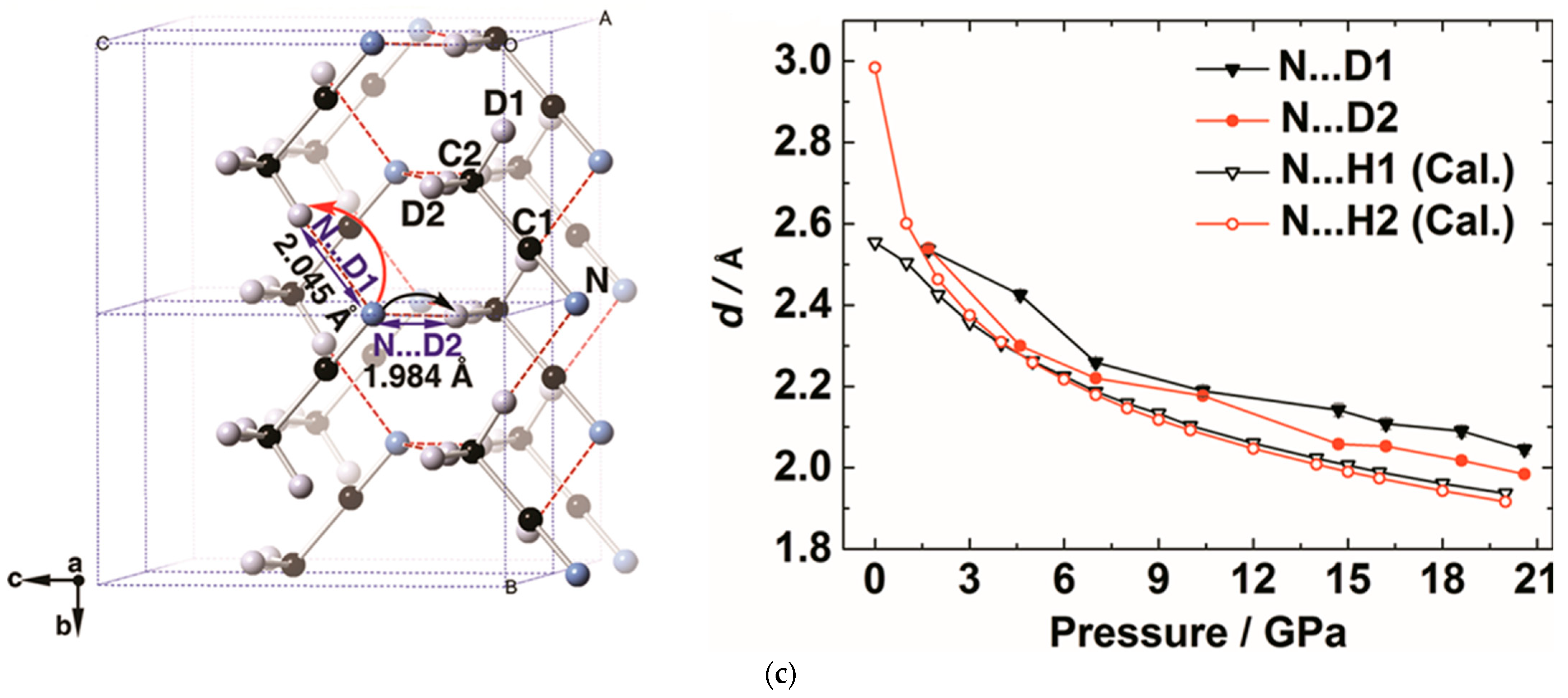
2.1.3. Alkynlphenyl Systems
2.2. PIP: Condensation Reaction with Hydrogen Transfer
2.2.1. Alkane
2.2.2. Nitriles
3. Characterization Methods
3.1. In Situ Neutron Diffraction under High Pressure
3.2. High Resolution Gas-Chromatography-Mass-Spectrometry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akaishi, M.; Kanda, H.; Yamaoka, S. Synthesis of diamond from graphite-carbonate system under very high temperature and pressure. J. Cryst. Growth 1990, 104, 578–581. [Google Scholar] [CrossRef]
- Irifune, T.; Kurio, A.; Sakamoto, S.; Inoue, T.; Sumiya, H.; Funakoshi, K.-I. Formation of pure polycrystalline diamond by direct conversion of graphite at high pressure and high temperature. Phys. Earth Planet. Inter. 2004, 143, 593–600. [Google Scholar] [CrossRef]
- Isobe, F.; Ohfuji, H.; Sumiya, H.; Irifune, T. Nanolayered diamond sintered compact obtained by direct conversion from highly oriented graphite under high pressure and high temperature. J. Nanomater. 2013, 2013, 380165. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, X.; Tang, X.; Wang, X.; Li, Y.; Lin, X.; Dong, X.; Yang, D.; Zheng, H.; Li, K. Pressure gradient squeezing hydrogen out of MnOOH: Thermodynamics and electrochemistry. J. Phys. Chem. Lett. 2021, 12, 10893–10898. [Google Scholar] [CrossRef] [PubMed]
- Schettino, V.; Bini, R. Molecules under extreme conditions: Chemical reactions at high pressure. Phys. Chem. Chem. Phys. 2003, 5, 1951–1965. [Google Scholar] [CrossRef]
- Schettino, V.; Bini, R. Constraining molecules at the closest approach: Chemistry at high pressure. Chem. Soc. Rev. 2007, 36, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Wang, Y.; Li, K.; Zheng, H. From molecules to carbon materials-High pressure induced polymerization and bonding mechanisms of unsaturated compounds. Crystals 2019, 9, 490. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.-S. Chemistry under extreme conditions: Pressure evolution of chemical bonding and structure in dense solids. Matter Radiat. Extrem. 2020, 5, 018202. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, K.; Zheng, H.; Zhang, P. Chemical reactions of molecules under high pressure. Chem. Bull. 2019, 82, 387–398. [Google Scholar] [CrossRef]
- Miao, M.; Sun, Y.; Zurek, E.; Lin, H. Chemistry under high pressure. Nat. Rev. Chem. 2020, 4, 508–527. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, M.; Xiao, G.; Zou, B. Thinking about the development of high-pressure experimental chemistry. J. Phys. Chem. Lett. 2020, 11, 7297–7306. [Google Scholar] [CrossRef]
- Mao, H.K.; Chen, B.; Chen, J.; Li, K.; Lin, J.F.; Yang, W.; Zheng, H. Recent advances in high-pressure science and technology. Matter Radiat. Extrem. 2016, 1, 59–75. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 17005. [Google Scholar] [CrossRef]
- Zheng, H.; Li, K.; Cody, G.D.; Tulk, C.A.; Dong, X.; Gao, G.; Molaison, J.J.; Liu, Z.; Feygenson, M.; Yang, W. Polymerization of acetonitrile via a hydrogen transfer reaction from CH3 to CN under extreme conditions. Angew. Chem. Int. Ed. 2016, 55, 12040–12044. [Google Scholar] [CrossRef]
- Aoki, K.; Kakudate, Y.; Yoshida, M.; Usuba, S.; Tanaka, K.; Fujiwara, S. Raman scattering observations of phase transitions and polymerizations in acetylene at high pressure. Solid State Commun. 1987, 64, 1329–1331. [Google Scholar] [CrossRef]
- Aoki, K.; Kakudate, Y.; Usuba, S.; Yoshida, M.; Tanaka, K.; Fujiwara, S. High-pressure Raman study of liquid and crystalline C2H2. J. Chem. Phys. 1988, 88, 4565–4568. [Google Scholar] [CrossRef]
- Aoki, K.; Usuba, S.; Yoshida, M.; Kakudate, Y.; Tanaka, K.; Fujiwara, S. Raman study of the solid-state polymerization of acetylene at high pressure. J. Chem. Phys. 1988, 89, 529–534. [Google Scholar] [CrossRef]
- Aoki, K.; Kakudate, Y.; Yoshida, M.; Usuba, S.; Tanaka, K.; Fujiwara, S. Solid-state polymerization of acetylene under pressure. Synth. Met. 1989, 28, D91–D98. [Google Scholar] [CrossRef]
- Trout, C.C.; Badding, J. Solid state polymerization of acetylene at high pressure and low temperature. J. Phys. Chem. A 2000, 104, 8142–8145. [Google Scholar] [CrossRef]
- Marshall, W.G.; Francis, D.J. Attainment of near-hydrostatic compression conditions using the Paris–Edinburgh cell. J. Appl. Crystallogr. 2002, 35, 122–125. [Google Scholar] [CrossRef]
- Klotz, S.; Besson, J.M.; Hamel, G.; Nelmes, R.J.; Loveday, J.S.; Marshall, W.G. High pressure neutron diffraction using the paris-edinburgh cell: Experimental possibilities and future prospects. High Press. Res. 1996, 14, 249–255. [Google Scholar] [CrossRef]
- Besson, J.; Nelmes, R. New developments in neutron-scattering methods under high pressure with the Paris-Edinburgh cells. Phys. B Condens. Matter 1995, 213, 31–36. [Google Scholar] [CrossRef]
- Sun, J.; Dong, X.; Wang, Y.; Li, K.; Zheng, H.; Wang, L.; Cody, G.D.; Tulk, C.A.; Molaison, J.J.; Lin, X. Pressure-induced polymerization of acetylene: Structure-directed stereoselectivity and a possible route to graphane. Angew. Chem. Int. Ed. 2017, 129, 6653–6657. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Fu, C.; Franchini, C. Polymeric forms of carbon in dense lithium carbide. J. Phys. Condens. Matter 2010, 22, 292201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, D.; Li, Y.; Luo, W.; Ahuja, R.; Svensson, G.; Haussermann, U. Lithium and calcium carbides with polymeric carbon structures. Inorg. Chem. 2013, 52, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Luo, W.; Zeng, Z.; Lin, H.-Q.; Mao, H.-k.; Ahuja, R. Pressure-induced superconductivity in CaC2. Proc. Natl. Acad. Sci. USA 2013, 110, 9289–9294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-L.; Wang, S.-N.; Oganov, A.R.; Gou, H.; Smith, J.S.; Strobel, T.A. Investigation of exotic stable calcium carbides using theory and experiment. Nat. Commun. 2015, 6, 6974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Strobel, T.A.; Cohen, R. Structural diversity in lithium carbides. Phys. Rev. B 2015, 92, 214106. [Google Scholar] [CrossRef] [Green Version]
- Efthimiopoulos, I.; Kunc, K.; Vazhenin, G.; Stavrou, E.; Syassen, K.; Hanfland, M.; Ruschewitz, U. Structural transformation and vibrational properties of BaC2 at high pressure. Phys. Rev. B 2012, 85, 054105. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Benson, D.E.; Konar, S.; Nylén, J.; Svensson, G.; Häussermann, U.; Liebig, S.; Ruschewitz, U.; Vazhenin, G.V.; Loa, I. Structural transformations of Li2C2 at high pressures. Phys. Rev. B 2015, 92, 064111. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Wang, L.; Li, K.; Yang, Y.; Wang, Y.; Wu, J.; Dong, X.; Wang, C.-H.; Tulk, C.A.; Molaison, J.J. Pressure induced polymerization of acetylide anions in CaC2 and 107-fold enhancement of electrical conductivity. Chem. Sci. 2017, 8, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Dong, X.; Wang, Y.; Zheng, H.; Li, K.; Peng, X.; Mao, H.-k.; Jin, C.; Meng, Y.; Huang, M. Pressure-induced polymerization and disproportionation of Li2C2 accompanied with irreversible conductivity enhancement. J. Phys. Chem. Lett. 2017, 8, 4241–4245. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, L.; Li, K.; Zheng, H.; Wang, Y.; Meng, Y.; Shu, H.; Mao, H.-K.; Feng, S.; Jin, C. Tailored synthesis of the narrowest zigzag graphene nanoribbon structure by compressing the lithium acetylide under high temperature. J. Phys. Chem. C 2018, 122, 20506–20512. [Google Scholar] [CrossRef]
- Han, J.; Tang, X.; Wang, Y.; Wang, Y.; Han, Y.; Lin, X.; Dong, X.; Lee, H.H.; Zheng, H.; Li, K. Pressure-induced polymerization of monosodium acetylide: A radical reaction initiated topochemically. J. Phys. Chem. C 2019, 123, 30746–30753. [Google Scholar] [CrossRef]
- Ellenson, W.D.; Nicol, M. Raman spectra of solid benzene under high pressures. J. Chem. Phys. 1974, 61, 1380–1389. [Google Scholar] [CrossRef]
- Thiery, M.; Leger, J. High pressure solid phases of benzene. I. Raman and X-ray studies of C6H6 at 294 K up to 25 GPa. J. Chem. Phys. 1988, 89, 4255–4271. [Google Scholar] [CrossRef]
- Pruzan, P.; Chervin, J.; Thiery, M.; Itie, J.; Besson, J.; Forgerit, J.; Revault, M. Transformation of benzene to a polymer after static pressurization to 30 GPa. J. Chem. Phys. 1990, 92, 6910–6915. [Google Scholar] [CrossRef]
- Cansell, F.; Fabre, D.; Petitet, J.P. Phase transitions and chemical transformations of benzene up to 550 ℃ and 30 GPa. J. Chem. Phys. 1993, 99, 7300–7304. [Google Scholar] [CrossRef]
- Ciabini, L.; Santoro, M.; Bini, R.; Schettino, V. High pressure crystal phases of benzene probed by infrared spectroscopy. J. Chem. Phys. 2001, 115, 3742–3749. [Google Scholar] [CrossRef]
- Ciabini, L.; Santoro, M.; Bini, R.; Schettino, V. High pressure reactivity of solid benzene probed by infrared spectroscopy. J. Chem. Phys. 2002, 116, 2928–2935. [Google Scholar] [CrossRef]
- Ciabini, L.; Santoro, M.; Bini, R.; Schettino, V. High pressure photoinduced ring opening of benzene. Phys. Rev. Lett. 2002, 88, 085505. [Google Scholar] [CrossRef]
- Jackson, B.; Trout, C.; Badding, J. UV Raman analysis of the C: H network formed by compression of benzene. Chem. Mater. 2003, 15, 1820–1824. [Google Scholar] [CrossRef]
- Ciabini, L.; Gorelli, F.A.; Santoro, M.; Bini, R.; Schettino, V.; Mezouar, M. High-pressure and high-temperature equation of state and phase diagram of solid benzene. Phys. Rev. B 2005, 72, 094108. [Google Scholar] [CrossRef]
- Citroni, M.; Bini, R.; Foggi, P.; Schettino, V. Role of excited electronic states in the high-pressure amorphization of benzene. Proc. Natl. Acad. Sci. USA 2008, 105, 7658–7663. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.-D.; Hoffmann, R.; Ashcroft, N. Benzene under high pressure: A story of molecular crystals transforming to saturated networks, with a possible intermediate metallic phase. J. Am. Chem. Soc. 2011, 133, 9023–9035. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, T.C.; Guthrie, M.; Xu, E.-s.; Crespi, V.H.; Davidowski, S.K.; Cody, G.D.; Alem, N.; Badding, J.V. Benzene-derived carbon nanothreads. Nat. Mater. 2015, 14, 43–47. [Google Scholar] [CrossRef]
- Li, X.; Baldini, M.; Wang, T.; Chen, B.; Xu, E.-s.; Vermilyea, B.; Crespi, V.H.; Hoffmann, R.; Molaison, J.J.; Tulk, C.A. Mechanochemical synthesis of carbon nanothread single crystals. J. Am. Chem. Soc. 2017, 139, 16343–16349. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Duan, P.; Baldini, M.; Huang, H.T.; Chen, B.; Badding, J.V. Carbon nitride nanothread crystals derived from pyridine. J. Am. Chem. Soc. 2018, 140, 4969–4972. [Google Scholar] [CrossRef]
- Fanetti, S.; Santoro, M.; Alabarse, F.; Enrico, B.; Bini, R. Modulating the H-bond strength by varying the temperature for the high pressure synthesis of nitrogen rich carbon nanothreads. Nanoscale 2020, 12, 5233–5242. [Google Scholar] [CrossRef]
- Biswas, A.; Ward, M.D.; Wang, T.; Zhu, L.; Huang, H.-T.; Badding, J.V.; Crespi, V.H.; Strobel, T.A. Evidence for orientational order in nanothreads derived from thiophene. J. Phys. Chem. Lett. 2019, 10, 7164–7171. [Google Scholar] [CrossRef] [PubMed]
- Huss, S.; Wu, S.; Chen, B.; Wang, T.; Gerthoffer, M.C.; Ryan, D.J.; Smith, S.E.; Crespi, V.H.; Badding, J.V.; Elacqua, E. Scalable synthesis of crystalline one-dimensional carbon nanothreads through modest-pressure polymerization of furan. ACS Nano 2021, 15, 4134–4143. [Google Scholar] [CrossRef]
- Fanetti, S.; Nobrega, M.M.; Teixeira-Neto, E.; Temperini, M.L.; Bini, R. Effect of structural anisotropy in high-pressure reaction of aniline. J. Phys. Chem. C 2018, 122, 29158–29164. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Teixeira-Neto, E.; Cairns, A.B.; Temperini, M.L.; Bini, R. One-dimensional diamondoid polyaniline-like nanothreads from compressed crystal aniline. Chem. Sci. 2018, 9, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Hoffmann, R.; Ashcroft, N.; Badding, J.; Xu, E.; Crespi, V. Linearly polymerized benzene arrays as intermediates, tracing pathways to carbon nanothreads. J. Am. Chem. Soc. 2015, 137, 14373–14386. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Li, X.; Wang, T.; Chen, B.; Juhl, S.J.; Koeplinger, D.; Crespi, V.H.; Badding, J.V.; Schmidt-Rohr, K. The chemical structure of carbon nanothreads analyzed by advanced solid-state NMR. J. Am. Chem. Soc. 2018, 140, 7658–7666. [Google Scholar] [CrossRef]
- Wang, T.; Duan, P.; Xu, E.-S.; Vermilyea, B.; Chen, B.; Li, X.; Badding, J.V.; Schmidt-Rohr, K.; Crespi, V.H. Constraining carbon nanothread structures by experimental and calculated nuclear magnetic resonance spectra. Nano Lett. 2018, 18, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zheng, H.; Li, K.; Andrzejewski, M.; Hattori, T.; Sano-Furukawa, A.; Katrusiak, A.; Meng, Y.; Liao, F. Phase transitions and polymerization of C6H6–C6F6 cocrystal under extreme conditions. J. Phys. Chem. C 2016, 120, 29510–29519. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Tang, X.; Zheng, H.; Li, K.; Lin, X.; Fang, L.; Sun, G.A.; Chen, X.; Xie, L.; et al. Pressure-induced Diels-Alder reactions in C6H6-C6F6 cocrystal towards graphane structure. Angew. Chem. Int. Ed. 2019, 58, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Ciabini, L.; Santoro, M.; Gorelli, F.A.; Bini, R.; Schettino, V.; Raugei, S. Triggering dynamics of the high-pressure benzene amorphization. Nat. Mater. 2007, 6, 39–43. [Google Scholar] [CrossRef]
- Ward, M.D.; Tang, W.S.; Zhu, L.; Popov, D.; Cody, G.D.; Strobel, T.A. Controlled single-crystalline polymerization of C10H8· C10F8 under pressure. Macromolecules 2019, 52, 7557–7563. [Google Scholar] [CrossRef]
- Friedrich, A.; Collings, I.E.; Dziubek, K.F. Pressure-induced polymerization of polycyclic arene-perfluoroarene cocrystals: Single crystal X-ray diffraction studies, reaction kinetics, and design of columnar hydrofluorocarbons. J. Am. Chem. Soc. 2020, 142, 18907–18923. [Google Scholar] [CrossRef]
- Spaulding, D.K.; Weck, G.; Loubeyre, P.; Datchi, F.; Dumas, P.; Hanfland, M. Pressure-induced chemistry in a nitrogen-hydrogen host—Guest structure. Nat. Commun. 2014, 5, 5739. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, X.; Bao, K.; Zhao, Z.; Huang, Y.; Wang, L.; Wu, G.; Zhou, B.; Duan, D.; Li, F. A novel high-density phase and amorphization of nitrogen-rich 1H-tetrazole (CH2N4) under high pressure. Sci. Rep. 2017, 7, 39249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Tang, X.; Wang, X.; Yang, X.; Zhang, P.; Che, G.; Han, J.; Hattori, T.; Wang, Y.; Dong, X. Phase transition and chemical reactivity of 1H-tetrazole under high pressure up to 100 GPa. Phys. Chem. Chem. Phys. 2021, 23, 19503–19510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, X.; Wang, Y.; Wang, X.; Gao, D.; Li, Y.; Zheng, H.; Wang, Y.; Wang, X.; Fu, R. Distance-selected topochemical dehydro-Diels–Alder reaction of 1, 4-diphenylbutadiyne toward crystalline graphitic nanoribbons. J. Am. Chem. Soc. 2020, 142, 17662–17669. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Zhang, P.; Wang, Y.; Yang, X.; Wang, X.; Li, K.; Wang, Y.; Wu, N.; Tang, M. Scalable high-pressure synthesis of sp2–sp3 carbon nanoribbon via [4+2] polymerization of 1, 3, 5-triethynylbenzene. J. Phys. Chem. Lett. 2021, 12, 7140–7145. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.; Kutcherov, V.G.; Goncharov, A.F. Methane-derived hydrocarbons produced under upper-mantle conditions. Nat. Geosci. 2009, 2, 566–570. [Google Scholar] [CrossRef]
- Benedetti, L.R.; Nguyen, J.H.; Caldwell, W.A.; Liu, H.; Kruger, M.; Jeanloz, R. Dissociation of CH4 at high pressures and temperatures: Diamond formation in giant planet interiors? Science 1999, 286, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobanov, S.S.; Chen, P.-N.; Chen, X.-J.; Zha, C.-S.; Litasov, K.D.; Mao, H.-K.; Goncharov, A.F. Carbon precipitation from heavy hydrocarbon fluid in deep planetary interiors. Nat. Commun. 2013, 4, 2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, Y.; Wang, Y.; Zheng, H.; Li, K.; Mao, H.-k. Chemical transformations of n-hexane and cyclohexane under the upper mantle conditions. Geosci. Front. 2021, 12, 1010–1017. [Google Scholar] [CrossRef]
- Aoki, K.; Baer, B.; Cynn, H.; Nicol, M. High-pressure Raman study of one-dimensional crystals of the very polar molecule hydrogen cyanide. Phys. Rev. B 1990, 42, 4298. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Huang, J.; Lin, X.; Yang, D.; Wang, Y.; Zheng, H. Phase transitions and chemical reactions of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine under high pressure and high temperature. RSC Adv. 2019, 9, 5825–5833. [Google Scholar] [CrossRef] [Green Version]
- Klotz, S.; Besson, J.; Hamel, G.; Nelmes, R.; Loveday, J.; Marshall, W.; Wilson, R. Neutron powder diffraction at pressures beyond 25 GPa. Appl. Phys. Lett. 1995, 66, 1735–1737. [Google Scholar] [CrossRef]
- Hattori, T.; Sano-Furukawa, A.; Machida, S.; Abe, J.; Funakoshi, K.; Arima, H.; Okazaki, N. Development of a technique for high pressure neutron diffraction at 40 GPa with a Paris-Edinburgh press. High Press. Res. 2019, 39, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Boehler, R.; Guthrie, M.; Molaison, J.J.; dos Santos, A.M.; Sinogeikin, S.; Machida, S.-I.; Pradhan, N.; Tulk, C.A. Large-volume diamond cells for neutron diffraction above 90 GPa. High Press. Res. 2013, 33, 546–554. [Google Scholar] [CrossRef]
- Guthrie, M.; Boehler, R.; Tulk, C.A.; Molaison, J.J.; dos Santos, A.M.; Li, K.; Hemley, R.J. Neutron diffraction observations of interstitial protons in dense ice. Proc. Natl. Acad. Sci. USA 2013, 110, 10552–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Chen, X.; Fang, L.; Sun, G.; Xie, C.; Chen, B.; Li, H.; Ulyanov, V.; Solovei, V.; Kolkhidashvili, M. Fenghuang: High-intensity multi-section neutron powder diffractometer at CMRR. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2019, 915, 31–35. [Google Scholar] [CrossRef]
- Gabriel, T.A.; Haines, J.R.; McManamy, T.J. Overview of the Spallation Neutron Source (SNS) with emphasis on target systems. J. Nucl. Mater. 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Machida, S. Neutron diffraction experiments at high pressure in SNS. Rev. High Press. Sci. Technol. 2016, 26, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Utsumi, W.; Kagi, H.; Komatsu, K.; Arima, H.; Nagai, T.; Okuchi, T.; Kamiyama, T.; Uwatoko, Y.; Matsubayashi, K.; Yagi, T. Neutron powder diffraction under high pressure at J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2009, 600, 50–52. [Google Scholar] [CrossRef]
- Arima, H.; Hattori, T.; Komatsu, K.; Abe, J.; Utsumi, W.; Kagi, H.; Suzuki, A.; Suzuya, K.; Kamiyama, T.; Arai, M. Designing PLANET: Neutron beamline for high-pressure material science at J-PARC. J. Phys. Conf. Ser. 2010, 215, 012025. [Google Scholar] [CrossRef]
- Hattori, T.; Sano-Furukawa, A.; Arima, H.; Komatsu, K.; Yamada, A.; Inamura, Y.; Nakatani, T.; Seto, Y.; Nagai, T.; Utsumi, W. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2015, 780, 55–67. [Google Scholar] [CrossRef]
- Bull, C.; Funnell, N.; Tucker, M.; Hull, S.; Francis, D.; Marshall, W. PEARL: The high pressure neutron powder diffractometer at ISIS. High. Press. Res. 2016, 36, 493–511. [Google Scholar] [CrossRef] [Green Version]
- Proffen, T.; Billinge, S.; Egami, T.; Louca, D. Structural analysis of complex materials using the atomic pair distribution function-a practical guide. Z. Kristallogr. Cryst. Mater. 2003, 218, 132–143. [Google Scholar] [CrossRef]
- Xian, F.; Hendrickson, C.L.; Marshall, A.G. High resolution mass spectrometry. Anal. Chem. 2012, 84, 708–719. [Google Scholar] [CrossRef] [PubMed]













| Compounds | DC (Å) | Reaction at Initiation | Pressure (GPa) |
|---|---|---|---|
| Acetylene (C2H2) | 3.1 [23] | free radical reaction | 5.7 |
| Sodium Monoacetylene (NaHC2) | 2.9 [34] | 11 | |
| Calcium Acetylene (CaC2) | 2.9 [31] | 20 | |
| Lithium Acetylene (Li2C2) | - | 33 | |
| Benzene (C6H6) | 2.8 [59] | Diels-Alder/1-1′ coupling, etc. reactions | 18 |
| Benzene-hexafluorobenzene (C6H6-C6F6) | 2.8 [58] | 20 | |
| 1,4-diphenylbutadiyne (DPB) | 3.2 [65] | Diels-Alder reaction | 10 |
| 1,3,5-Triethynylbenzene (TEB) | 3.3 [66] | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Xu, J.; Wang, Y.; Zheng, H.; Li, K. Pressure-Induced Polymerization: Addition and Condensation Reactions. Molecules 2021, 26, 7581. https://doi.org/10.3390/molecules26247581
Li F, Xu J, Wang Y, Zheng H, Li K. Pressure-Induced Polymerization: Addition and Condensation Reactions. Molecules. 2021; 26(24):7581. https://doi.org/10.3390/molecules26247581
Chicago/Turabian StyleLi, Fang, Jingqin Xu, Yajie Wang, Haiyan Zheng, and Kuo Li. 2021. "Pressure-Induced Polymerization: Addition and Condensation Reactions" Molecules 26, no. 24: 7581. https://doi.org/10.3390/molecules26247581





