Imidazole Processing of Wheat Straw and Eucalyptus Residues—Comparison of Pre-Treatment Conditions and Their Influence on Enzymatic Hydrolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Biomass and Pre-Treated Solid Characterization
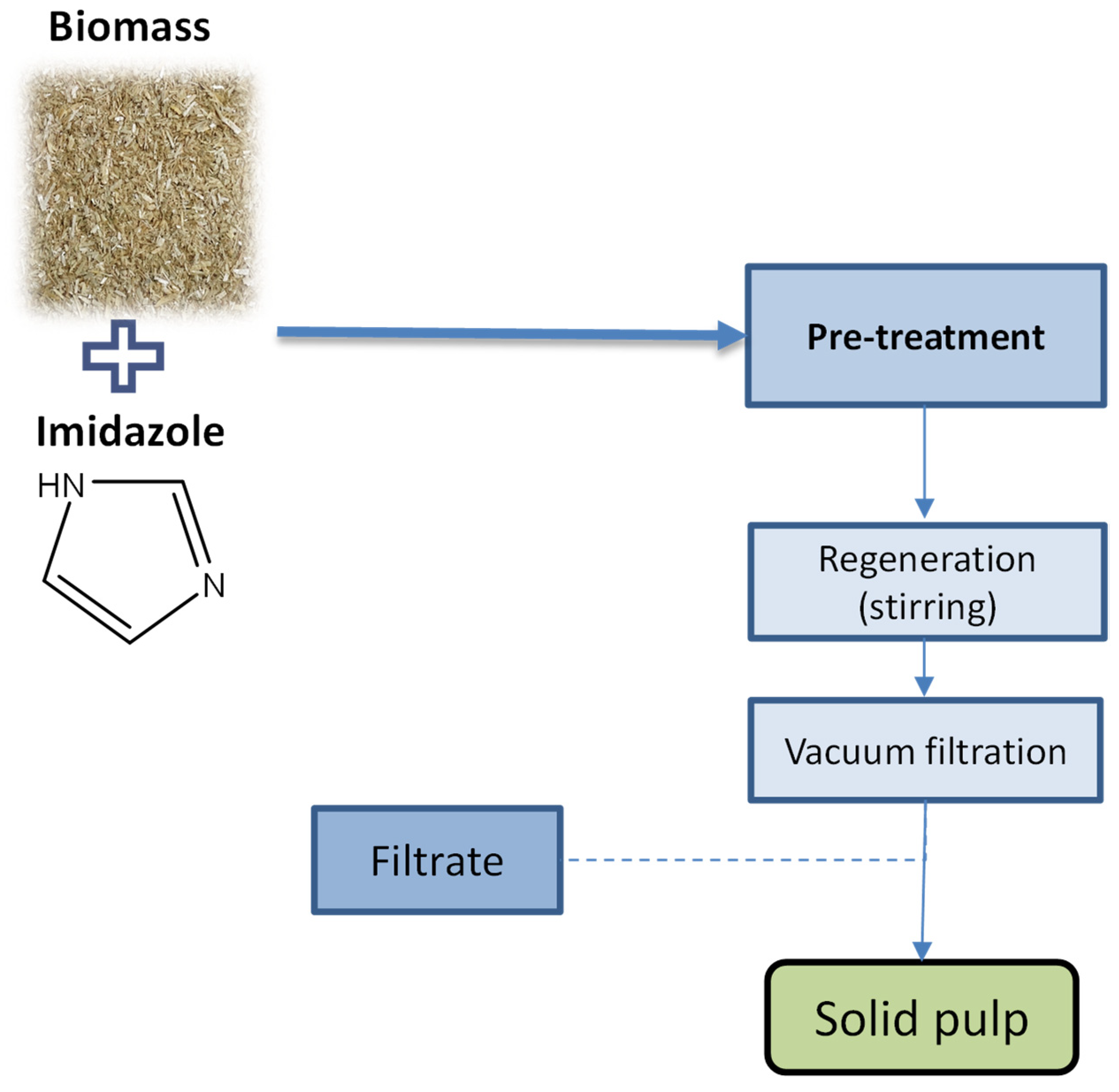
2.3. Biomass Processing
2.4. Enzymatic Hydrolysis of Solids
2.5. Chemical Analysis
3. Results and Discussion
3.1. Biomass Composition
3.2. Pre-Treatment with Imidazole
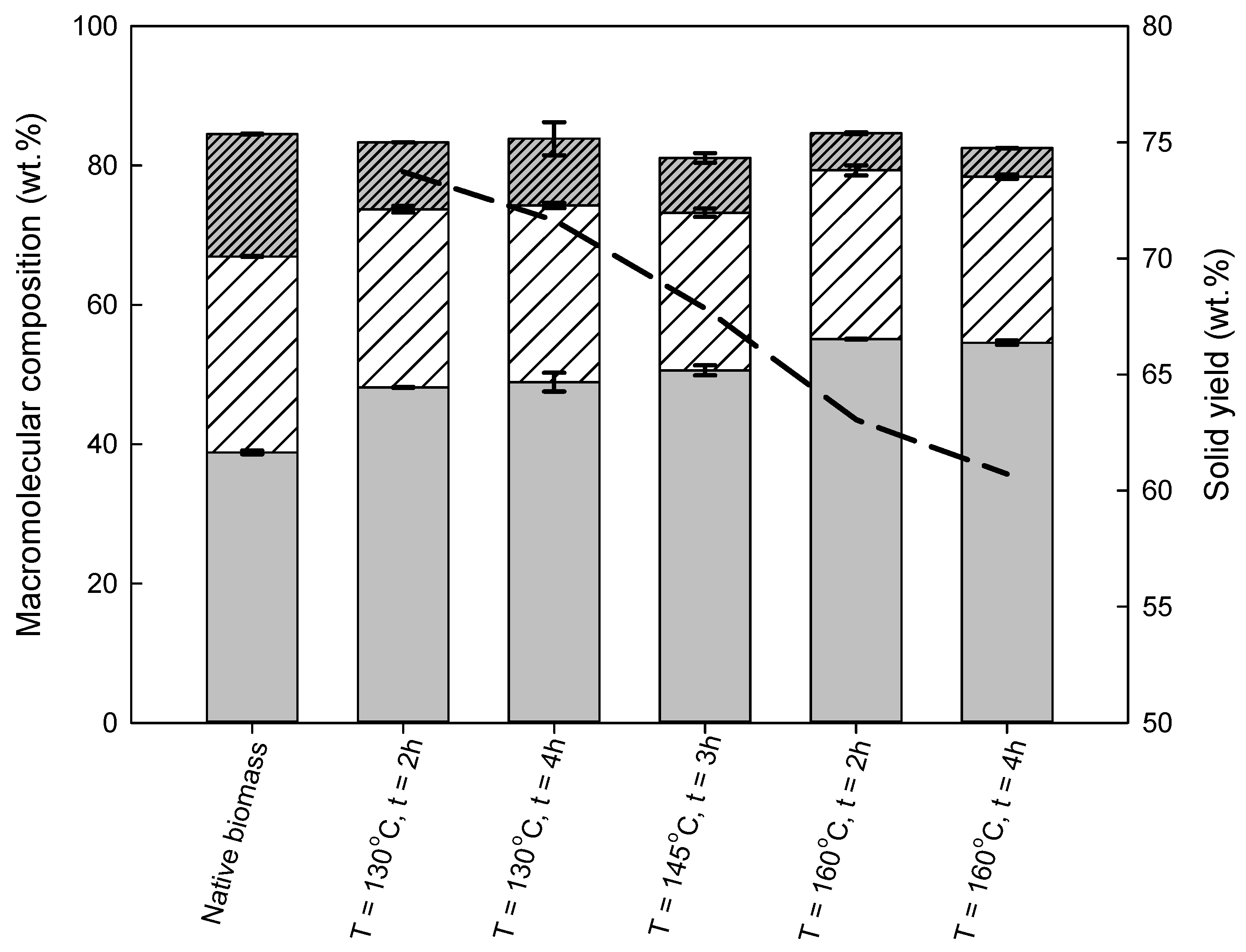
3.2.1. Wheat Straw
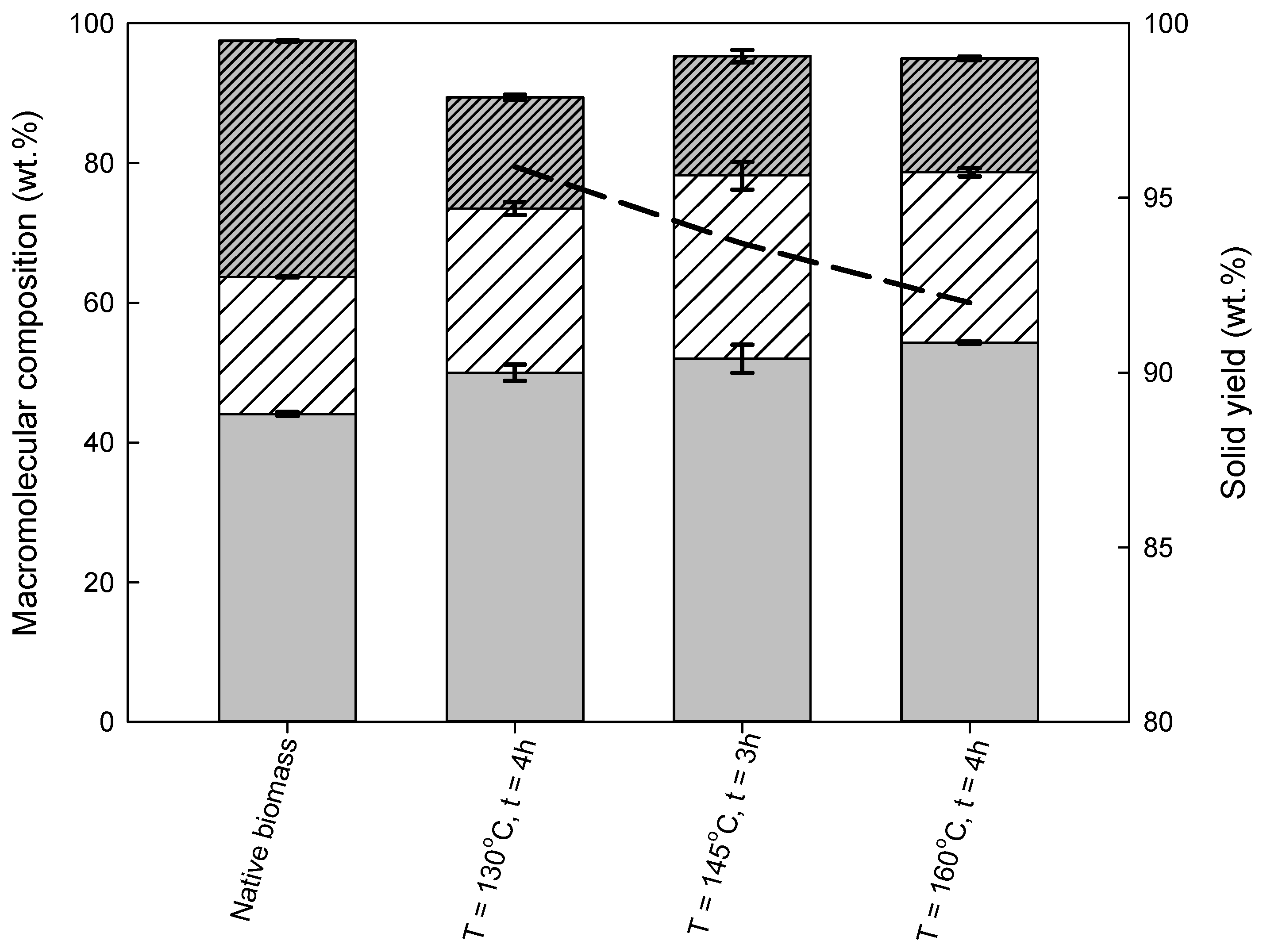
3.2.2. Eucalyptus Residues
3.2.3. Comparison between Wheat Straw and Eucalyptus Residues Pre-Treatments
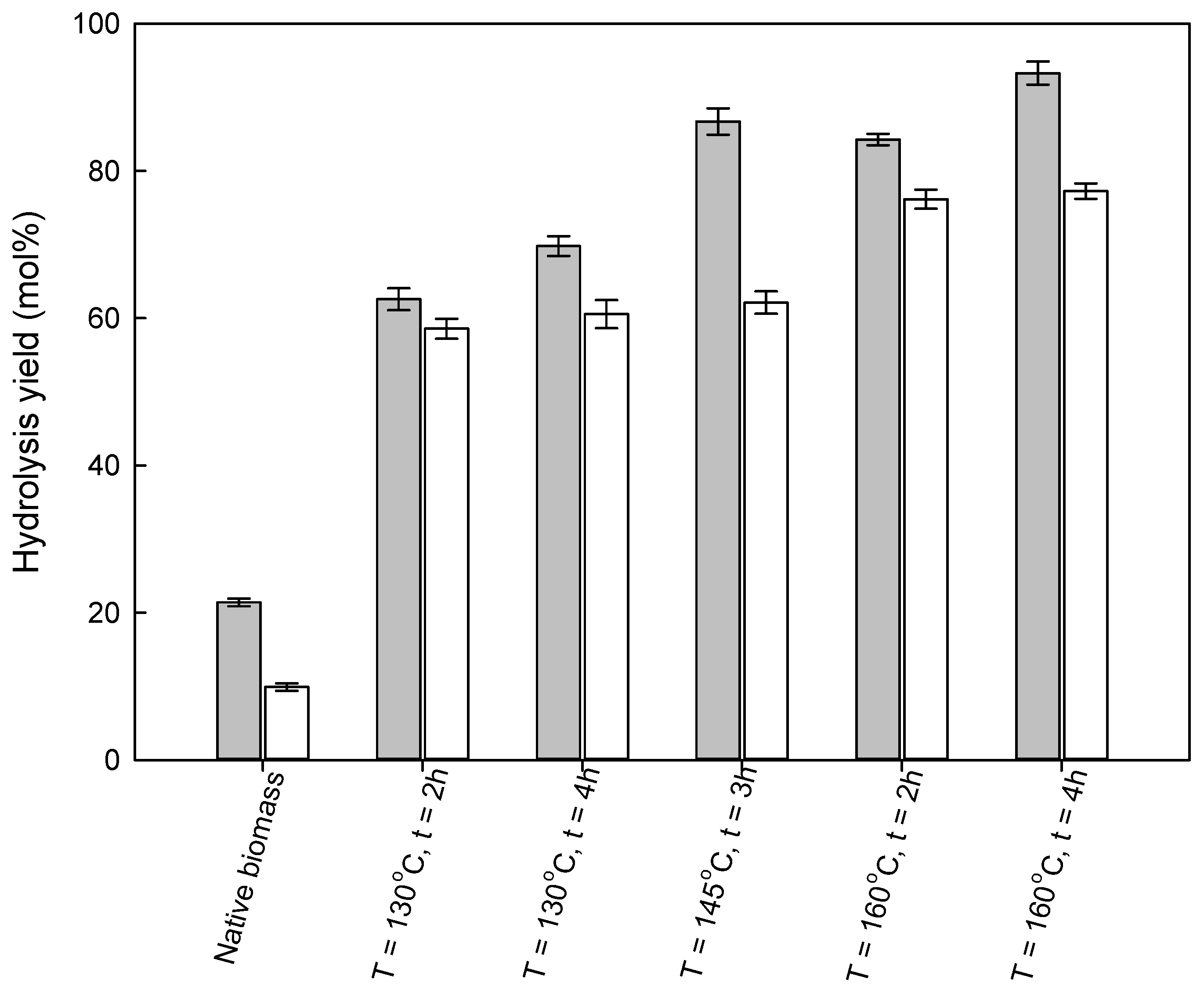
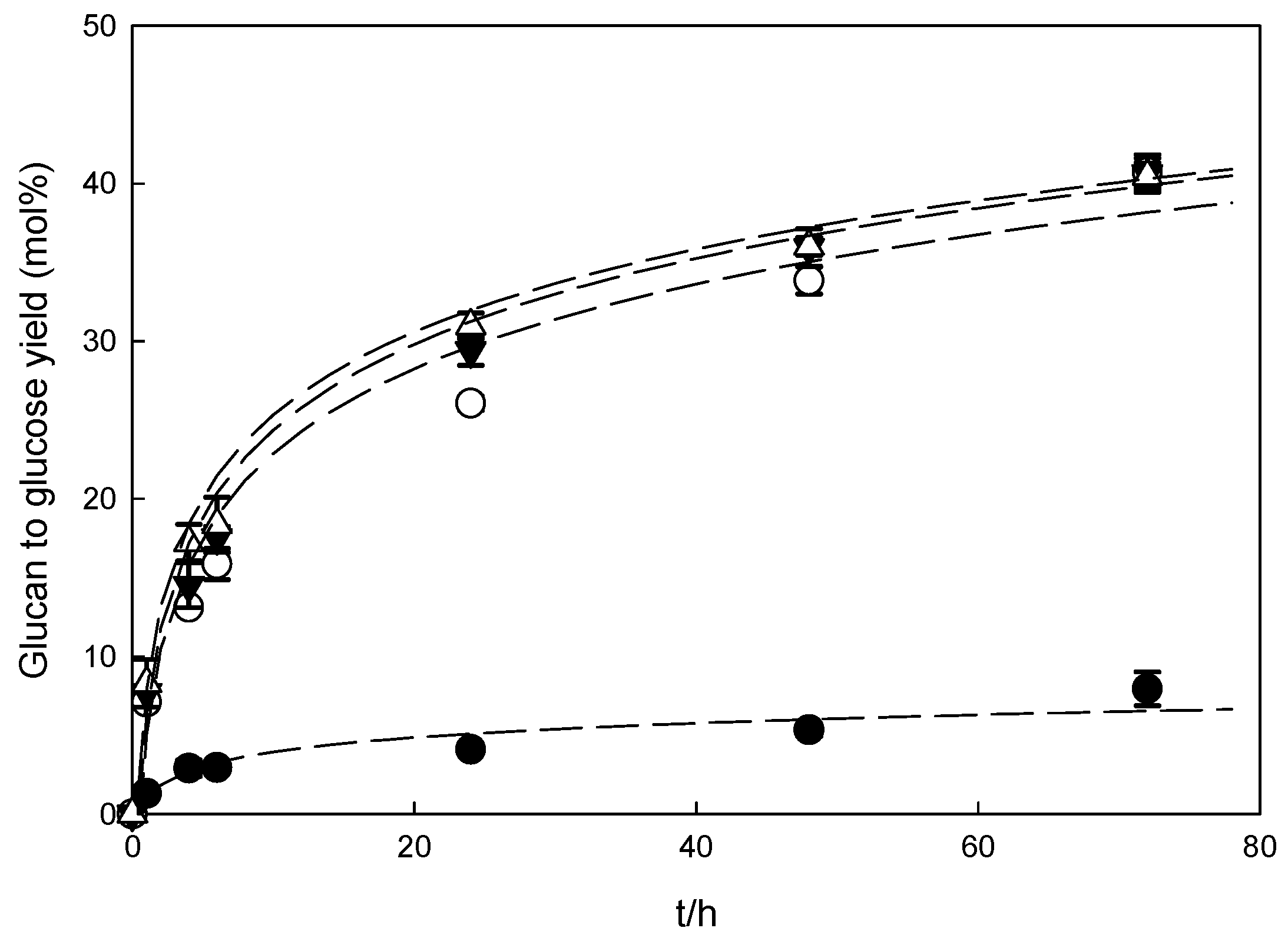
3.3. Enzymatic Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nurek, T.; Gendek, A.; Roman, K. Forest residues as a renewable source of energy: Elemental composition and physical properties. BioResources 2019, 14, 6–20. [Google Scholar] [CrossRef]
- Hrbek, J. Past, present and future of thermal gasification of biomass and waste. Acta Innov. 2020, 35, 5–20. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Rzodkiewicz, W.; Dawidowski, M. Evaluation of mechanical and energetic properties of the forest residues shredded chips during briquetting process. Energies 2021, 14, 3270. [Google Scholar] [CrossRef]
- Manzanares, P. The role of biorefinering research in the development of a modern bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Harmsen, P.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen UR-Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahluwalia, V.; Kundu, P.; Sangwan, R.S.; Kansal, S.K.; Runge, T.M.; Elumalai, S. Improved levulinic acid production from agri-residue biomass in biphasic solvent system through synergistic catalytic effect of acid and products. Bioresour. Technol. 2018, 251, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kochepka, D.M.; Dill, L.P.; Fockink, D.H.; Łukasik, R.M. Contribution to the production and use of biomass-derived solvents—A review. Acta Innov. 2020, 35, 29–56. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.X.; Yu, H.B. Conversion of xylan and xylose into furfural in biorenewable deep eutectic solvent with trivalent metal chloride added. Bioresources 2013, 8, 6014–6025. [Google Scholar] [CrossRef]
- Jordan, T.; Schmidt, S.; Liebert, T.; Heinze, T. Molten imidazole—A starch solvent. Green Chem. 2014, 16, 1967–1973. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect solvent for lignocellulosic biomass fractionation and delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids: [HSO4]−-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef] [Green Version]
- Da Costa Lopes, A.M.; Lins, R.M.G.G.; Rebelo, R.A.; Lukasik, R.M.; Łukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057. [Google Scholar] [CrossRef] [Green Version]
- Hyvarinen, S.; Mikkola, J.P.; Murzin, D.Y.; Vaher, M.; Kaljurand, M.; Koel, M. Sugars and sugar derivatives in ionic liquid media obtained from lignocellulosic biomass: Comparison of capillary electrophoresis and chromatographic analysis. Catal. Today 2014, 223, 18–24. [Google Scholar] [CrossRef]
- Rodríguez, H. Ionic liquids in the pretreatment of lignocellulosic biomass. Acta Innov. 2021, 38, 23–36. [Google Scholar] [CrossRef]
- Fockink, D.H.; Morais, A.R.C.; Ramos, L.P.; Łukasik, R.M. Insight into the high-pressure CO2 pre-treatment of sugarcane bagasse for a delivery of upgradable sugars. Energy 2018, 151, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Özbek, H.N.; Fockink, D.H.; Yanık, D.K.; Göğüş, F.; Lukasik, R. The green biorefinery concept for the valorisation of pistachio shell by high-pressure CO2/H2O system. J. Clean. Prod. 2018, 196, 842–851. [Google Scholar] [CrossRef] [Green Version]
- Morais, A.R.C.; Matuchaki, M.D.D.J.; Andreaus, J.; Bogel-Lukasik, R. A green and efficient approach to selective conversion of xylose and biomass hemicellulose into furfural in aqueous media using high-pressure CO2 as a sustainable catalyst. Green Chem. 2016, 18, 2985–2994. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Pereira, P.M.A.; Bernardo, J.R.; Oliveira, M.C.; Roseiro, L.B.; Łukasik, R.M. Depolymerization of lignin from extracted solid waste of cupressus lusitanica Mill. Biomass using imidazole. Waste Biomass Valorization 2021, 12, 1341–1355. [Google Scholar] [CrossRef]
- Toscan, A.; Fontana, R.C.; Andreaus, J.; Camassola, M.; Lukasik, R.M.; Dillon, A.J.P. New two-stage pretreatment for the fractionation of lignocellulosic components using hydrothermal pretreatment followed by imidazole delignification: Focus on the polysaccharide valorization. Bioresour. Technol. 2019, 285, 121346. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. NREL Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- International Organization for Standarization. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation; International Organization for Standarization: London, UK, 2014. [Google Scholar]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory Analytical Procedure (LAP): Issue Date, 3/21/2008; National Renewable Energy Laboratory—NREL: Golden, CO, USA, 2008.
- Bernardo, J.R.; Gírio, F.M.; Łukasik, R.M. The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules 2019, 24, 808. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, M.G.; Mendonça, R.T.; Martínez, P.; Rodríguez, J.; Pereira, M. Chemical characteristics and kraft pulping of tension wood from Eucalyptus globulus Labill. Rev. Arvore 2012, 36, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.; Parajo, J.C. Non-isothermal autohydrolysis of Eucalyptus wood. Wood Sci. Technol. 2002, 36, 111–123. [Google Scholar] [CrossRef]
- Magalhães da Silva, S.P.; Morais, A.R.C.; Bogel-Łukasik, R. The CO2-assisted autohydrolysis of wheat straw. Green Chem. 2014, 16, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, G.; Singh, L.K.; Ghosh, S. Alkaline pretreatment methods followed by acid hydrolysis of Saccharum spontaneum for bioethanol production. Bioresour. Technol. 2012, 124, 111–118. [Google Scholar] [CrossRef]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. Effective extraction of lignin from elephant grass using imidazole and its effect on enzymatic saccharification to produce fermentable sugars. Ind. Eng. Chem. Res. 2017, 56, 5138–5145. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S.; Kim, T.H. Pretreatment of corn stover using organosolv with hydrogen peroxide for effective enzymatic saccharification. Energies 2018, 11, 1301. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zong, M.H.; Wu, H.; Li, N. Efficient pretreatment of wheat straw using novel renewable cholinium ionic liquids to improve enzymatic saccharification. Ind. Eng. Chem. Res. 2016, 55, 1788–1795. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; João, K.G.; Bogel-Łukasik, E.; Roseiro, L.B.; Bogel-Łukasik, R. Pretreatment and fractionation of wheat straw using various ionic liquids. J. Agric. Food Chem. 2013, 61, 7874–7882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa Lopes, A.M.; João, K.G.; Rubik, D.F.; Bogel-Łukasik, E.; Duarte, L.C.; Andreaus, J.; Bogel-Łukasik, R. Pre-treatment of lignocellulosic biomass using ionic liquids: Wheat straw fractionation. Bioresour. Technol. 2013, 142, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Elmacı, S.B.; Ozcelik, F. Ionic liquid pretreatment of yellow pine followed by enzymatic hydrolysis and fermentation. Biotechnol. Prog. 2018, 34, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Magalhães da Silva, S.P.; da Costa Lopes, A.M.; Roseiro, L.B.; Bogel-Łukasik, R. Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids. RSC Adv. 2013, 3, 16040. [Google Scholar] [CrossRef] [Green Version]


| Components (Dry Weight %) | Wheat Straw | Eucalyptus Residues |
|---|---|---|
| Glucan | 35.9 ± 0.3 | 44.1 ± 0.9 |
| Hemicellulose | 26.7 | 19.6 |
| Xylan | 22.1 ± 0.6 | 15.7 ± 0.2 |
| Arabinosyl group | 2.0 ± 0.7 | 0.5 ± 0.1 |
| Acetyl group | 2.6 ± 0.9 | 3.4 ± 0.9 |
| Lignin | 16.7 | 33.8 |
| Acid-insoluble | 15.5 ± 0.4 | 26.4 ± 0.1 |
| Acid-soluble | 1.2 ± 0.1 | 7.4 ± 0.1 |
| Ash | 11.4 ± 0.1 | 1.0 ± 0.1 |
| Extractives | ||
| Water | 9.4 ± 1.3 | 3.3 ± 0.4 |
| Ethanol | 1.4 ± 0.1 | 1.5 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.M.A.; Bernardo, J.R.; Roseiro, L.B.; Gírio, F.; Łukasik, R.M. Imidazole Processing of Wheat Straw and Eucalyptus Residues—Comparison of Pre-Treatment Conditions and Their Influence on Enzymatic Hydrolysis. Molecules 2021, 26, 7591. https://doi.org/10.3390/molecules26247591
Pereira PMA, Bernardo JR, Roseiro LB, Gírio F, Łukasik RM. Imidazole Processing of Wheat Straw and Eucalyptus Residues—Comparison of Pre-Treatment Conditions and Their Influence on Enzymatic Hydrolysis. Molecules. 2021; 26(24):7591. https://doi.org/10.3390/molecules26247591
Chicago/Turabian StylePereira, Pedro M. A., Joana R. Bernardo, Luisa Bivar Roseiro, Francisco Gírio, and Rafał M. Łukasik. 2021. "Imidazole Processing of Wheat Straw and Eucalyptus Residues—Comparison of Pre-Treatment Conditions and Their Influence on Enzymatic Hydrolysis" Molecules 26, no. 24: 7591. https://doi.org/10.3390/molecules26247591
APA StylePereira, P. M. A., Bernardo, J. R., Roseiro, L. B., Gírio, F., & Łukasik, R. M. (2021). Imidazole Processing of Wheat Straw and Eucalyptus Residues—Comparison of Pre-Treatment Conditions and Their Influence on Enzymatic Hydrolysis. Molecules, 26(24), 7591. https://doi.org/10.3390/molecules26247591








