GC-MS Analysis and Biomedical Therapy of Oil from n-Hexane Fraction of Scutellaria edelbergii Rech. f.: In Vitro, In Vivo, and In Silico Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis
2.2. Antibacterial Activity
2.3. Antifungal Assay
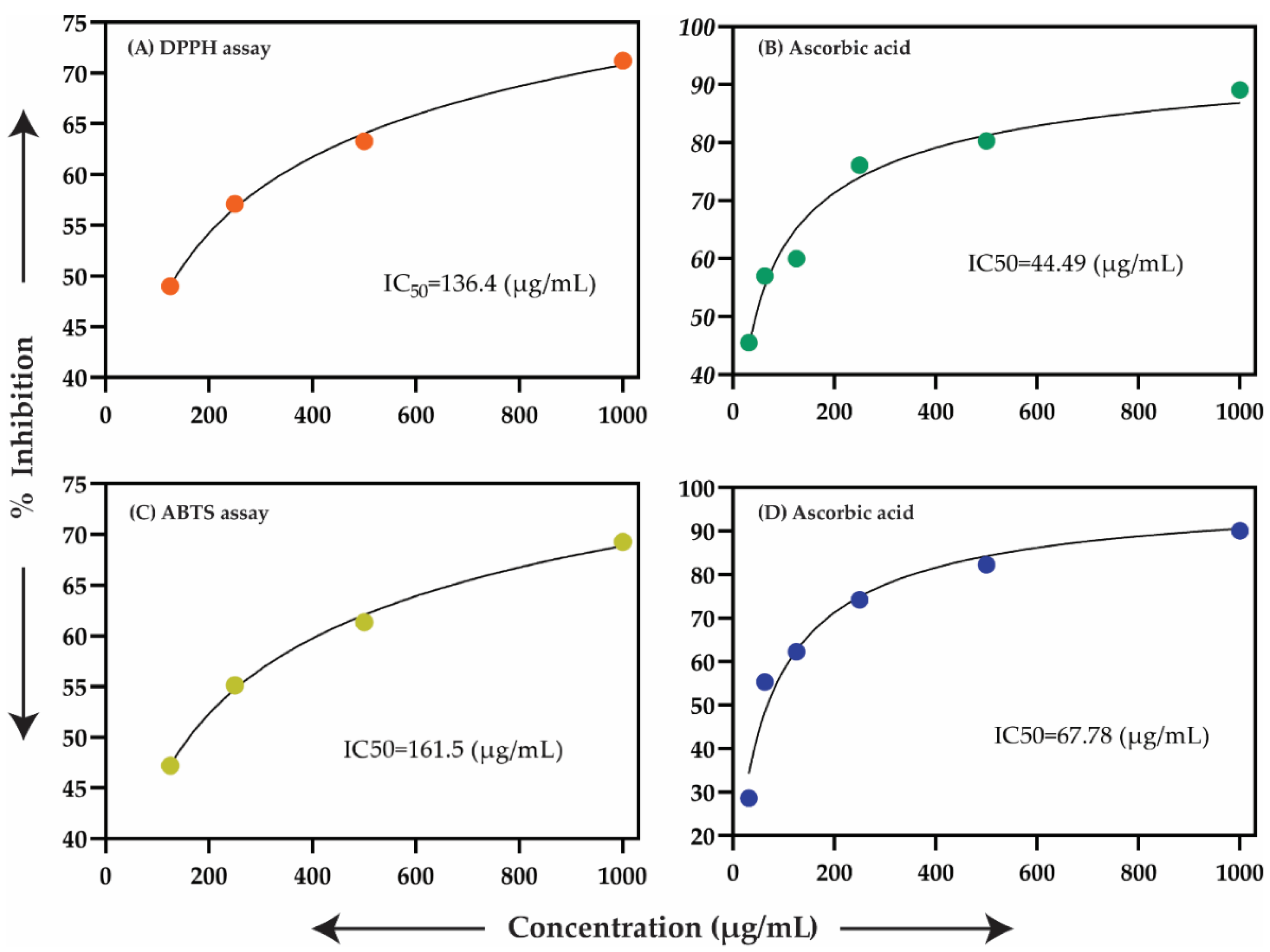
2.4. Antioxidant Assay
2.5. In Vitro Antidiabetic Assay
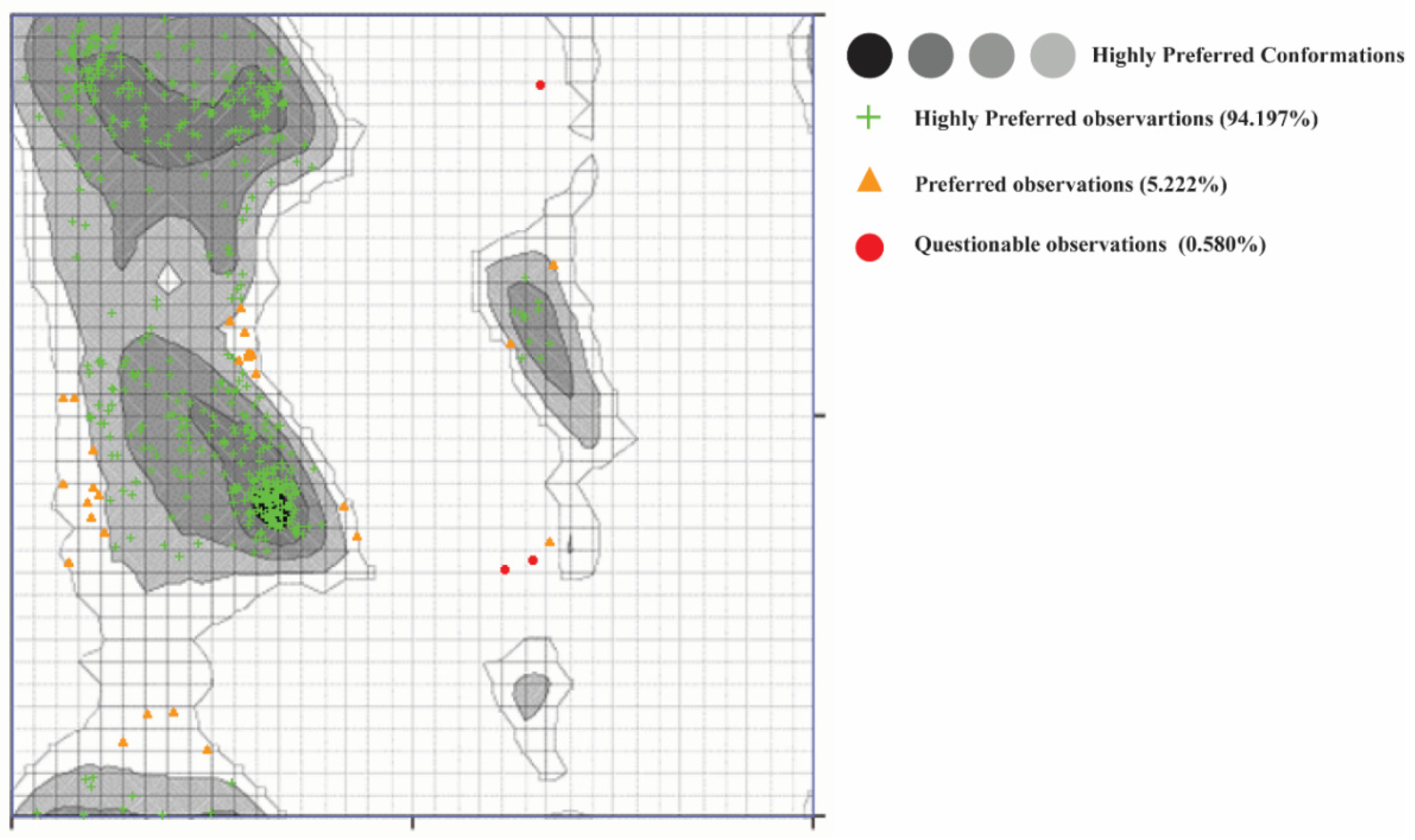
2.6. α-Glucosidase Structure Modeling
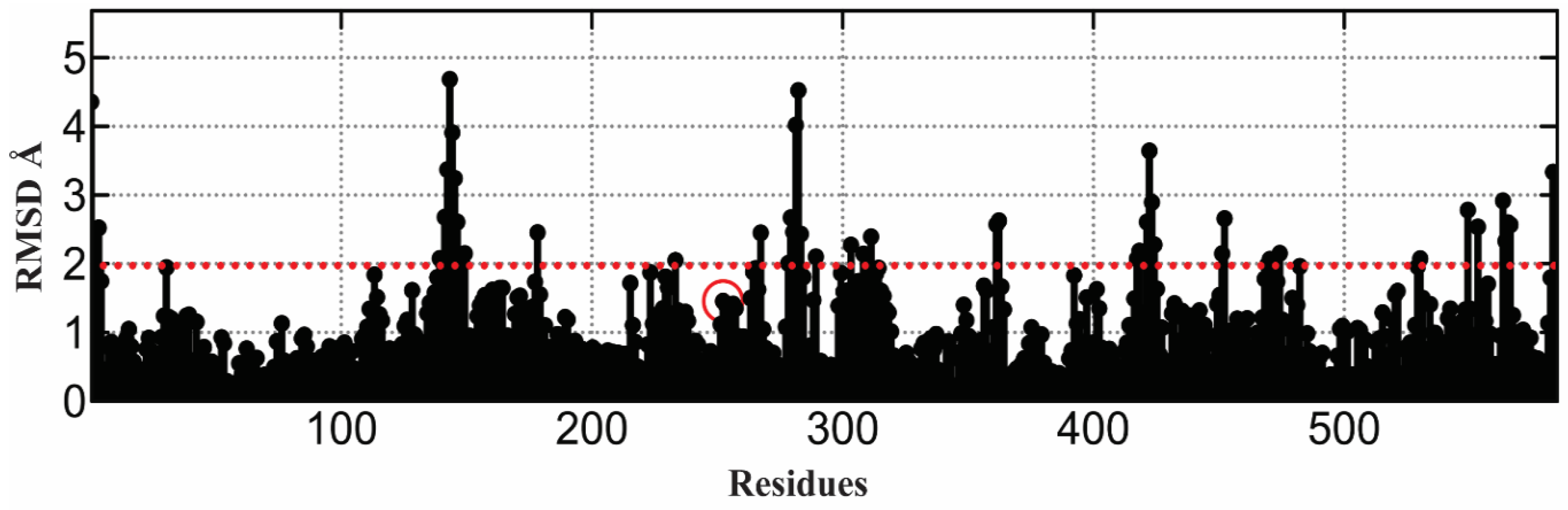
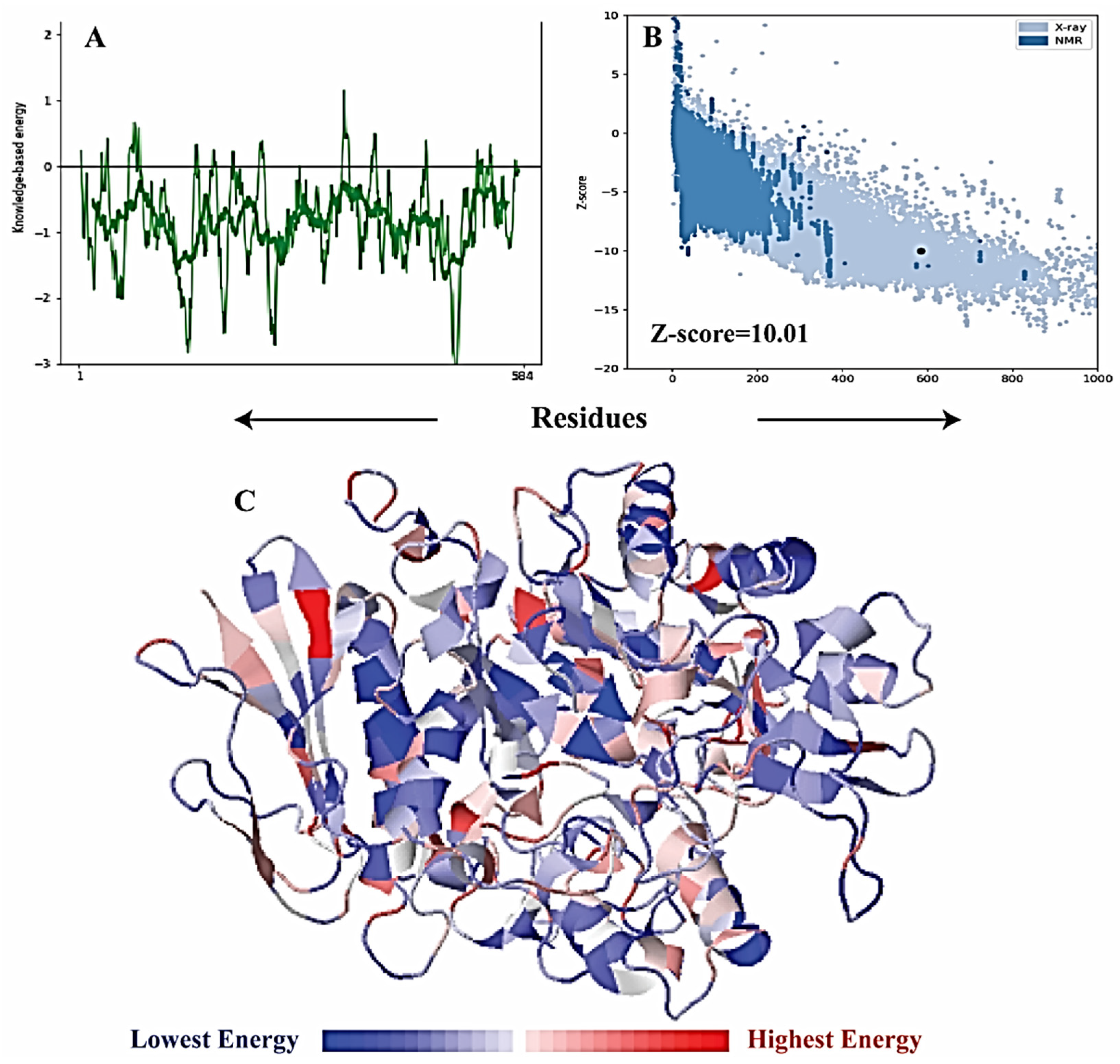
2.7. α-Glucosidase Model Validation
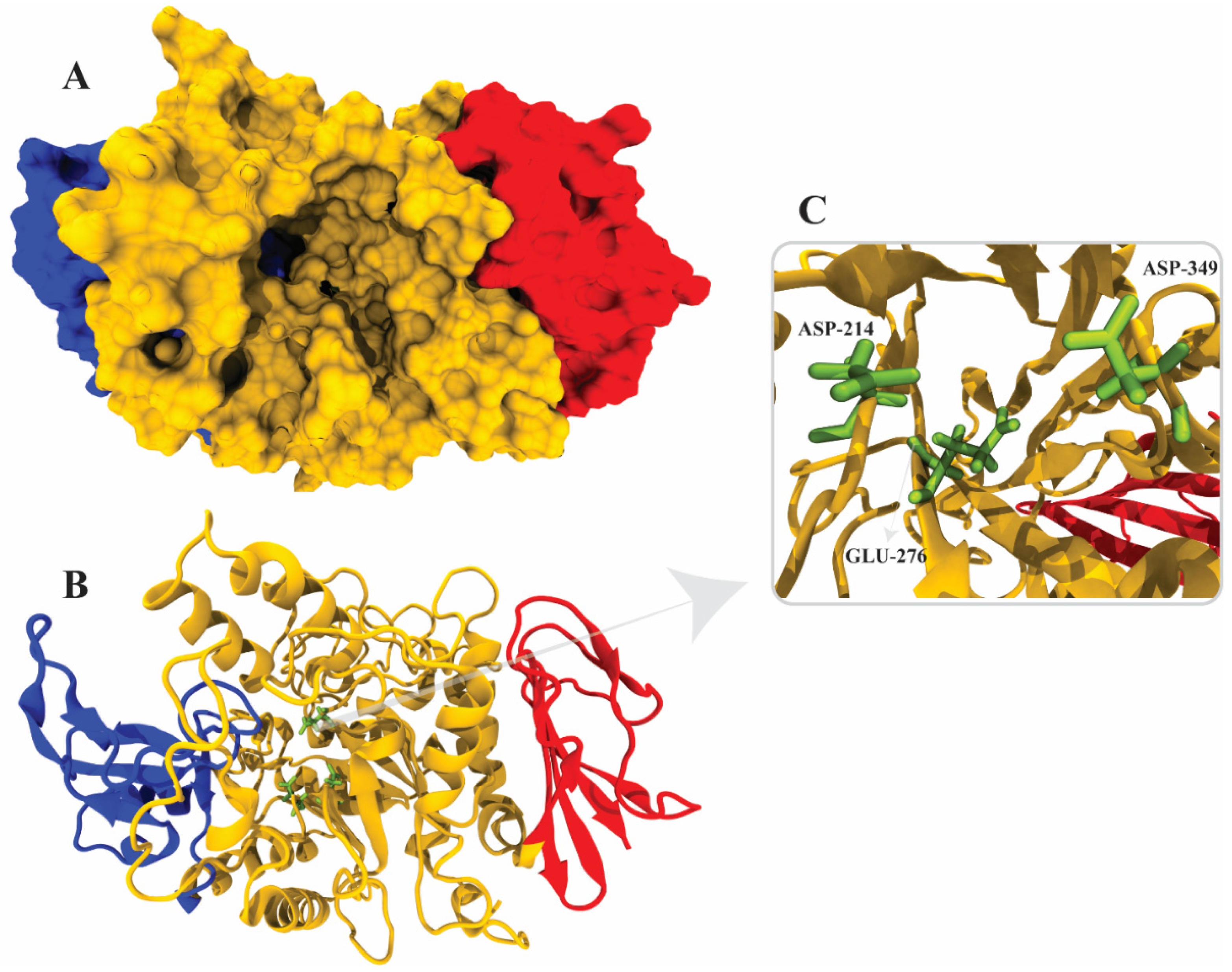
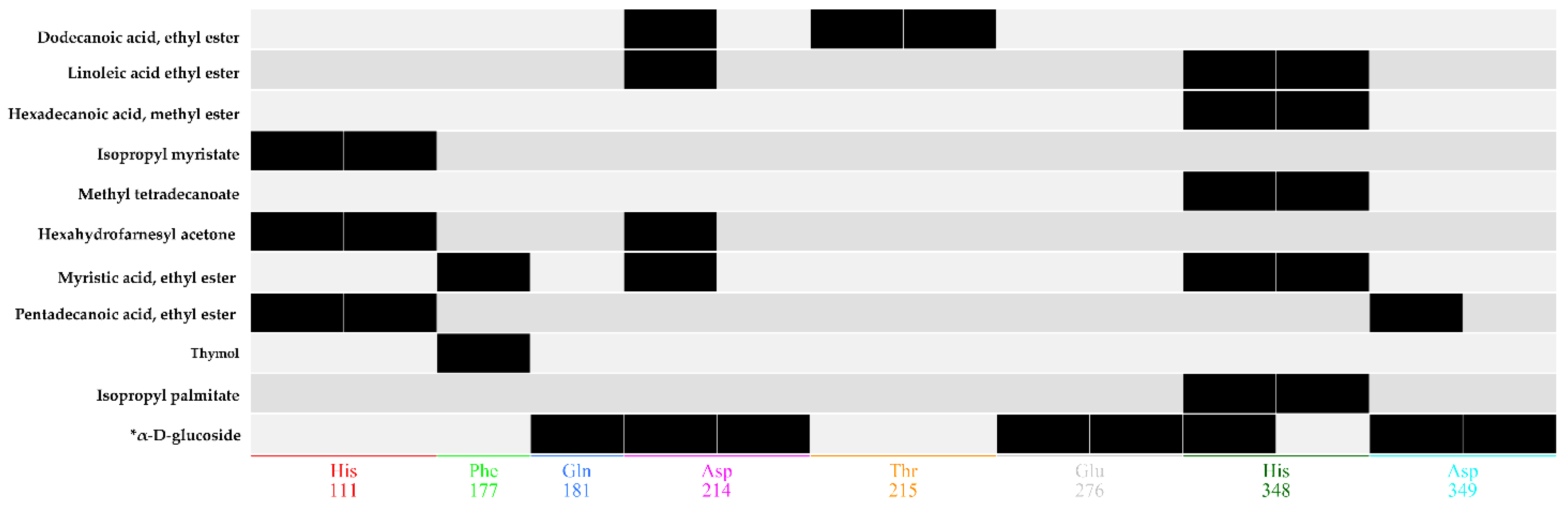
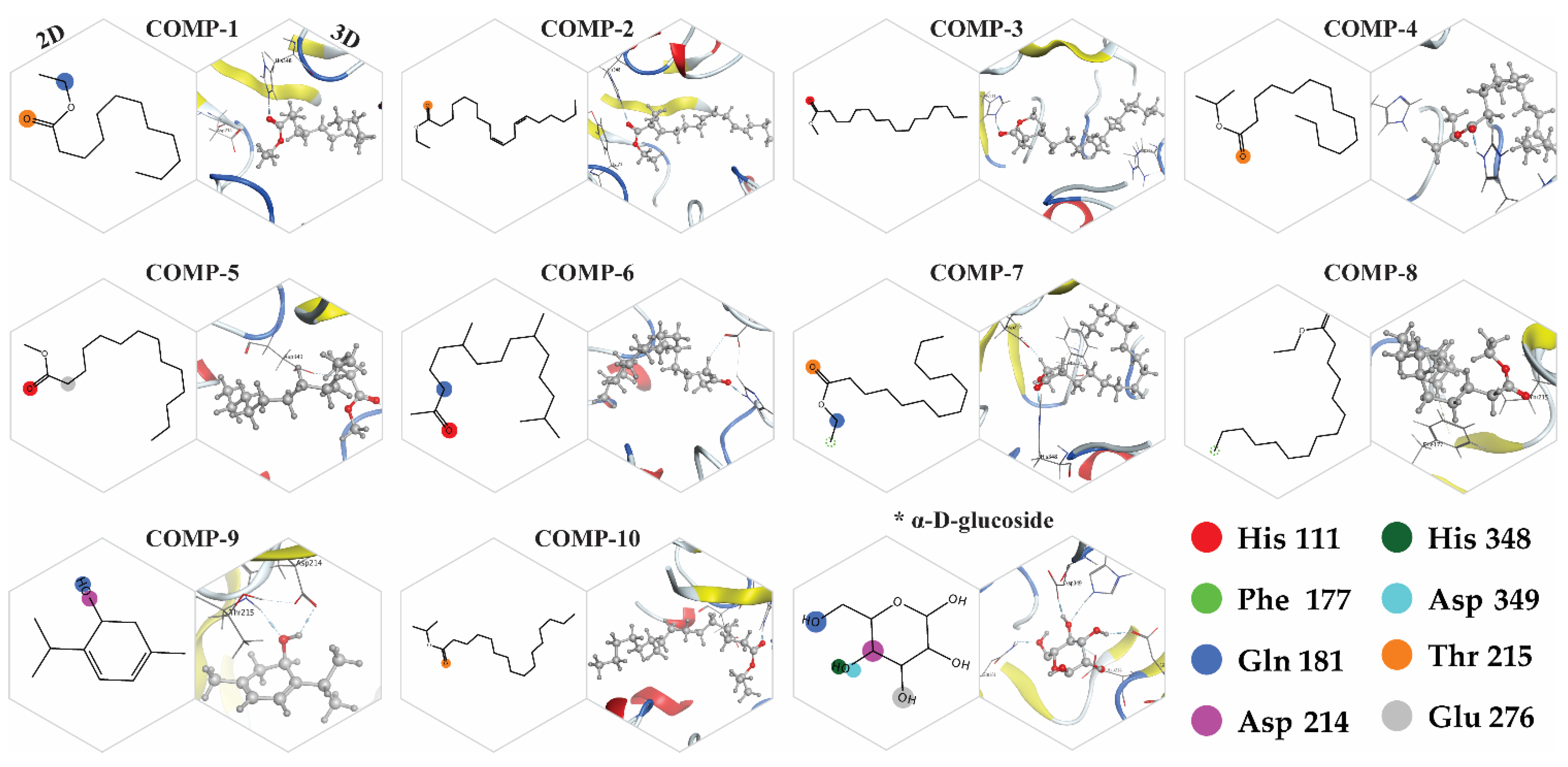
2.8. Docking Simulations
2.9. Binding Energy and Binding Affinity
2.10. Anti-Inflammatory Activity
2.11. AnalgesicActivity
3. Material and Methods
3.1. General Instrumentation
3.2. Plant Material
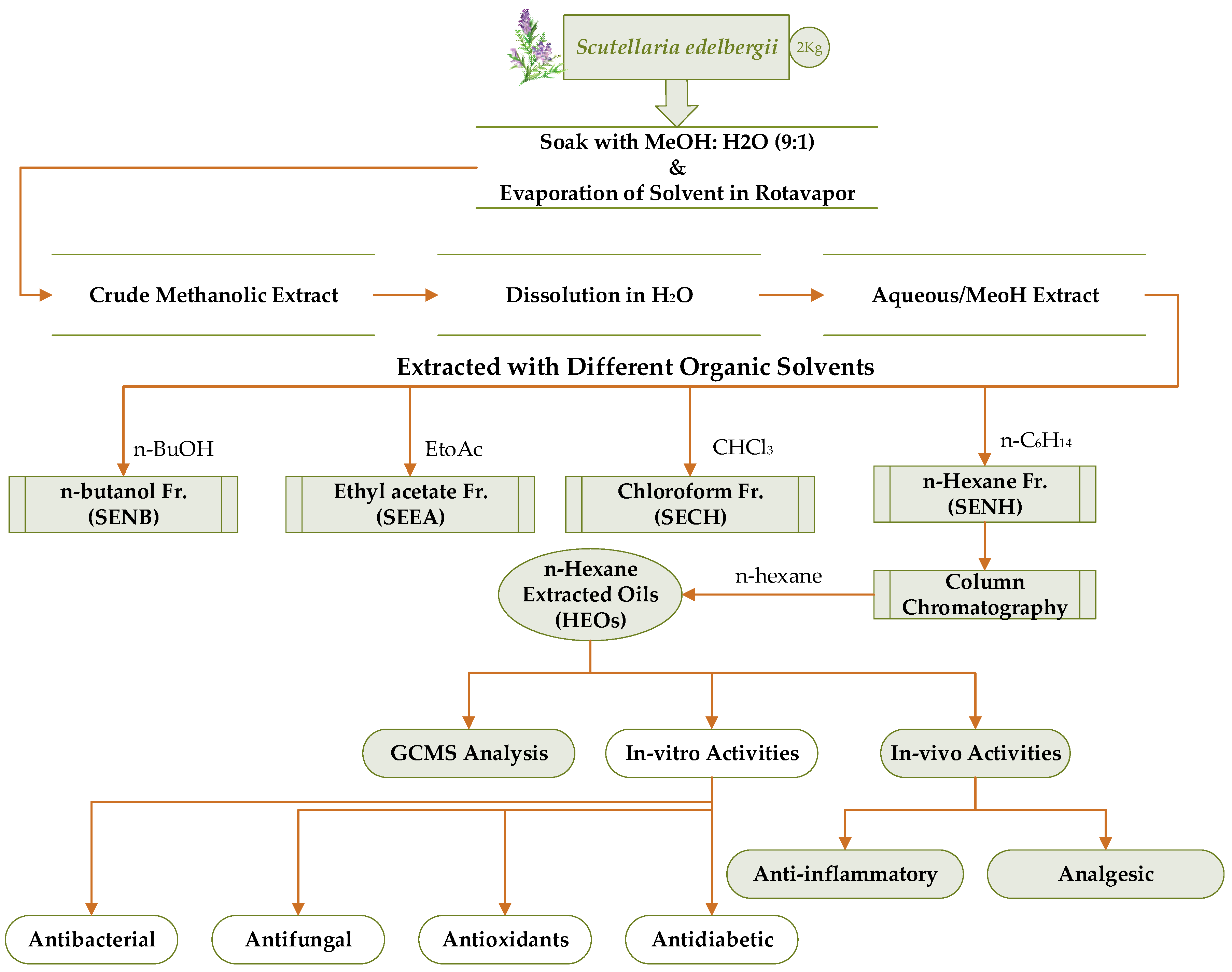
3.3. Soaking, Extraction, and Fractionation
3.4. Oil Extraction and Preparation of FAEs
3.5. GC-MS Analysis
3.6. Detection and Identification of Compounds
3.7. Stock Solution Preparation
3.8. Antimicrobial Screening
3.8.1. MIC/MBC Assay
3.8.2. Zone of Inhibitions
3.9. Antifungalassay
3.10. Antioxidant Activity
3.10.1. DPPH Assay
3.10.2. ABTS Assay
3.11. Antidiabetic Activity (α-Glucosidase Assay)
3.12. 3 D Structure of α-Glucosidase
3.13. Docking Simulations
3.14. Binding Energy and Binding Affinity Calculation
3.15. In Vivo Pharmacological Activities
Experimental Animals
3.16. Anti-Inflammatory Activities
3.17. Analgesic Activities
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; De Soyza, S.; Pace, S.; Lorenz, S.; Qader, M.; Witharana, S.; Nagahawatte, A.; Wijayaratne, G. The anti-inflammatory and antimicrobial potential of selected ethnomedicinal plants from Sri Lanka. Molecules 2020, 25, 1894. [Google Scholar] [CrossRef]
- Shah, M.; Murad, W.; Ur Rehman, N.; Halim, S.A.; Ahmed, M.; Rehman, H.; Zahoor, M.; Mubin, S.; Khan, A.; Nassan, M.A. biomedical applications of Scutellaria edelbergii Rech. f.: In-vitro and in-vivo Approach. Molecules 2021, 26, 3740. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Khan, I.; Zeb, A.; Sahibzada, M.U.K.; Naz, S.; Bari, W.U.; Kamran, A.W. Pharmacological evaluation and in-silico modeling study of compounds isolated from Ziziphus oxyphylla. Heliyon 2021, 7, e06367. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, J.; Alesheikh, P.; Mohammadi, A. Chemical compositions and biological activities of scutellaria genus essential oils (lamiaceae). Jundishapur J. Nat. Pharm. Prod. 2020, 15, e62279. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Kyriazopoulos, P.; Golegou, S.; Triantaphyllidis, S.; Sokovic, M.; Kypriotakis, Z. Composition and antimicrobial activity of the essential oils of Scutellaria sieberia Benth. and Scutellaria rupestris Boiss. et Heldr. ssp. adenotricha (Boiss. et Heldr.) Greuter et Burdet from Greece. J. Essent. Oil-Res. 2005, 17, 232–235. [Google Scholar]
- Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Simmonds, M.S.; Rosselli, S.; Bruno, M.; Loziene, K. Essential oils of three species of Scutellaria and their influence on Spodoptera littoralis. Biochem. Syst. Ecol. 2013, 48, 206–210. [Google Scholar] [CrossRef]
- Mohammad hosseini, M. Essential oils extracted using microwave-assisted hydrodistillation from aerial parts of eleven Artemisia species: Chemical compositions and diversities in different geographical regions of Iran. Rec. Nat. Prod. 2017, 11, 114. [Google Scholar]
- Nazir, N.; Zahoor, M.; Uddin, F.; Nisar, M. Chemical composition, in vitro antioxidant, anticholinesterase, and antidiabetic potential of essential oil of Elaeagnus umbellata Thunb. BMC Complement. Med. Ther. 2021, 21, 73. [Google Scholar] [CrossRef]
- Kidane, Y.; Bokrezion, T.; Mebrahtu, J.; Mehari, M.; Gebreab, Y.B.; Fessehaye, N.; Achila, O.O. In vitro inhibition of-amylase and-glucosidase by extracts from Psiadia punctulata and Meriandra bengalensis. Evid. Based Complement. Altern. Med. 2018, 2018, 2164345. [Google Scholar] [CrossRef] [Green Version]
- Akhter, S. Low to no cost remedies for the management of diabetes mellitus; global health concern. J. Diabetes Metab. Disord. 2021, 20, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Liu, E.-H.; Chu, C.; Peng, Y.-B.; Cai, H.-X.; Li, P. Anti-diabetic agents from natural products—An update from 2004 to 2009. Curr. Top. Med. Chem. 2010, 10, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Babkov, D.A.; Prokhorova, T.Y.; Sturova, E.A.; Muleeva, D.R.; Demidov, M.R.; Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Synthesis and biological evaluation of 2-acylbenzofuranes as novel α-glucosidase inhibitors with hypoglycemic activity. Chem. Biol. Drug Des. 2017, 90, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Montgomery, P.A. Acarbose: An α-glucosidase inhibitor. Am. J. Health Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef]
- Pan, B.; Ge, L.; Xun, Y.-Q.; Chen, Y.-J.; Gao, C.-Y.; Han, X.; Zuo, L.-Q.; Shan, H.-Q.; Yang, K.-H.; Ding, G.-W. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Hafez, A.; Nassef, E.; Fahmy, M.; Elsabagh, M.; Bakr, A.; Hegazi, E. Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ. Sci. Pollut. Res. 2020, 27, 19108–19114. [Google Scholar] [CrossRef]
- Farhat, U.; Shah, S.M.M.; Shah, S.M.H.; Zahoor, M.; Sadiq, A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the north west of Pakistan. BMC Complement. Altern. Med. 2012, 12, 244–254. [Google Scholar]
- Delazar, A.; Nazemiyeh, H.; Afshar, F.H.; Barghi, N.; Esnaashari, S.; Asgharian, P. Chemical compositions and biological activities of Scutellaria pinnatifida A. Hamilt aerial parts. Res. Pharm. Sci. 2017, 12, 187. [Google Scholar] [PubMed] [Green Version]
- von Bothmer, R. Differentiation patterns in the Scutellaria albida group (Lamiaceae) in the Aegean area. Nord. J. Bot. 1985, 5, 421–439. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Furukawa, H.; Fujioka, T.; Fujii, H.; Mihashi, K.; Shimomura, K.; Ishimaru, K. Flavone production in transformed root cultures of Scutellaria baicalensis Georgi. Phytochemistry 1999, 52, 885–890. [Google Scholar] [CrossRef]
- Rehman, N.U.; Alsabahi, J.N.; Alam, T.; Khan, A.; Rafiq, K.; Khan, M.; Al-Harrasi, A. chemical constituents and carbonic anhydrase II activity of essential oil of Acridocarpus orientalis A. Juss. in comparison with stem and leaves. J. Essent. Oil-Bear. Plants 2021, 24, 68–74. [Google Scholar] [CrossRef]
- He, M.; Li, Y.; Yan, J.; Cao, D.; Liang, Y. Analysis of essential oils and fatty acids from Platycodi radix using chemometric methods and retention indices. J. Chromatogr. Sci. 2013, 51, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Shabi, M.M.; Gayathri, K.; Venkatalakshmi, R.; Sasikala, C. Chemical constituents of hydro alcoholic extract and phenolic fraction of Cynodon dactylon. Int. J. ChemTech Res. 2010, 2, 149–154. [Google Scholar]
- Kim, B.-R.; Kim, H.M.; Jin, C.H.; Kang, S.-Y.; Kim, J.-B.; Jeon, Y.G.; Park, K.Y.; Lee, I.-S.; Han, A.-R. Composition and antioxidant activities of volatile organic compounds in radiation-bred Coreopsis cultivars. Plants 2020, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Rossellia, S.; Maggio, A.; Formisano, C.; Napolitano, F.; Senatore, F.; Spadaro, V.; Bruno, M. Chemical composition and antibacterial activity of extracts of Helleborus bocconei Ten. subsp. intermedius. Nat. Prod. Commun. 2007, 2, 578–611. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Valencia, V.A.; Us-Vázquez, R.A.; Larqué-Saavedra, F.A.; Barahona-Pérez, L.F. Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonas reinhardtii. Ann. Microbiol. 2012, 62, 865–870. [Google Scholar] [CrossRef]
- Lawal, O.A.; Ogunwande, I.A.; Ibirogba, A.E.; Layode, O.M.; Opoku, A.R. Chemical constituents of essential oils from Catharanthus roseus (L.) G. Don grown in Nigeria. J. Essent. Oil-Bear. Plants 2015, 18, 57–63. [Google Scholar] [CrossRef]
- Udourioh, G.A.; Etokudoh, M.F. Essential oils and fatty acids composition of dry fruits of Tetrapleura tetraptera. J. Appl. Sci. Environ. Manag. 2014, 18, 419–424. [Google Scholar]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264. 7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef]
- Jelenko, C.; Wheeler, M.; Anderson, A.; Callaway, B.; McKinley, J. Studies in burns: XIV, Heling in burn wounds treated with Ethyl Linoleate alone or in combination with selected topical antibacterial agents. Ann. Surg. 1975, 182, 562. [Google Scholar] [CrossRef]
- Charakida, A.; Charakida, M.; Chu, A. Double-blind, randomized, placebo-controlled study of a lotion containing triethyl citrate and ethyl linoleate in the treatment of acne vulgaris. Br. J. Dermatol. 2007, 157, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.-A.; Cho, S.K. Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway. Korean J. Physiol. Pharm. 2018, 22, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Zahra, G.; Khadijeh, B.; Hossein, D.; Ali, S. Essential oil composition of two Scutellaria species from Iran. J. Tradit. Chin. Med. Sci. 2019, 6, 244–253. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Sharopov, F.; Satyal, P.; Azimova, S.S.; Wink, M. Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat. Prod. Res. 2017, 31, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Kim, M.-G.; Lee, H.-S. Acaricidal toxicities of 1-hydroxynaphthalene from Scutellaria barbata and its derivatives against house dust and storage mites. Planta Med. 2013, 79, 946–951. [Google Scholar] [CrossRef]
- Cicek, M.; Demirci, B.; Yilmaz, G.; Ketenoglu, O.; Baser, K.H.C. Composition of the essential oils of subspecies of Scutellaria albida L. from Turkey. J. Essent. Oil-Bear. Res. 2010, 22, 55–58. [Google Scholar] [CrossRef]
- Escobar, A.; Perez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 17, 12–16. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W. Characterization of antimicrobial, antioxidant, anticancer properties and chemical composition of Malaysian Andrographis paniculata leaf extract. Pharmacol. Online 2011, 2, 996–1002. [Google Scholar]
- Elshafie, H.S.; Racioppi, R.; Bufo, S.A.; Camele, I. In vitro study of biological activity of four strains of Burkholderia gladioli pv. agaricicola and identification of their bioactive metabolites using GC–MS. Saudi J. Biol. Sci. 2017, 24, 295–301. [Google Scholar]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Han, C.; Gao, T.; Shao, H. Chemical composition, phytotoxic and antimicrobial activities of the essential oil of Scutellaria strigillosa Hemsley. J. Essent. Oil-Bear. Plants 2016, 19, 664–670. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2, 4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, P.S.; Arunachalam, K.; Saikumar, S.; Kasipandi, M.; Dhivya, S.; Murugan, R.; Parimelazhagan, T. Gastroprotective effect and mode of action of methanol extract of Sphenodesme involucrata var. paniculata (CB Clarke) Munir (Lamiaceae) leaves on experimental gastric ulcer models. Biomed. Pharmacother. 2018, 97, 1109–1118. [Google Scholar]
- Luís, Â.; Gallardo, E.; Ramos, A.; Domingues, F. Design and characterization of bioactive bilayer films: Release kinetics of isopropyl palmitate. Antibiotics 2020, 9, 443. [Google Scholar] [CrossRef]
- Ya’ni, A.; Eldahshan, O.; Hassan, S.; Elwan, Z.; Ibrahim, H. Antidiabetic effects of essential oils of some selected medicinal Lamiaceae plants from Yemen against αglucosidase enzyme. J. Phytochem. Biochem. 2018, 2, 2. [Google Scholar]
- Tahir, H.U.; Sarfraz, R.A.; Ashraf, A.; Adil, S. Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int. J. Food Prop. 2016, 19, 2156–2164. [Google Scholar] [CrossRef]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In-vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ali, A.; Warsi, M.H.; Ahmad, W. Chemical characterization, antidiabetic and anticancer activities of Santolina chamaecyparissus. Saudi J. Biol. Sci. 2021, 12, 7–9. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Piozzi, F.; Arnold, N.A. Analysis of essential oils from Scutellaria orientalis ssp. alpina and S. utriculata by GC and GC-MS. Nat. Prod. Commun. 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Jelassi, A.; Hassine, M.; Besbes Hlila, M.; Ben Jannet, H. Chemical composition, antioxidant properties, α-glucosidase inhibitory, and antimicrobial activity of essential oils from Acacia mollissima and Acacia cyclops cultivated in Tunisia. Chem. Biodivers. 2017, 14, e1700252. [Google Scholar] [CrossRef] [PubMed]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential oil-bearing plants from Balkan Peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Santos, F.; Rao, V. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Kumar, N.R.; Reddy, J.S.; Gopikrishna, G.; Solomon, K.A. GC-MS determination of bioactive constituents of Cycas beddomei cones. Int. J. Pharm. Bio Sci. 2012, 3, 344–350. [Google Scholar]
- Whittle, B. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br. J. Pharmacol. Chemother. 1964, 22, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Quintao, N.L.; da Silva, G.F.; Antonialli, C.S.; Rocha, L.W.; Cechinel Filho, V.; Cicció, J.F. Chemical composition and evaluation of the anti-hypernociceptive effect of the essential oil extracted from the leaves of Ugni myricoides on inflammatory and neuropathic models of pain in mice. Planta Med. 2010, 76, 1411–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajhashemi, V.; Sajjadi, S.E.; Heshmati, M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J. Ethnopharmacol. 2009, 124, 475–480. [Google Scholar] [CrossRef]
- Akin-Osanaiye, C.; Gabriel, A.; Alebiosu, R. Characterization and antimicrobial screening of ethyl oleate isolated from Phyllanthus amarus (Schum and Thonn). Ann. Biol. Res. 2011, 2, 298–305. [Google Scholar]
- Kumar, R.S.; Anburaj, G.; Subramanian, A.; Vasantha, S.; Selvam, A.P. Preliminary phytochemical investigation, Antimicrobial activity and GC-MS analysis of leaf extract of Capparis zeylanica Linn. J. Pharm. Phytochem. 2019, 8, 1399–1405. [Google Scholar]
- Yin, H.; Bhattarai, J.P.; Oh, S.M.; Park, S.J.; Ahn, D.K.; Han, S.K. Baicalin activates glycine and γ-aminobutyric acid receptors on substantia gelatinosa neurons of the trigeminal subsnucleus caudalis in juvenile mice. Am. J. Chin. Med. 2016, 44, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.-X.; Ji, X.-Y.; Tan, B.-B.; Liang, Y.-Z.; Liang, N.-N.; Wang, X.-L.; Dai, H. Identification of the composition of fatty acids in Eucommia ulmoides seed oil by fraction chain length and mass spectrometry. Food Chem. 2010, 121, 815–819. [Google Scholar] [CrossRef]
- Aobuli, A.; Maitusong, J.; Bakri, M.; Lu, X.; Maiwulanjiang, M.; Aisa, H.A. The effect of volatile oil from Vernonia anthelmintica seeds on melanin synthesis in B16 cells and its chemical analysis by GC-QTOF-MS. Evid. Based Complement. Altern. Med. 2018, 2018, 6291281. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Kausar, N.; Ullah, S.; Khan, M.A.; Zafar, H.; Choudhary, M.I.; Yousuf, S. Celebrex derivatives: Synthesis, α-glucosidase inhibition, crystal structures, and molecular docking studies. Bioorg. Chem. 2021, 106, 104499. [Google Scholar] [CrossRef]
- Feldmann, H.; Aigle, M.; Aljinovic, G.; Andre, B.; Baclet, M.; Barthe, C.; Baur, A.; Becam, A.; Biteau, N.; Boles, E. Complete DNA sequence of yeast chromosome II. EMBO J. 1994, 13, 5795–5809. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.-Y.; Sali, A. Protein structure modeling with MODELLER. In Structural Proteomics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 145–159. [Google Scholar]
- Anderson, R.J.; Weng, Z.; Campbell, R.K.; Jiang, X. Main-chain conformational tendencies of amino acids. Proteins Struct. Funct. Bioinform. 2005, 60, 679–689. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
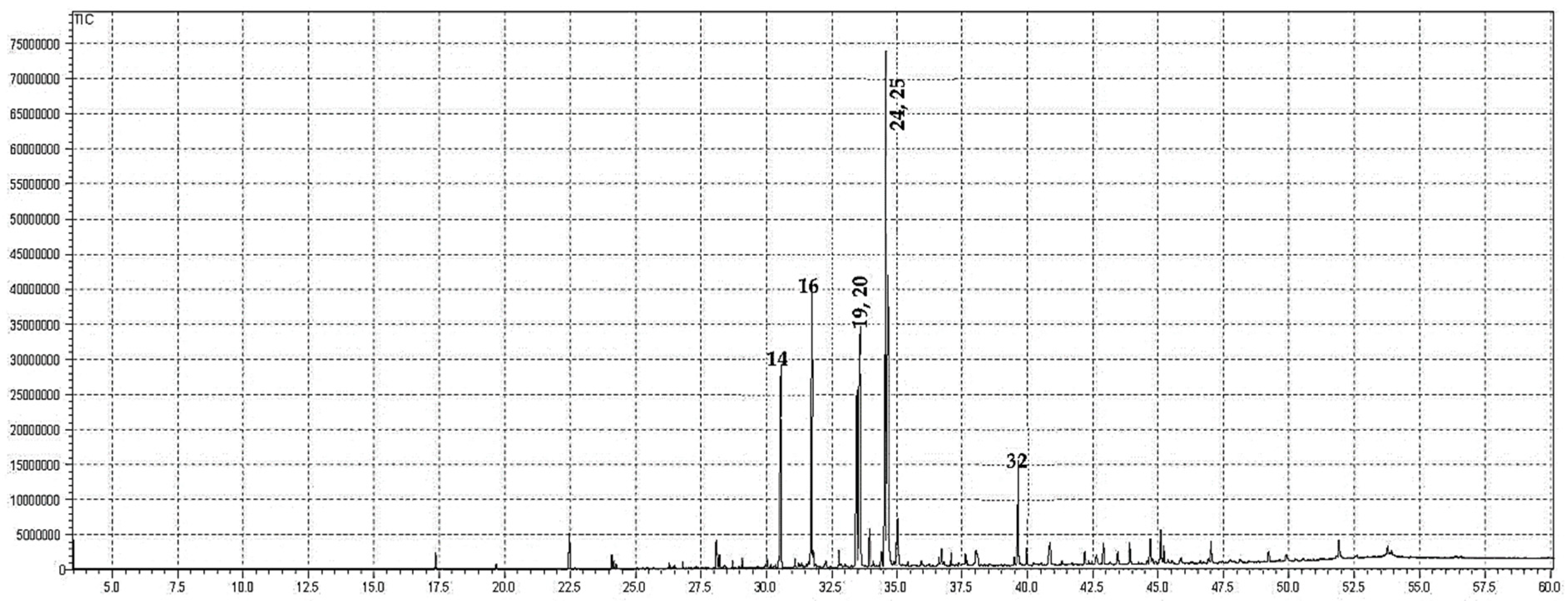




| Compounds | Contents (%) | RIcalc. | RIrep. |
|---|---|---|---|
| Thymol | 0.61 | 1295 | 1266 |
| 1-Tridecene | 0.18 | 1307 | 1287 |
| 2,4-Di-tert-butylphenol | 1.29 | 1518 | 1519 |
| Cetene | 0.47 | 1593 | 1587 |
| Dodecanoic acid, ethyl ester | 0.29 | 1596 | 1581 |
| Methyl tetradecanoate | 0.23 | 1727 | 1714 |
| 1-Octadecene | 1.01 | 1793 | 1788 |
| Myristic acid, ethyl ester | 0.47 | 1796 | 1778 |
| Octadecane | 0.52 | 1800 | 1800 |
| Isopropyl myristate | 0.26 | 1824 | 1827 |
| Hexahydrofarnesyl acetone | 0.34 | 1845 | 1842 |
| Pentadecanoic acid, ethyl ester | 0.11 | 1896 | 1878 |
| Methyl hexadec-9-enoate | 0.09 | 1908 | 1892 |
| Hexadecanoic acid, methyl ester | 7.16 | 1929 | 1908 |
| Ethyl 9-hexadecenoate | 0.126 | 1976 | 1955 |
| Palmitic acid, ethyl ester | 11.01 | 1997 | 1968 |
| Isopropyl palmitate | 0.13 | 2026 | 2011 |
| Heptadecanoic acid, methyl ester | 0.18 | 2029 | 2008 |
| Linoleic acid, methyl ester | 7.02 | 2099 | 2071 |
| Linolenic acid, methyl ester | 11.67 | 2107 | 2077 |
| Oleic acid, methyl ester | 0.38 | 2111 | 2082 |
| Methyl stearate | 1.41 | 2130 | 2133 |
| Dodecyl nonyl ether | 0.66 | 2158 | 2158 |
| Linoleic acid ethyl ester | 19.67 | 2168 | 2177 |
| Ethyl oleate | 18.45 | 2173 | 2171 |
| Hexadecanoic acid, butyl ester | 0.15 | 2189 | 2174 |
| Octadecanoic acid, ethyl ester | 2.33 | 2197 | 2181 |
| Heneicosane | 0.41 | 2100 | 2100 |
| Phytol, acetate | 0.13 | 2123 | 2222 |
| cis-11-Eicosenoic acid, methyl ester | 0.64 | 2306 | 2284 |
| Eicosanoic acid, methyl ester | 0.48 | 2331 | 2310 |
| 13-Docosenoic acid, methyl ester, (Z) | 3.98 | 2509 | 2473 |
| Docosanoic acid, methyl ester | 0.71 | 2533 | 2502 |
| Docosanoic acid, ethyl ester | 1.19 | 2598 | 2576 |
| Tricosanoic acid, methyl ester | 0.12 | 2634 | 2612 |
| 2-Methylhexacosane | 0.61 | 2700 | 2664 |
| 15-Tetracosenoic acid, methyl ester, (Z) | 0.23 | 2712 | 2709 |
| Tetracosanoic acid, methyl ester | 0.38 | 2735 | 2712 |
| (E)-3,7-Dimethylocta-2,6-dien-1-yl palmitate | 0.75 | 2756 | 2747 |
| Squalene | 0.82 | 2837 | 2808 |
| 1,6,10,14,18,22-Tetracosahexaen-3-ol, 2,6,10,15,19,23-hexamethyl-, (all-E)- | 0.14 | 2890 | 3059 |
| n-Nonacosane | 0.98 | 2900 | 2900 |
| Identified compounds | 97.79 |
| Strains | MIC (µg/mL) | MBC (µg/mL) | |
|---|---|---|---|
| Bacteria | K. pneumoniae | 100 | 150 |
| P. aeruginosa | 100 | 150 | |
| E. coli | 100 | 150 | |
| E. faecalis | 50 | 100 | |
| Fungi | C. albicans | 100 | 100 |
| F. oxysporum | 50 | 100 | |
| Model | MolPDF | DOPE Score |
|---|---|---|
| Model-1 | 3649.48315 | 72,730.43750 |
| Model-2 | 3525.77832 | 73,327.53906 |
| Model-3 | 3936.56714 | 72,413.56250 |
| Model-4 | 3989.72241 | 72,646.79688 |
| Model-5 | 3680.53467 | 72,542.36719 |
| S. No | Name | CID | Energies = Kcal/mol | ||
|---|---|---|---|---|---|
| Docking Score | Binding Energy | Binding Affinity | |||
| 1 | Dodecanoic acid, ethyl ester | 7800 | −6.27 | −39.87 | −8.33 |
| 2 | Linoleic acid ethyl ester | 5,282,184 | −5.89 | −50.55 | −9.74 |
| 3 | Hexadecanoic acid, methyl ester | 8181 | −5.28 | −41.29 | −8.88 |
| 4 | Isopropyl myristate | 8042 | −4.82 | −41.10 | −9.1 |
| 5 | Methyl tetradecanoate | 31,284 | −4.73 | −38.65 | −8.64 |
| 6 | Hexahydrofarnesyl acetone | 10,408 | −4.45 | −40.55 | −9.2 |
| 7 | Myristic acid, ethyl ester | 31,283 | −4.22 | −40.40 | −8.68 |
| 8 | Pentadecanoic acid, ethyl ester | 38,762 | −4.01 | −43.75 | −8.76 |
| 9 | Thymol | 6989 | −3.61 | −25.49 | −6.39 |
| 10 | Isopropyl palmitate | 8907 | −3.19 | −47.79 | −9.81 |
| 11 | α-D-glucose | 7,9025 | −3.66 | −33.49 | −6.35 |
| Changes in Paw Diameter (Mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Treatment | Conc. (mg/kg) | After 1 h | After 2 h | After 3 h | Aver. Measurement | % Inhibition |
| Inducer (Carrag) | 1 mL | 1.15 ± 0.01 | 1.39 ± 0.03 | 1.71 ± 0.03 | 1.41 ± 0.03 | |
| NS | 1 mL | 1.14 ± 0.03 | 1.39 ± 0.03 | 1.70 ± 0.03 | 1.41 ± 0.03 | - |
| DS | 10 | 0.54 ± 0.05 | 0.41 ± 0.03 | 0.29 ± 0.5 | 0.41 ± 0.02 | 70.92 |
| HEO | 5 | 0.79 ± 0.07 | 0.73 ± 0.01 | 0.67 ± 0.03 | 0.73 ± 0.05 * | 48.22 |
| 10 | 0.71 ± 0.04 | 0.59 ± 0.02 | 0.47 ± 0.06 | 0.59 ± 0.04 * | 58.15 | |
| 15 | 0.68 ± 0.06 | 0.56 ± 0.03 | 0.42 ± 0.01 | 0.55 ± 0.03 * | 61 | |
| Treatment | Dosage (mg/kg) | Counted Writhes Mean ± SEM | % Reduction (Writhes) |
|---|---|---|---|
| AA | 1 mL | 26.3 ± 0.05 | |
| NS | 1 mL | 26.1 ± 0.05 | - |
| Aspirin | 1 mL | 10.6 ± 0.03 | 59.69 |
| HEO | 5 | 19.3 ± 0.04 ** | 26.61 |
| 10 | 15.3 ± 0.05 ** | 41.82 | |
| 15 | 13.6 ± 0.02 ** | 48.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Murad, W.; Ur Rehman, N.; Mubin, S.; Al-Sabahi, J.N.; Ahmad, M.; Zahoor, M.; Ullah, O.; Waqas, M.; Ullah, S.; et al. GC-MS Analysis and Biomedical Therapy of Oil from n-Hexane Fraction of Scutellaria edelbergii Rech. f.: In Vitro, In Vivo, and In Silico Approach. Molecules 2021, 26, 7676. https://doi.org/10.3390/molecules26247676
Shah M, Murad W, Ur Rehman N, Mubin S, Al-Sabahi JN, Ahmad M, Zahoor M, Ullah O, Waqas M, Ullah S, et al. GC-MS Analysis and Biomedical Therapy of Oil from n-Hexane Fraction of Scutellaria edelbergii Rech. f.: In Vitro, In Vivo, and In Silico Approach. Molecules. 2021; 26(24):7676. https://doi.org/10.3390/molecules26247676
Chicago/Turabian StyleShah, Muddaser, Waheed Murad, Najeeb Ur Rehman, Sidra Mubin, Jamal Nasser Al-Sabahi, Manzoor Ahmad, Muhammad Zahoor, Obaid Ullah, Muhammad Waqas, Saeed Ullah, and et al. 2021. "GC-MS Analysis and Biomedical Therapy of Oil from n-Hexane Fraction of Scutellaria edelbergii Rech. f.: In Vitro, In Vivo, and In Silico Approach" Molecules 26, no. 24: 7676. https://doi.org/10.3390/molecules26247676
APA StyleShah, M., Murad, W., Ur Rehman, N., Mubin, S., Al-Sabahi, J. N., Ahmad, M., Zahoor, M., Ullah, O., Waqas, M., Ullah, S., Kamal, Z., Almeer, R., Bungau, S. G., & Al-Harrasi, A. (2021). GC-MS Analysis and Biomedical Therapy of Oil from n-Hexane Fraction of Scutellaria edelbergii Rech. f.: In Vitro, In Vivo, and In Silico Approach. Molecules, 26(24), 7676. https://doi.org/10.3390/molecules26247676










