Abstract
Objective: The use of platelet concentrates (PCs) in oral and maxillofacial surgery, periodontology, and craniofacial surgery has been reported. While PCs provide a rich reservoir of autologous bioactive growth factors for tissue regeneration, their drawbacks include lack of utility for long-term application, low elastic modulus and strength, and limited storage capability. These issues restrict their broader application. This review focuses on the lyophilization of PCs (LPCs) and how this processing approach affects their biological and mechanical properties for application as a bioactive scaffold for craniofacial tissue regeneration. Materials and Methods: A comprehensive search of five electronic databases, including Medline, PubMed, EMBASE, Web of Science, and Scopus, was conducted from 1946 until 2019 using a combination of search terms relating to this topic. Results: Ten manuscripts were identified as being relevant. The use of LPCs was mostly studied in in vitro and in vivo craniofacial bone regeneration models. Notably, one clinical study reported the utility of LPCs for guided bone regeneration prior to dental implant placement. Conclusions: Lyophilization can enhance the inherent characteristics of PCs and extends shelf-life, enable their use in emergency surgery, and improve storage and transportation capabilities. In light of this, further preclinical studies and clinical trials are required, as LPCs offer a potential approach for clinical application in craniofacial tissue regeneration.
1. Introduction
Platelet concentrates (PCs) typically refer to a group of materials produced from autologous blood designed to improve tissue regeneration [1]. PCs were initially created as fresh plasma preparations for autologous application as a regenerative scaffold to enable optimal growth factor release [2]. Several terms have been used for PCs according to their final form used, including plasma-enriched platelets (PEPs), platelet-rich plasma (PRP), platelet releasate (PR), preparation rich in growth factor (PRGF) [1], platelet-rich fibrin (PRF) [2], and concentrated growth factor (CGF) [3]. They provide the potential for simultaneous delivery of an abundance of bioactive molecules, including transforming growth factor beta-1 (TGF-β1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF) (Kobayashi et al., 2017). These PC preparations have been established as an affordable, safer and more potent way to release high concentrations of cocktails of growth factors locally for enhanced tissue regeneration [4].
Marx [5] published the initial report on the first-generation of PCs as PRPs. These PCs were used as bone substitutes to treat osseous defects in a variety of areas of dentistry, including oral and maxillofacial surgery, periodontology, and plastic surgery [5,6]. Despite their early promise, several drawbacks of PCs were identified mainly as a result of the inclusion of anticoagulants and bovine thrombin [7].
PRF is a second-generation PC, which offers the benefit of being synthesized without the need for anticoagulants or other artificial by-products that inhibit the coagulation cascade. PRF is autologous and naturally derived and meets all three essential tissue regeneration requirements including:
- (a)
- Provision of a three-dimensional fibrin scaffold;
- (b)
- Containing autologous cells;
- (c)
- Functioning as a store of endogenous growth factors for release for up to 14 days [8].
Notably, this PRF has been found to improve the deposition and regeneration of bone [9]. In this review, the term PCs will be used for all types of platelet concentrates, such as PRP, PRF, PRGF, CGF, etc.
PCs have gained popularity in dentistry and craniofacial surgery as a therapeutic alternative for tissue regeneration. Jeon [10] performed a bone regeneration study using PCs in twenty-four New Zealand White rabbit cranial defect models. The research showed that bone regeneration is higher in PCs classes (collagen sponges without PCs). They observed that the PC was efficacious in bone regeneration and could be used as an adjunct therapy for bone regeneration. Moreover, Anitua [1] presented the biocompatibility of PC preparation, as this method has encouraged clinical use in regenerative therapies, particularly in oral and maxillofacial surgery. They emphasized that the emergence and progress of novel biomaterials can revolutionize medicine. The combination of platelet-rich preparations and biomaterials has increased the therapeutic value of this biomaterial. PC extract can bridge the gap between scaffolds and cell biology, adding the biologic stimulus required for functional tissue regeneration. Furthermore, PCs also can be used in the treatment of recurrent ulcers, as the cavity can be filled with activated PCs. permitting the fibrin matrix to form inside the ulcer bed.
However, PCs were designed for immediate and short-term use (for up to 14-days); thus, this approach somewhat limits their therapeutic value [11,12]. As longer periods are reportedly required for the enhancement of bone regeneration, more effective methods for the delivery of growth factors over longer time-periods have been sought [13]. Furthermore, PCs are processed from autologous blood prior to clinical use, and, therefore, their bioactivity can vary between individuals. Their low viscosity, elastic modulus and strength are also issues, which mean PCs are technically challenging to handle in the clinical setting. Finally, due to the requirement for the need for their use within a short time-frame, as they cannot be easily stored or transported, their application is not well suited for emergency care medicine [14]. These issues restrict the use of PCs for broader therapeutic applications [15].
Notably, it has recently been reported that in addition to improved durability, lyophilized PC (LPC) formulations provide better handling, storage, and transport capabilities. They are, therefore, now being preferred for large-scale production [16]. Several researchers have specifically reported protocols to enable the generation of LPCs which exhibit longer-term survival and preservation characteristics [17,18,19,20]. This review reports on how lyophilization approaches affect the handling and biological properties of PCs and identify their potential for application in craniofacial tissue regenerative medicine.
2. Results and Discussion
2.1. Overview of Lyophilization for Platelet Concentrates
Lyophilization, or freeze-drying, has been developed as a method for transforming solutions containing labile substances into more stable solids to enable their distribution as well as preserving bioactivity for different applications. This process is widely used to enhance the stability and long-term storage of proteins in the pharmaceutical, biotechnology and food industries [16]. Indeed, freeze-drying offers storage and processing benefits over conventional methods, including (1) longer storage times at room temperature; (2) rapid transformation by rehydration, which enables practical application in emergency medicine; and (3) increased stability to enable transport and application at distant sites [21,22].
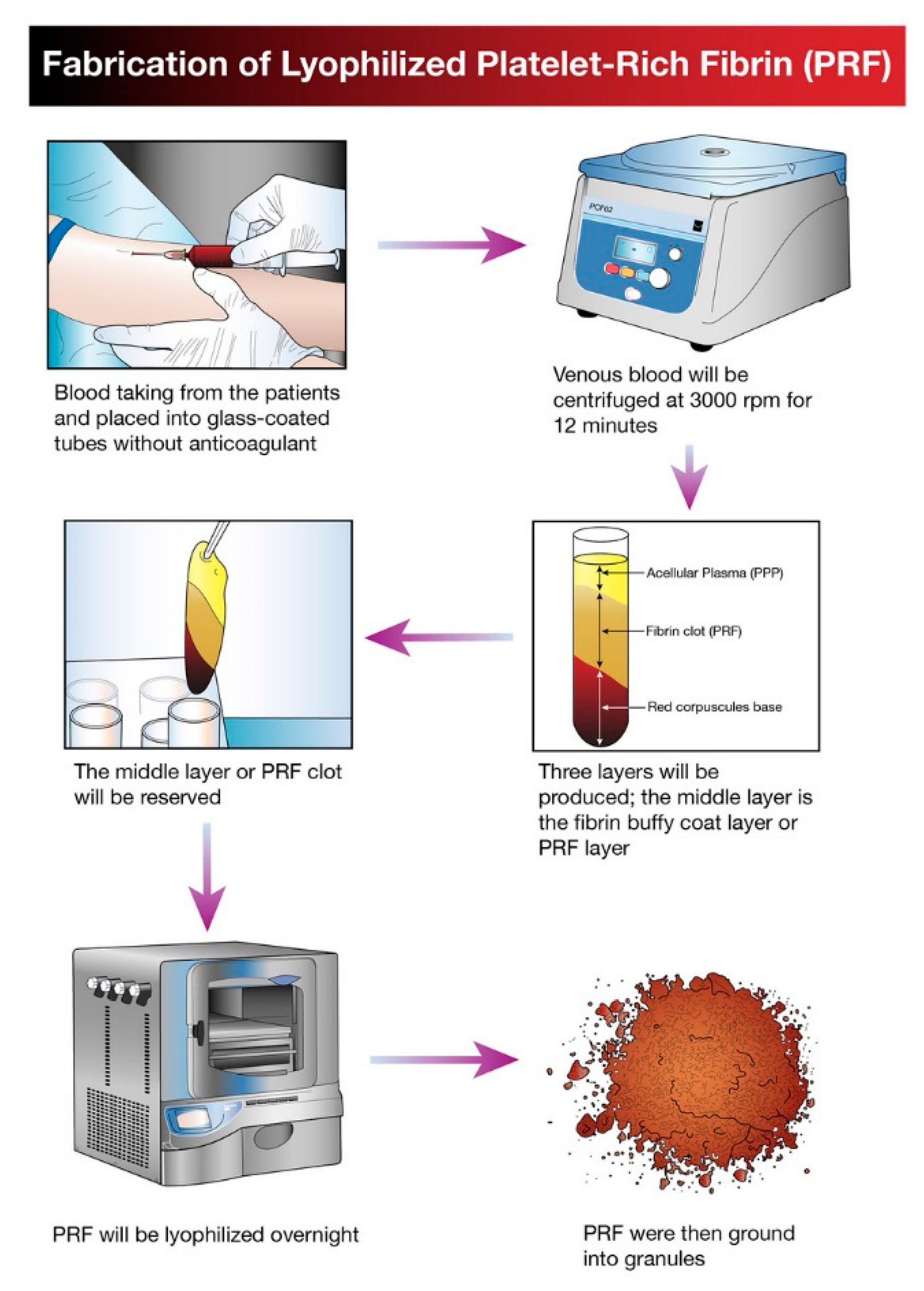
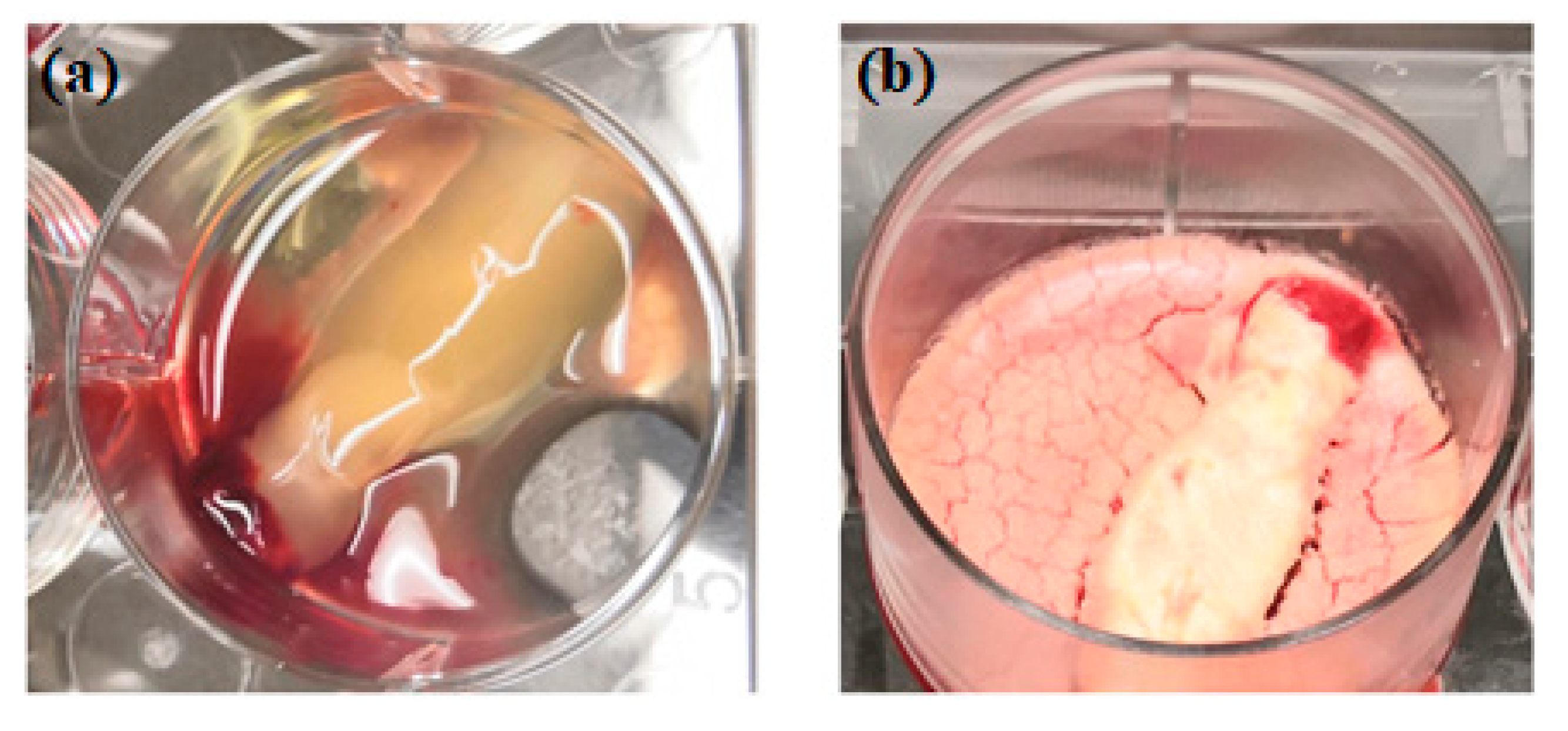
The idea of platelet lyophilization was originally proposed by Wolkers [23], who initially indicated that freeze-dried platelets functioned biologically similarly to endogenous platelets. Indeed, these lyophilized platelets not only exhibit increased storage stability but are also able to rapidly release bioactive growth factors, such as platelet-derived growth factor-BB (PDGF-BB), TGF-β1, and VEGF at the surgical site [24,25,26]. It does not have a detrimental effect on PCs’ ability to facilitate tissue regeneration, suggesting that a range of cytokines and fibrin networks in the PCs are preserved and have the capacity to promote chemotaxis and cell proliferation [27]. LPCs express their growth factors more slowly than PCs. This was discovered in a study by Zheng [13] when they attempted to combine nano-hydroxyapatite (nHA), L-lactic acid-co-glycolic acid (PLGA), and the hydrogel (nHA/PLGA/Gel) scaffold with LPCs. The in vitro release experiments showed that the composite scaffold allowed for gradual and continuous release of PRF-derived growth factors. They examine the pattern of growth factors released from the composite scaffolds over 12 weeks and illustrated that the concentration of IGF-I, TGF-β1, and PDGF-AB in the four weeks reached 66%, 67%, and 65%, respectively. It was demonstrated that there was a higher release rate in the first 4 weeks followed by a relatively steady rate over the following weeks. Figure 1 provides a schematic diagram of the method used for producing lyophilized PCs, and Figure 2 provides a physical comparison of PCs and lyophilized PCs.
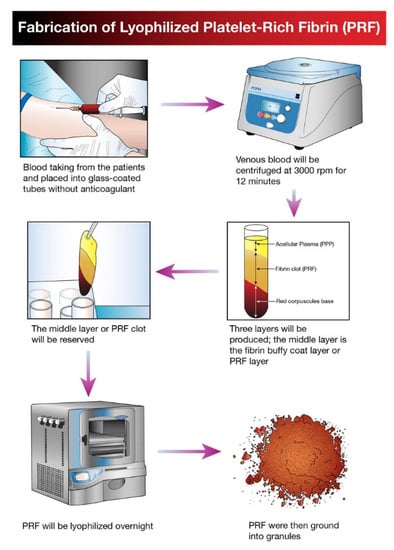
Figure 1.
Schematic diagram illustrating the processing and development of lyophilized platelet concentrate (LPC) in the form of platelet-rich fibrin (PRF).
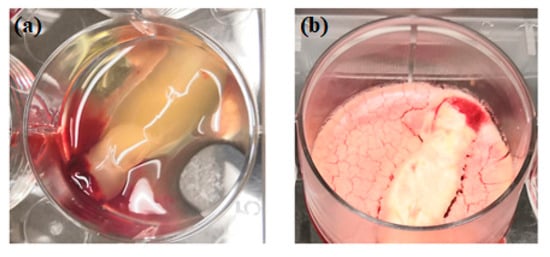
Figure 2.
Physical comparison of traditionally prepared platelet concentrates (a) versus lyophilized platelet concentrates (b).
The advantages of lyophilized platelet concentrates are further highlighted in a study that recently documented lyophilized PCs exhibiting enhanced osteogenic capability and improved tissue compatibility at the injury site, compared with freshly isolated PCs [17].
2.2. Lyophilized Platelet Concentrates in Craniofacial Tissue Regeneration
The use of PCs to deliver growth factors to defective areas to stimulate tissue regeneration in both the medical and dental fields has gained considerable interest over recent years. However, despite the considerable advantages offered by LPCs, only a limited number of studies, summarized below, have been reported. This review reports on the current application and potential of LPCs in craniofacial tissue regeneration. Table 1 provides details of studies reporting the different preparation methods of LPCs for potential use as craniofacial scaffolds for tissue regeneration.

Table 1.
Different preparation methods of lyophilized platelet concentrates as craniofacial bioactive scaffolds.
Despite its excellent safety and efficacy, PCs suffer from several significant disadvantages that limit their broader clinical applications. Current drawbacks include the processing requiring open membrane handling and a reduced elastic modulus that does not support suturing. Recent research has attempted to address these issues. One recent analysis compared the biological and mechanical properties of fresh, frozen, and lyophilized PC membranes produced by the traditional bottle-tube approach and those produced in a singular-syringe closed system. The data demonstrated that a relatively small number of mesenchymal stem cells could attach to fresh PC membranes compared with those that were frozen or lyophilized [32]. Furthermore, frozen and lyophilized PCs exhibited a more compact structure with an uneven texture compared with fresh PCs, and this may explain the differences observed in the properties of the tensile strength reported [32]. In 2017, Nakatani [24] performed a bone engineering study on mice calvarias using an LPC product. The researchers showed preservation of biological properties of LPCs even after exposure to lyophilization, as they observed that the sum of PDGF-BB and TGF-B1 was maintained by lyophilization. They concluded that the LPC can increase cranial bone regeneration similar to the process observed in traditionally prepared PCs. They postulated that the biological function of growth factor during the bone healing period was preserved.
Periodontitis is an important disease in dentistry that can result in both hard and soft tissue loss. Periodontal regeneration and healing can be enhanced by utilizing growth factors that modulate cellular responses, and, therefore, LPCs have been incorporated into chitosan hydrogel scaffolds containing encapsulated periodontal ligament stem cells (PDLSCs) [28]. PDLSC proliferation and migration rate increased dramatically with LPC inclusion as compared with fresh PC-containing hydrogel controls. This outcome was reportedly potentially due to both the increased pore diameter in the LPC containing scaffolds as well as enhanced growth factor release [17].
For several decades, barrier membranes have been used in oral and maxillofacial surgery. The most popular materials for the manufacture of these membranes are collagen, fresh PCs, and chitosan compounds. Recently, Ansarizadeh [29] reported the integration of chitosan and LPCs into a composite collagen membrane, and the results revealed that both the chitosan/collagen weight percentage and the concentration of LPCs enhanced mechanical strength and membrane biodegradability. Furthermore, increased cell proliferation, higher cell viability, and osteogenic differentiation were enabled by the incorporation of LPCs.
LPCs have also been assessed clinically to determine their applicability as a scaffolding material for other craniofacial tissue regeneration applications [27]. The data demonstrated that constructs exhibited a consistent fibrin structure with a significant leukocyte infiltrate. Transmission electron microscopy (TEM) studies verified the preservation of their potency in promoting tissue healing, even at 1-week following lyophilization at −196 °C. For the assessment of general healing at the surgical site, color, swelling, bleeding, and post-operative pain were assessed to determine the effect of the LPCs. No apparent differences were identified compared with the control group, which was composed of freshly isolated PCs [27]. Consequently, the authors concluded that the LPC constructs promoted bone regeneration and that they also promoted chemotaxis and proliferation of neighboring osteoblast progenitors similarly to the fresh PC isolate control.
Thus far, there have been only a relatively small number of studies analyzing the effects of PCs for pulp regenerative therapies. Two recent reports have, however, aimed at determining the potential application of LPCs in regenerative endodontic procedures [33,34]. The authors reported that the lyophilization approach still enabled comprehensive local release of cytokines. Xu [34], conducting a study on beagle dogs, showed that LPC application was capable of inhibiting the release of pro-inflammatory cytokines while promoting proliferation, migration, and odonto-/osteo-genic differentiation of human dental stem pulp cells (hDPSCs). Furthermore, LPCs also induced dentine–pulp complex regeneration as well as enabling continued root growth in the immature teeth treated [34].
In contrast, a recent study [33] reported that both LPCs and fresh PCs inhibited the mineralization potential during the first week of application. The authors speculated that the stem cells from the apical papilla (SCAP) used in the study were unique compared to other types of mesenchymal stem cells. This is because SCAPs have been certified to regenerate into vascularized dentine–pulp complex in vivo, and it is a stem cell niche that is known to be a source of odontoblasts during dental development. Notably, however, this study still highlighted the potential for application of LPCs in dental tissue engineering, particularly for the treatment of pulp necrosis and periapical periodontitis in immature teeth.
2.2.1. Craniofacial Wound Healing
The interest in the application of tissue engineering and PC strategies for craniofacial wound repair has increased in interest in recent years. Nakajima [35] published a report on a LPC hybrid scaffold preparation using a polyglactin mesh for use as a wound dressing. One of its drawbacks, however, was the increased host inflammatory response that occurred due to the polyglactin degradation. Consequently, the use of a collagen sponge as a carrier for the LPCs has been explored [14]. It was found that the LPC coating improved the collagen sponge’s ability to attract and become infiltrated by fibroblasts, as well as promoting neo-angiogenesis in the surrounding tissue. The data indicated that the LPC-coated collagen sponge exhibited enhanced wound healing and regenerative capacity by stimulation of angiogenesis and infiltration of cells from surrounding tissue without causing a substantial inflammatory response. Furthermore, in the LPC-coated collagen sponge group, they identified considerably thicker capillary blood vessels compared to the noncoated sponge group at 4 weeks and 12 weeks post-implantation. This result is in agreement with Pietramaggiori [36], who noted that LPCs facilitated wound healing in a chronic wound model developed in diabetic mice. Interestingly, in contrast to the nontreated group, this study revealed a significant (p < 0.01) fivefold increase in blood vessel density in the LPC group. Recently, Xu et al. [15] suggested that the vascularization process was accelerated in their skin wound study in mice after using a scaffold composed of polyvinyl alcohol (PVA) hydrogel and LPCs as a dressing within 9 days post-surgery. Hence, they believe the neo-angiogenesis could provide oxygen and nutrient to the surgical site, thus promoting protein synthesis.
2.2.2. Lyophilized Platelet Concentrates as a Craniofacial Bioactive Scaffold
Guided tissue repair approaches are dependent upon growth factor activity. Incorporating PCs into a carrier system, such as a 3D scaffold, can enable controlled release of these molecules as well as enhancing their bioavailability. Kutlu [31] demonstrated that a chitosan scaffold loaded with PCs provided an excellent tool for multiple concurrent releases of PC-derived growth factors, and hence recommended its use in tissue repair purposes. Further research has assessed the applicability of LPCs as a scaffold for regeneration of craniofacial tissue and compared their biological effects with fresh PCs [17]. Dental follicle cells, periodontal progenitor cells, and alveolar bone cells were used in comparisons of their cellular activity in response to fresh and lyophilized PCs. The data indicated that LPCs exhibited superior effects compared with fresh PCs as a scaffolding material in terms of induction of proliferation, osteogenic differentiation, and tissue integration [17]. The authors concluded that the LPC preparations not only increased the capacity of cells to migrate and proliferate within the scaffold due to an increased pore size, but the construct also enabled a gradual release of growth factors, such as TGF-β1, PDGF, and VEGF from the biomaterial surface. Similarly, Liu [37] more recently reported that the lyophilization process enhanced the fibrin and platelet structures, enabling improved bioactivity.
As LPCs reportedly continuously release bone regenerating growth factors [13], Li and colleagues [30] compared the utility of 3D-printed polycaprolactone (PCL) scaffolds containing fresh PCs and LPCs for bone repair. The LPC–PCL scaffold exhibited superior stimulation of bone growth as compared with the conventional PC–PCL scaffold. Furthermore, their results indicated that in vivo, mineralization and osteogenesis could be promoted by coating 3D-printed PCL scaffolds with LPCs. The authors postulated that this outcome could be directly linked to the sustained release of PC-derived growth factors, including VEGF, PDGF, basic fibroblast growth factor (bFGF), TGF-β1, EGF, and IGF-1 [30]. Table 2 summarizes the in vitro, in vivo and one randomized clinical trial application of LPCs to enhance tissue regeneration. Table 3 summarises the strengths and limitations of LPC.

Table 2.
Summary findings of in-vitro, in-vivo and clinical trial studies using lyophilized platelet concentrates (LPCs).

Table 3.
Strengths and limitations of the lyophilized platelet concentrate (LPC) [38].
2.2.3. Limitations of the Study
The dynamic biology of PCs and their role in tissue regeneration and inflammation have provided the basis for PC and LPC therapies for a wide variety of medical and dental treatments. However, scientific advancements remain hindered by the lack of standardization of PC and LPC products, doses, and fabrication protocols [38]. The situation continues to be ambiguous as the different approaches and materials used do not seem to produce the same material as original PCs. Moreover, heterogeneous processing methods, unstandardized nomenclature, and vague classifications complicate comparisons among studies; thus, we recommend a comprehensive, detailed and step-by-step explanation of the preparation procedure for the LPC to allow for comparison between studies, ensuring reproducibility to prevent misunderstanding and misleading assumptions in the literature (Table 3) [38,39,40,41].
3. Materials and Methods
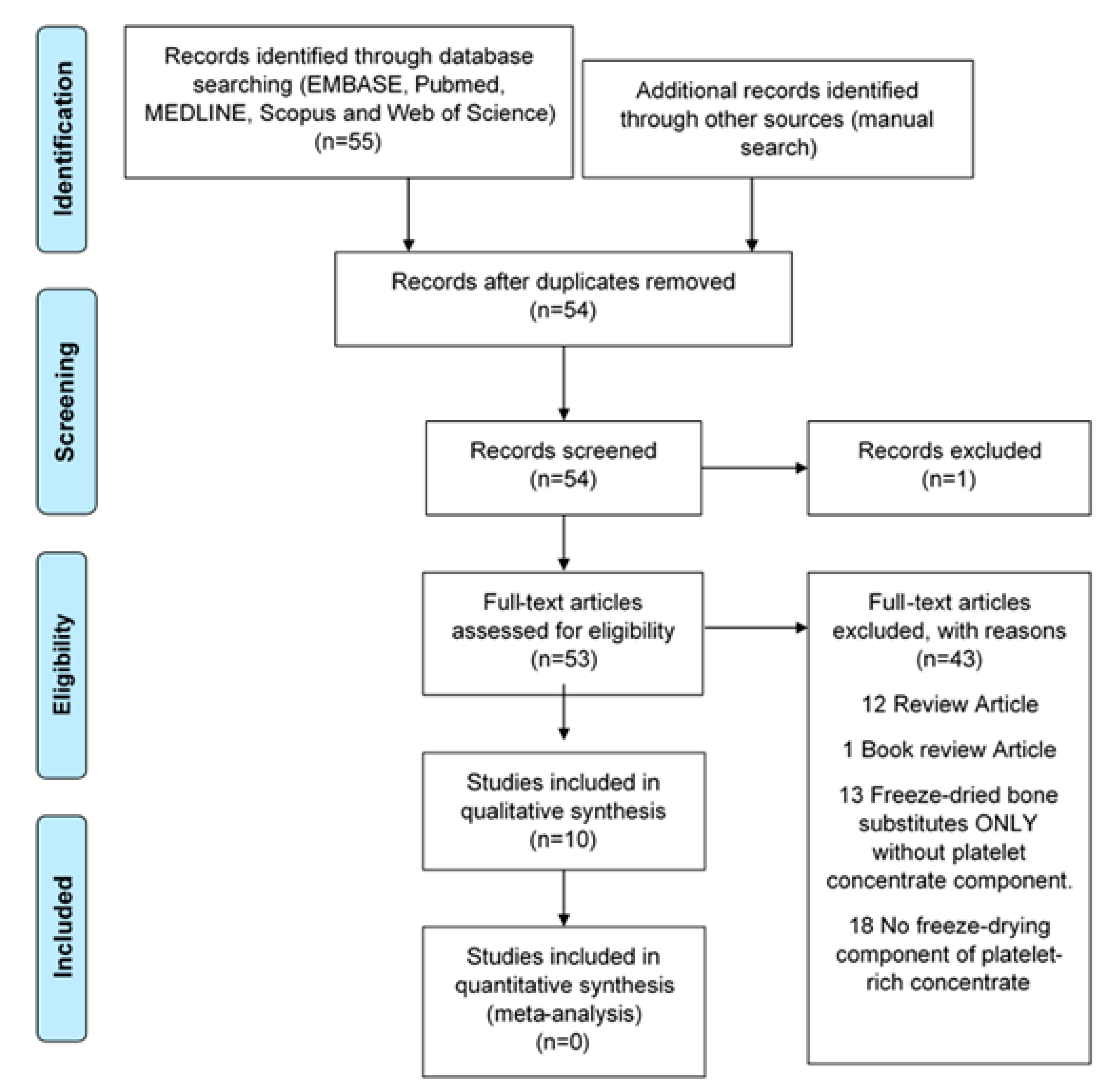
The relevant literature was searched for electronically up to 30 September 2019 to identify reports evaluating LPCs in EMBASE, PubMed, MEDLINE, Scopus, and the Web of Science. The following keywords were used for MEDLINE and EMBASE as MESH terms or free words: (Platelet-rich plasma OR platelet-rich fibrin OR platelet concentrate* OR concentrated growth factor*) AND (Freeze-drying OR lyophilisation OR lyophilization) AND (Craniofacial OR facial bone* OR face* OR oral OR dentist*) AND (Bone regeneration OR tissue regeneration OR tissue engineering). Publications were identified from citations of selected papers to improve search sensitivity. Only studies published using the English language were included, and duplicates were eliminated (Figure 3).
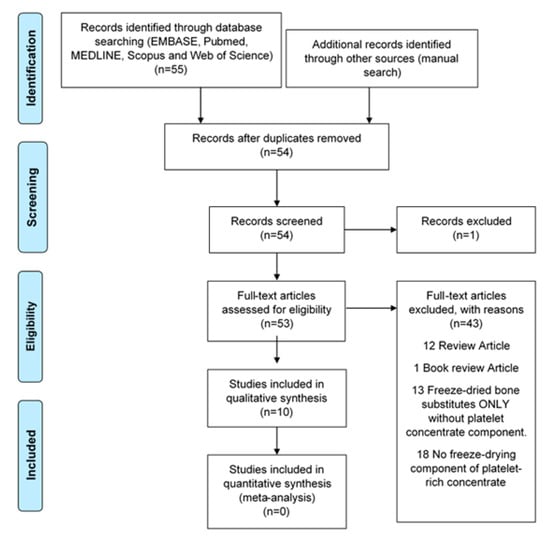
Figure 3.
Flowchart of manuscript screening process and inclusion in the review.
Selected research papers describing (1) the outcomes of lyophilization of PCs; (2) the preservation status of growth factors after the lyophilization process; (3) the biological properties of LPCs in in vitro and in vivo models; and (4) novel directions for LPC research in craniofacial regeneration were included.
The information provided in this review indicates that lyophilization provides a potential benefit for PCs for use in craniofacial tissue regeneration approaches. This is due to the increased preservation of growth factor bioactivity following freeze-drying due to its transformation of the fibrin and PC structure. Combined, these characteristics of LPCs for use in clinical practice extend shelf-life, enable emergency medicine usage, improve sample storage, and facilitate transportation management. However, despite these improvements, research is still required to improve the mechanical properties of LPCs as well as providing efficient delivery and release systems.
Nevertheless, the drawbacks of lyophilization include fabrication cost and the possibility of LPCs contamination. Additionally, a standardization protocol of all PC products has to be addressed, including standardizing the temperature, duration of lyophilization, and storage of the material [37]. In light of this, further preclinical studies and clinical trials are still required to enable LPCs to be routinely applied in craniofacial tissue regenerative therapies.
Author Contributions
All authors contributed extensively to the work presented in this paper. Conceptualization, N.A.N., J.R. and H.M.H.; methodology, N.A.N., S.N.F.M.N., P.R.C. and J.R.; validation, N.A.N., D.C.T. and J.R.; formal analysis, N.A.N., J.R., P.R.C. and H.M.H.; resources, D.C.T., G.J.D. and H.M.H.; writing—original draft preparation, N.A.N., J.R., P.R.C. and H.M.H.; writing—review and editing, J.R., P.R.C., G.J.D., S.N.F.M.N. and H.M.H. visualization, J.R. and P.R.C.; supervision, H.M.H., J.R., P.R.C., D.C.T. and G.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Malaysian government and Universiti Teknologi MARA for scholarship funding of this project. We acknowledge the John Walsh Research Institute, Faculty of Dentistry, University of Otago for their support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Trish Leishman; Subject Librarian, Dentistry, Information Services, University of Otago Library for her expert help and assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Anitua, E.; Sánchez, M.; Orive, G.; Andía, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ai, H. Concentrated growth factor promotes proliferation, osteogenic differentiation, and angiogenic potential of rabbit periosteum-derived cells in vitro. J. Orthop. Surg. Res. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical ap-plications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor en-hancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Huber, S.C.; Cunha, J.L.; Montalvao, S.A.L.; da Silva, L.Q.; Paffaro, A.U.; da Silva, F.A.R.; Rodrigues, B.L.; Santos Duarte Lana, J.F.; Annichino-Bizzacchi, J.M. In vitro study of the role of thrombin in platelet rich plasma (PRP) preparation: Utility for gel formation and impact in growth factors release. J. Stem Cells Regen. Med. 2016, 12, 2–9. [Google Scholar]
- Miron, R.J.; Choukroun, J. Platelet Rich Fibrin in Regenerative Dentistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Kim, M.J.; Kim, Y.O.; Roh, T.S.; Lee, W.J.; Kang, E.H.; Yun, I.S. Scaffold Free Bone Regeneration Using Platelet-Rich Fibrin in Calvarial Defect Model. J. Craniofac. Surg. 2018, 29, 251–254. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Diss, A.; Odin, G.; Doglioli, P.; Hippolyte, M.-P.; Charrier, J.-B. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 341–352. [Google Scholar] [CrossRef]
- Yung, Y.-L.; Fu, S.-C.; Cheuk, Y.-C.; Qin, L.; Ong, M.T.-Y.; Chan, K.-M.; Yung, P.S. Optimisation of platelet concentrates therapy: Composition, localisation, and duration of action. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, L.; Qin, J.; Sun, X.; Yang, T.; Ni, Y.; Zhou, Y. New Biodegradable Implant Material Containing Hydrogel with Growth Factors of Lyophilized PRF in Combination with an nHA/PLGA Scaffold. J. Hard Tissue Biol. 2015, 24, 54–60. [Google Scholar] [CrossRef]
- Horimizu, M.; Kawase, T.; Nakajima, Y.; Okuda, K.; Nagata, M.; Wolff, L.F.; Yoshie, H. An improved freeze-dried PRP-coated biode-gradable material suitable for connective tissue regenerative therapy. Cryobiology 2013, 66, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, D.; Dai, T.; Xu, H.; An, R.; Liu, Y.; Liu, B. Effects of incorporation of granule-lyophilised platelet-rich fibrin into pol-yvinyl alcohol hydrogel on wound healing. Sci. Rep. 2018, 8, 14042. [Google Scholar] [CrossRef]
- Nireesha, G.; Divya, L.; Sowmya, C.; Venkateshan, N.; Babu, M.; Lavakumar, V. Lyophilization/Freeze Drying—A Review. Int. J. Nov. Trends Pharm. Sci. 2013, 3, 87–98. [Google Scholar]
- Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang-Boquiren, M.T.; Gajendrareddy, P.; et al. Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef]
- Pan, L.; Yong, Z.; Yuk, K.S.; Hoon, K.Y.; Yuedong, S.; Xu, J. Growth Factor Release from Lyophilized Porcine Platelet-Rich Plasma: Quantitative Analysis and Implications for Clinical Applications. Aesthetic Plast. Surg. 2015, 40, 157–163. [Google Scholar] [CrossRef]
- Valeri, C.R.; Feingold, H.; Marchionni, L.D. A Simple Method for Freezing Human Platelets Using 6% Dimethylsulfoxide and Storage at −80 °C. Blood 1974, 43, 131–136. [Google Scholar] [CrossRef]
- Read, M.S.; Reddick, R.L.; Bode, A.P.; Bellinger, D.A.; Nichols, T.C.; Taylor, K.; Smith, S.V.; McMahon, D.K.; Griggs, T.R.; Brinkhous, K.M. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: Potential for long-term storage of dried platelets for transfusion. Proc. Natl. Acad. Sci. USA 1995, 92, 397–401. [Google Scholar] [CrossRef]
- Fan, J.-L.; Xu, X.-G.; Zhang, S.-Z.; Zhu, F.-M.; Chen, G.-M.; Yan, L.-X. Experimental study on rehydration conditions of freeze-dried platelets. J. Zhejiang Univ. Sci. A 2009, 10, 697–703. [Google Scholar] [CrossRef]
- Muraglia, A.; Ottonello, C.; Spanò, R.; Dozin, B.; Strada, P.; Grandizio, M.; Cancedda, R.; Mastrogiacomo, M. Biological activity of a standardized freeze-dried platelet derivative to be used as cell culture medium supplement. Platelets 2014, 25, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Walker, N.J.; Tablin, F.; Crowe, J.H. Human Platelets Loaded with Trehalose Survive Freeze-Drying. Cryobiology 2001, 42, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Agata, H.; Sumita, Y.; Koga, T.; Asahina, I. Efficacy of freeze-dried platelet-rich plasma in bone engineering. Arch. Oral Biol. 2017, 73, 172–178. [Google Scholar] [CrossRef]
- Sum, R.; Hager, S.; Pietramaggiori, G.; Orgill, D.P.; Dee, J.; Rudolph, A.; Orser, C.; Fitzpatrick, G.M.; Ho, D. Wound-healing properties of trehalose-stabilized freeze-dried outdated platelets. Transfusion 2007, 47, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Vocetkova, K.; Buzgo, M.; Sovkova, V.; Rampichová, M.; Staffa, A.; Filova, E.; Lukasova, V.; Doupnik, M.; Fiori, F.; Amler, E. A comparison of high throughput core–shell 2D electrospinning and 3D centrifugal spinning techniques to produce platelet lyophilisate-loaded fibrous scaffolds and their effects on skin cells. RSC Adv. 2017, 7, 53706–53719. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, X.; Luo, X.; Li, D.; Wang, H.; Li, T. Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin. Implant. Dent. Relat. Res. 2017, 19, 466–477. [Google Scholar] [CrossRef]
- Ammar, M.M.; Waly, G.H.; Saniour, S.H.; Moussa, T.A. Growth factor release and enhanced encapsulated periodontal stem cells viability by freeze-dried platelet concentrate loaded thermo-sensitive hydrogel for periodontal regeneration. Saudi Dent. J. 2018, 30, 355–364. [Google Scholar] [CrossRef]
- Ansarizadeh, M.; Mashayekhan, S.; Saadatmand, M. Fabrication, modeling and optimization of lyophilized advanced platelet rich fibrin in combination with collagen-chitosan as a guided bone regeneration membrane. Int. J. Biol. Macromol. 2019, 125, 383–391. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef]
- Kutlu, B.; Aydın, R.S.T.; Akman, A.C.; Gümüşderelioğlu, M.; Nohutcu, R.M. Platelet-rich plasma-loaded chitosan scaffolds: Preparation and growth factor release kinetics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 101, 28–35. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Jiang, B. A Comparative Evaluation of Concentrated Growth Factor and Platelet-rich Fibrin on the Proliferation, Migration, and Differentiation of Human Stem Cells of the Apical Papilla. J. Endod. 2018, 44, 977–983. [Google Scholar] [CrossRef]
- Xu, F.; Qiao, L.; Zhao, Y.; Chen, W.; Hong, S.; Pan, J.; Jiang, B. The potential application of concentrated growth factor in pulp re-generation: An in vitro and in vivo study. Stem Cell Res. Ther. 2019, 10, 134. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kawase, T.; Kobayashi, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. Bioactivity of freeze-dried platelet-rich plasma in an adsorbed form on a biodegradable polymer material. Platelets 2012, 23, 594–603. [Google Scholar] [CrossRef]
- Pietramaggiori, G.; Kaipainen, A.; Czeczuga, J.M.; Christopher, T.W.; Orgill, D.P. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006, 14, 573–580. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, H.; Xie, Q.; Jiang, Z.; Guo, S.; Li, Y.; Zhang, B. Controlled Release Strategies for the Combination of Fresh and Lyophilized Platelet-Rich Fibrin on Bone Tissue Regeneration. BioMed Res. Int. 2019, 2019, 4923767. [Google Scholar] [CrossRef]
- Andia, I.; Perez-Valle, A.; Del Amo, C.; Maffulli, N. Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. Int. J. Mol. Sci. 2020, 21, 6904. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2017, 29, 171–184. [Google Scholar] [CrossRef]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of relative centrifugal forces in studies related to plate-let-rich fibrin. J. Periodontol. 2019, 90, 817–820. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. Am. 2017, 99, 1769–1979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).