The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging
Abstract
:1. Introduction
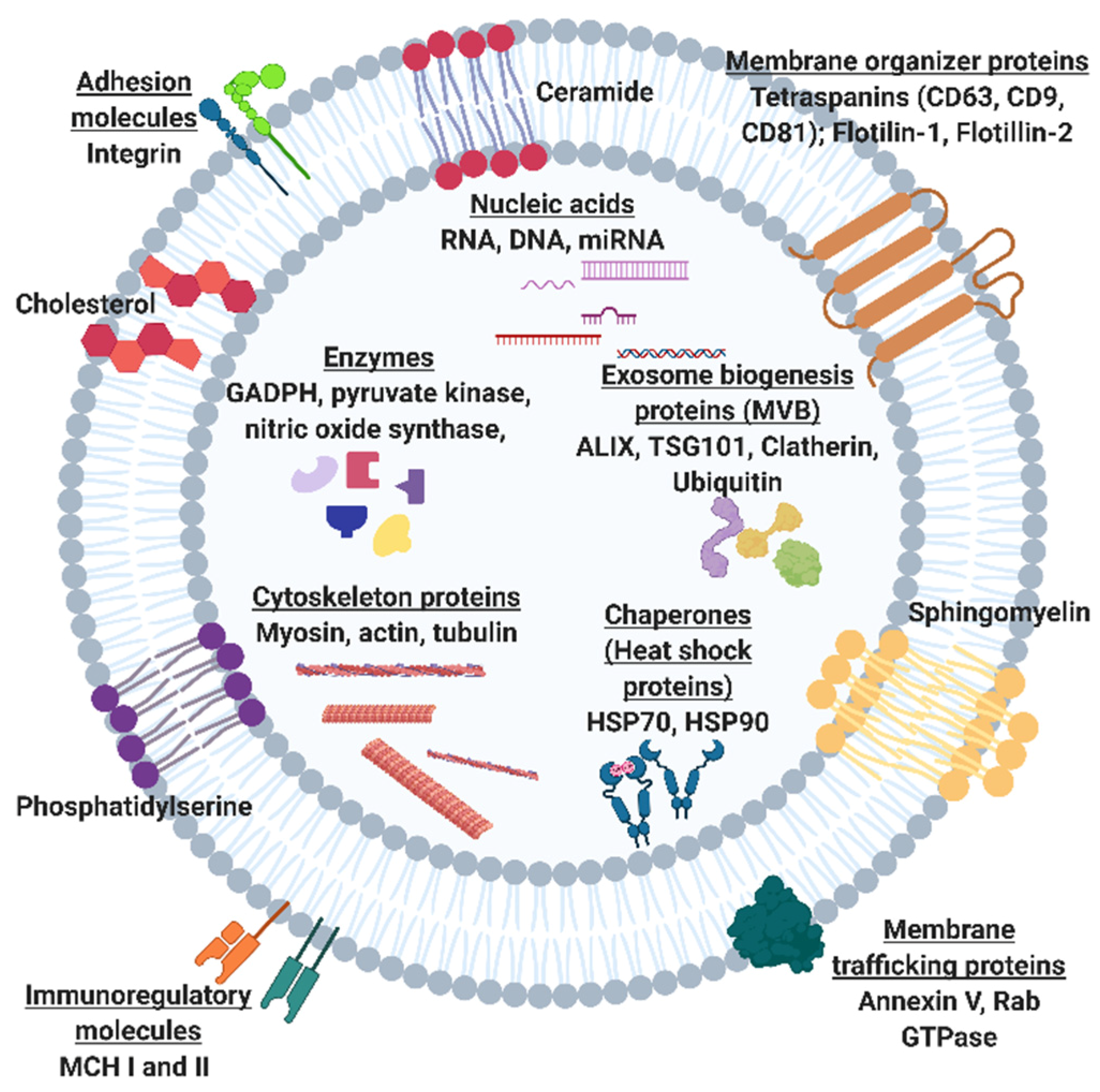
2. The Potential of Exosomal Biomarkers for Precision Medicine and Liquid Biopsies
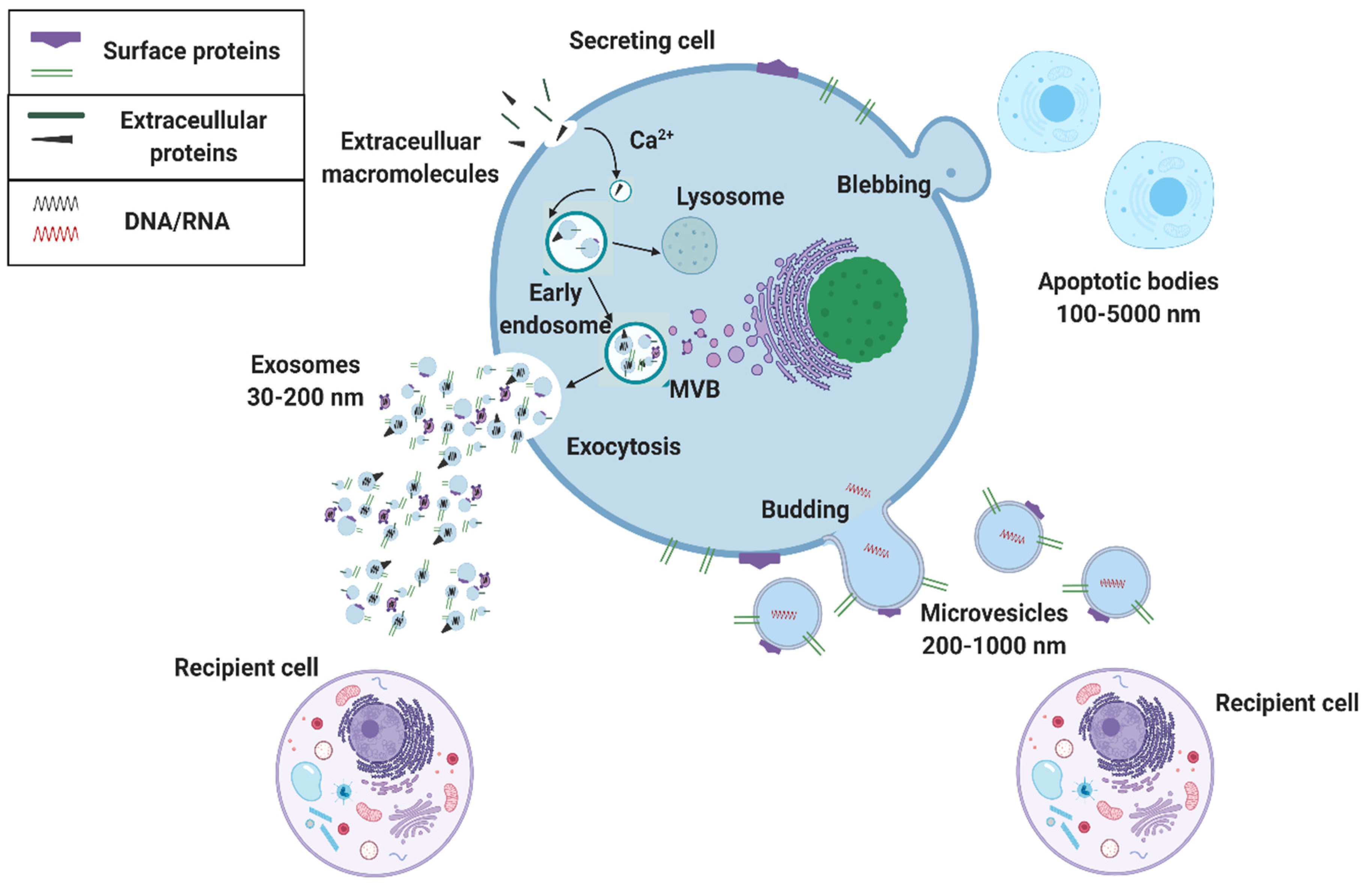
3. Microfluidic Solutions for Exosome Isolations
3.1. Field-Based Isolation of Exosomes
3.2. Surface-Based Isolation of Exosomes
4. Exosomal Detection Systems to Monitor Age-Associated Pathologies
4.1. Technologies for Profiling of Antigen-Specific Exosomal Biomarkers
4.1.1. Immunoassay-Based Technologies
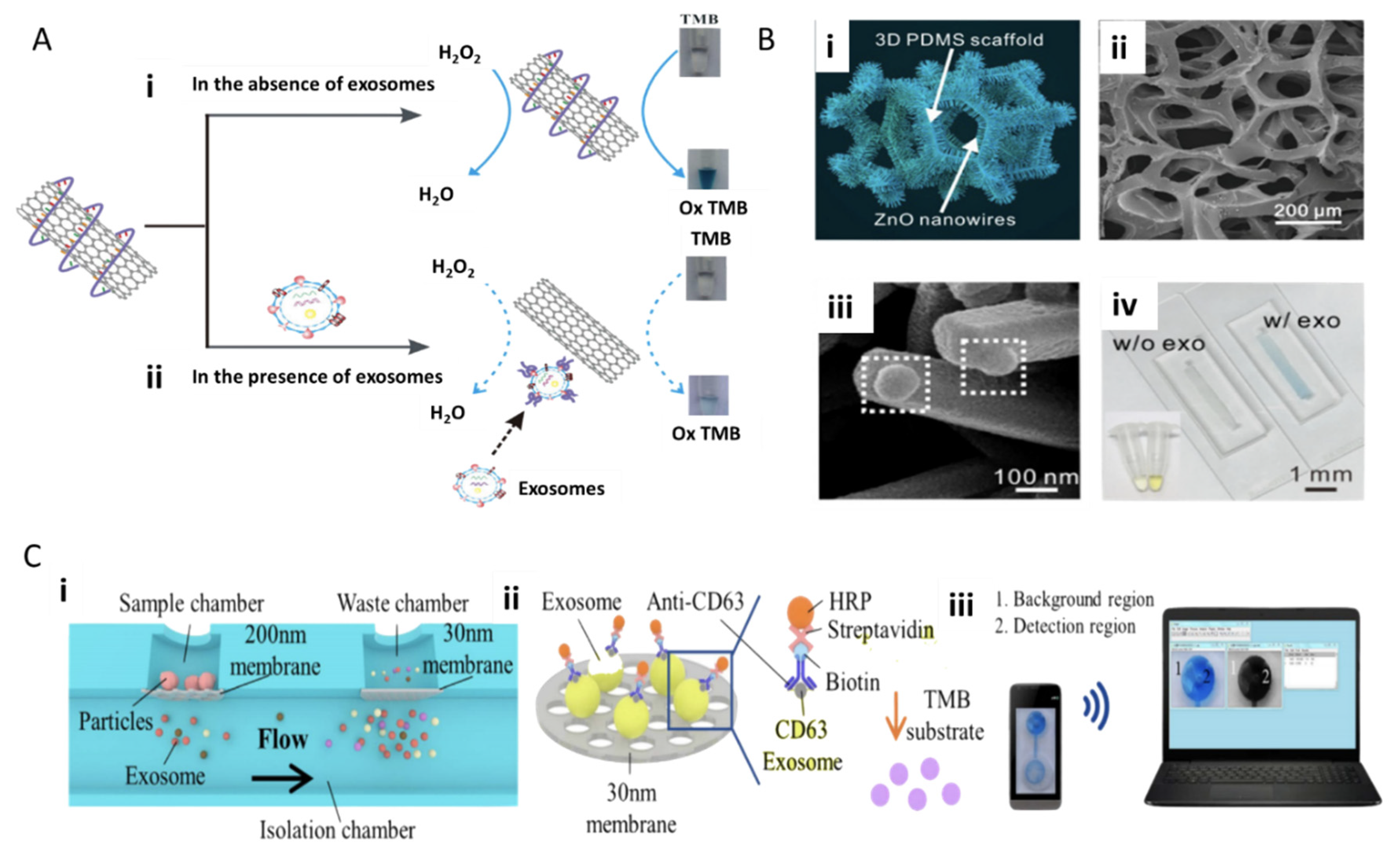
4.1.2. Fluorescence and Field-Based Technologies
4.2. Microfluidic Approaches for Screening Neurotoxic Biomarkers
4.2.1. Microfluidic Detection of Alzheimer’s Disease Biomarkers: Tau Protein and Amyloid-Beta
4.2.2. Opportunities to Develop Technologies for Profiling of Exosomal Cargo Biomarkers
5. Challenges to Commercialization
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.J.; Shin, Y.; Chung, S.; Hwang, D.W.; Lee, D.S. Convective Exosome-Tracing Microfluidics for Analysis of Cell-Non-Autonomous Neurogenesis. Biomaterials 2017, 112, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. CtDNA and CTCs in Liquid Biopsy – Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Lowes, L.E.; Bratman, S.V.; Dittamore, R.; Done, S.; Kelley, S.O.; Mai, S.; Morin, R.D.; Wyatt, A.W.; Allan, A.L. Circulating Tumor Cells (CTC) and Cell-Free DNA (CfDNA) Workshop 2016: Scientific Opportunities and Logistics for Cancer Clinical Trial Incorporation. Int. J. Mol. Sci. 2016, 17, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, Gina Exosomes: Promising Targets for Liquid Biopsy. Available online: http://www.biocompare.com/Editorial-Articles/357781-Exosomes-Promising-Targets-for-Liquid-Biopsy/ (accessed on 19 May 2020).
- Lang, J.E. Advantages and Disadvantages of CtDNA vs CTC Assays: How to Move the Needle Forward towards Clinical Application; Keck Medicine of USC: Los Angeles, CA, USA, 2007. [Google Scholar]
- Contreras-Naranjo, J.C.; Wu, H.-J.; Ugaz, V.M. Microfluidics for Exosome Isolation and Analysis: Enabling Liquid Biopsy for Personalized Medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- De Vrij, J.; Maas, S.L.N.; van Nispen, M.; Sena-Esteves, M.; Limpens, R.W.A.; Koster, A.J.; Leenstra, S.; Lamfers, M.L.; Broekman, M.L.D. Quantification of Nanosized Extracellular Membrane Vesicles with Scanning Ion Occlusion Sensing. Nanomed. (Lond.) 2013, 8, 1443–1458. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Heon, M.; Zhao, Z.; He, M. Microfluidic Engineering of Exosomes: Editing Cellular Messages for Precision Therapeutics. Lab Chip 2018, 18, 1690–1703. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of Exosomes from Whole Blood by Integrating Acoustics and Microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Vassalli, G. Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Bianco, M.; Nigro, A.; Primiceri, E.; Ferrara, F.; Romano, A.; Quattrini, A.; Furlan, R.; Arima, V.; Maruccio, G. Lab-on-Chip for Exosomes and Microvesicles Detection and Characterization. Sensors 2018, 18, 3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cell Guidance Systems Exospin: Trusted Exosome Purification. Available online: https://www.cellgs.com/products/exo-spinand8482-midi-columns.html (accessed on 16 December 2020).
- System Biosciences ExoQuick Exosome Preciptiation Solution. Available online: https://systembio.com/wp-content/uploads/MANUAL_EXOQXXA-1-1.pdf (accessed on 16 December 2020).
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of Membrane Affinity-Based Method with Size-Exclusion Chromatography for Isolation of Exosome-like Vesicles from Human Plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of Distinct Nanoparticles and Subsets of Extracellular Vesicles by Asymmetric Flow Field-Flow Fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Eskelin, K.; Poranen, M.M.; Oksanen, H.M. Asymmetrical Flow Field-Flow Fractionation on Virus and Virus-Like Particle Applications. Microorganisms 2019, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Qiu, G.; Ng, S.-P.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.-M.L. Direct Detection of Two Different Tumor-Derived Extracellular Vesicles by SAM-AuNIs LSPR Biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef]
- Dudani, J.S.; Gossett, D.R.; Tse, H.T.K.; Lamm, R.J.; Kulkarni, R.P.; Carlo, D.D. Rapid Inertial Solution Exchange for Enrichment and Flow Cytometric Detection of Microvesicles. Biomicrofluidics 2015, 9, 014112. [Google Scholar] [CrossRef] [Green Version]
- Gossett, D.R.; Tse, H.T.K.; Dudani, J.S.; Goda, K.; Woods, T.A.; Graves, S.W.; Di Carlo, D. Inertial Manipulation and Transfer of Microparticles across Laminar Fluid Streams. Small 2012, 8, 2757–2764. [Google Scholar] [CrossRef]
- Cho, S.; Jo, W.; Heo, Y.; Kang, J.Y.; Kwak, R.; Park, J. Isolation of Extracellular Vesicle from Blood Plasma Using Electrophoretic Migration through Porous Membrane. Sens. Actuators B Chem. 2016, 233, 289–297. [Google Scholar] [CrossRef]
- Tao, D.; Shui, B.; Gu, Y.; Cheng, J.; Zhang, W.; Jaffrezic-Renault, N.; Song, S.; Guo, Z. Development of a Label-Free Electrochemical Aptasensor for the Detection of Tau381 and Its Preliminary Application in AD and Non-AD Patients’ Sera. Biosensors 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Liu, M.; Wang, L.; Yan, A.; He, W.; Chen, M.; Lan, J.; Xu, J.; Guan, L.; Chen, J. A Visible and Colorimetric Aptasensor Based on DNA-Capped Single-Walled Carbon Nanotubes for Detection of Exosomes. Biosens. Bioelectron. 2017, 92, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Cheng, S.-B.; Cao, P.; Qiu, Q.-F.; Chen, Y.; Xie, M.; Xu, Y.; Huang, W.-H. Detection of Exosomes by ZnO Nanowires Coated Three-Dimensional Scaffold Chip Device. Biosens. Bioelectron. 2018, 122, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-G.; Kong, M.-Q.; Zhou, S.; Sheng, Y.-F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.-J.; Demirci, U.; et al. An Integrated Double-Filtration Microfluidic Device for Isolation, Enrichment and Quantification of Urinary Extracellular Vesicles for Detection of Bladder Cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemically Functionalised Graphene FET Biosensor for the Label-Free Sensing of Exosomes | Scientific Reports. Available online: https://www.nature.com/articles/s41598-019-50412-9 (accessed on 14 July 2020).
- Robbins, P.D. Extracellular Vesicles and Aging. Stem Cell Investig. 2017, 4, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sina, A.A.I.; Vaidyanathan, R.; Dey, S.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. Real Time and Label Free Profiling of Clinically Relevant Exosomes. Sci. Rep. 2016, 6, 30460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Ortiz, N.; Sahmoud, A.; Black, T.; Aiello, N.M.; McKenzie, L.; O’Hara, M.; Redlinger, C.; et al. Combining Machine Learning and Nanofluidic Technology To Diagnose Pancreatic Cancer Using Exosomes. ACS Nano 2017, 11, 11182–11193. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating Exosomes Measure Responses to Therapy in Head and Neck Cancer Patients Treated with Cetuximab, Ipilimumab, and IMRT. Oncoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.-Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C.; et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, X.; An, Z.; Yang, Y.; Eisenbaum, M.; Gu, X.; Jornet, J.M.; Dy, G.K.; Reid, M.E.; Gan, Q.; et al. Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sens. 2018. [Google Scholar] [CrossRef] [PubMed]
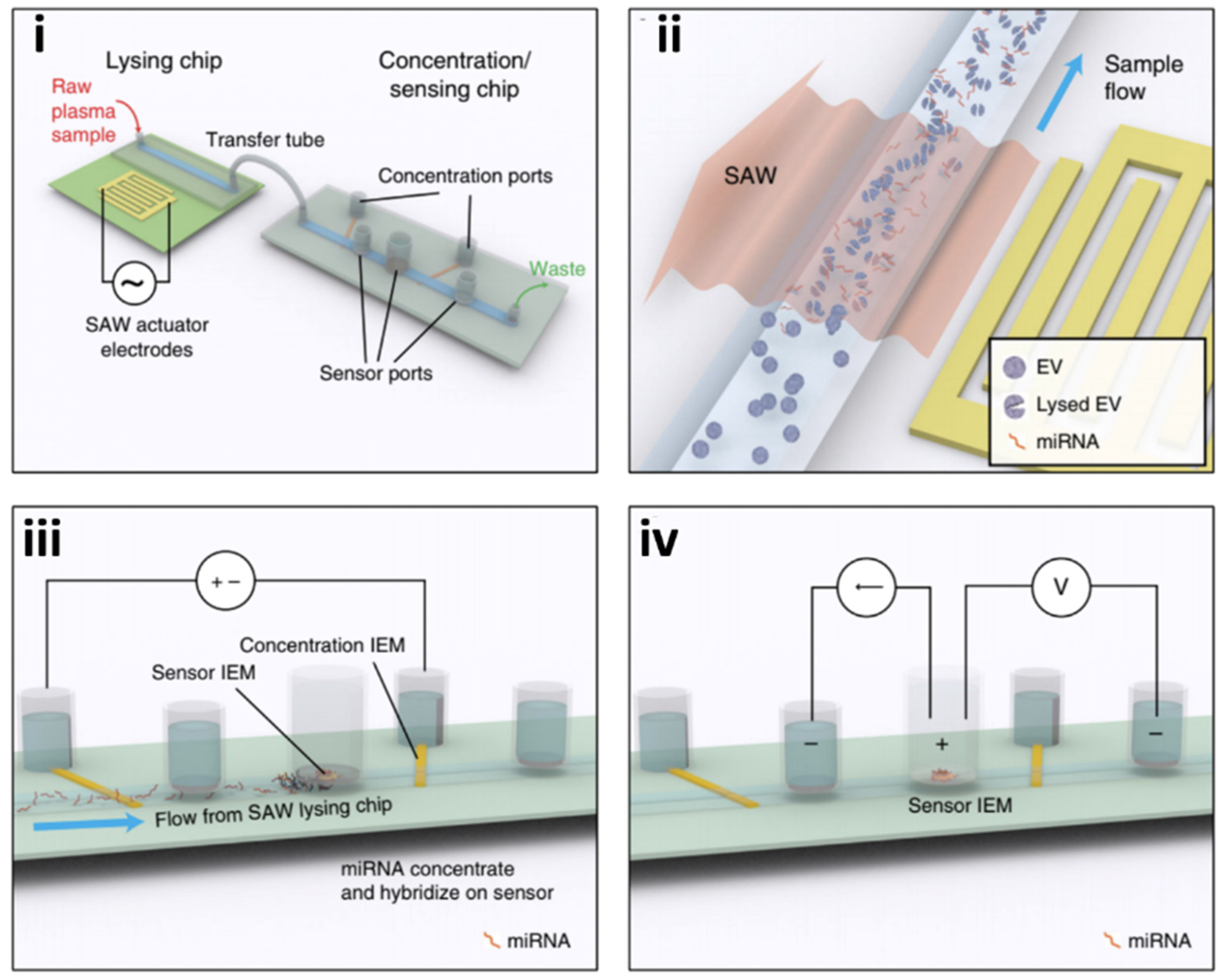
- Ramshani, Z.; Zhang, C.; Richards, K.; Chen, L.; Xu, G.; Stiles, B.L.; Hill, R.; Senapati, S.; Go, D.B.; Chang, H.-C. Extracellular Vesicle MicroRNA Quantification from Plasma Using an Integrated Microfluidic Device. Commun. Biol. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Go, D.B.; Atashbar, M.Z.; Ramshani, Z.; Chang, H.-C. Surface Acoustic Wave Devices for Chemical Sensing and Microfluidics: A Review and Perspective. Anal. Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef]
- Taller, D.; Richards, K.; Slouka, Z.; Senapati, S.; Hill, R.; Go, D.B.; Chang, H.-C. On-Chip Surface Acoustic Wave Lysis and Ion-Exchange Nanomembrane Detection of Exosomal RNA for Pancreatic Cancer Study and Diagnosis. Lab Chip 2015, 15, 1656–1666. [Google Scholar] [CrossRef]
- Rama, E.C.; González-García, M.B.; Costa-García, A. Competitive Electrochemical Immunosensor for Amyloid-Beta 1-42 Detection Based on Gold Nanostructurated Screen-Printed Carbon Electrodes. Sens. Actuators B Chem. 2014, 201, 567–571. [Google Scholar] [CrossRef]
- Tao, W.; Xie, Q.; Wang, H.; Ke, S.; Lin, P.; Zeng, X. Integration of a Miniature Quartz Crystal Microbalance with a Microfluidic Chip for Amyloid Beta-Aβ42 Quantitation. Sensors (Basel) 2015, 15, 25746–25760. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.K.; Kim, J.; Kim, G.; Kim, Y.S.; Kim, H.Y.; Lee, S.; Cho, W.W.; Kim, S.; Lee, S.-M.; Lee, B.C.; et al. A Highly Sensitive Plasma-Based Amyloid-β Detection System through Medium-Changing and Noise Cancellation System for Early Diagnosis of the Alzheimer’s Disease. Sci. Rep. 2017, 7, 8882. [Google Scholar] [CrossRef] [Green Version]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for Detection of Tau Protein as an Alzheimer’s Disease Marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef]
- Frost, B.; Götz, J.; Feany, M.B. Connecting the Dots Between Tau Dysfunction and Neurodegeneration. Trends Cell Biol. 2015, 25, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, M.; Kerman, K.; Kim, D.-K.; Hiep, H.M.; Tamiya, E. Detection of Alzheimer’s Tau Protein Using Localised Surface Plasmon Resonance-Based Immunochip. Talanta 2008, 74, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Scarano, S.; Lisi, S.; Palladino, P.; Minunni, M. Real-Time Tau Protein Detection by Sandwich-Based Piezoelectric Biosensing: Exploring Tubulin as a Mass Enhancer. Sensors 2018, 18, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeganathan, V.S.E.; Langford, T.; Sefo, L.A.-C.; Hewitt, A.W.; Verma, N. Screening for Diabetic Eye Disease among Samoan Adults: A Pilot Study. Ophthalmol. Ther. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courte, J.; Bousset, L.; Boxberg, Y.V.; Villard, C.; Melki, R.; Peyrin, J.-M. The Expression Level of Alpha-Synuclein in Different Neuronal Populations Is the Primary Determinant of Its Prion-like Seeding. Sci. Rep. 2020, 10, 4895. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, M.H.; Tosatto, L.; Dear, A.J.; Garcia, G.A.; Iljina, M.; Cremades, N.; Dalla Serra, M.; Knowles, T.P.J.; Dobson, C.M.; Klenerman, D. Fast Flow Microfluidics and Single-Molecule Fluorescence for the Rapid Characterization of α-Synuclein Oligomers. Anal. Chem. 2015, 87, 8818–8826. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.T.S.; Chutna, O.; Chu, V.; Conde, J.P.; Outeiro, T.F. A Novel Microfluidic Cell Co-Culture Platform for the Study of the Molecular Mechanisms of Parkinson’s Disease and Other Synucleinopathies. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Boukouris, S.; Mathivanan, S. Exosomes in Bodily Fluids Are a Highly Stable Resource of Disease Biomarkers. Proteomics Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Nik Mohamed Kamal, N.N.S.B.; Shahidan, W.N.S. Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers? Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of Preclinical Alzheimer’s Disease by a Profile of Pathogenic Proteins in Neurally Derived Blood Exosomes: A Case-Control Study. Alzheimers Dement 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [Green Version]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.Y.; et al. Exosome-Associated Tau Is Secreted in Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in Early Alzheimer Disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Scicluna, B.J.; Hill, A.F.; Götz, J. Extracellular Vesicles Isolated from the Brains of RTg4510 Mice Seed Tau Protein Aggregation in a Threshold-Dependent Manner. J. Biol. Chem. 2016, 291, 12445–12466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s Disease Pathology Propagation by Exosomes Containing Toxic Amyloid-Beta Oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Ngolab, J.; Trinh, I.; Rockenstein, E.; Mante, M.; Florio, J.; Trejo, M.; Masliah, D.; Adame, A.; Masliah, E.; Rissman, R.A. Brain-Derived Exosomes from Dementia with Lewy Bodies Propagate α-Synuclein Pathology. Acta Neuropathol. Commun. 2017, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, L.; Bao, H.; Premi, S.; Das, U.; Chapman, E.R.; Roy, S. Functional Cooperation of α-Synuclein and VAMP2 in Synaptic Vesicle Recycling. Proc. Natl. Acad. Sci. USA 2019, 116, 11113–11115. [Google Scholar] [CrossRef] [Green Version]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED Diagnostics to Inform Disease Control Strategies, Strengthen Health Systems and Improve Patient Outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Shamout, F.; Zhu, T.; Clifton, D.A. Machine Learning for Clinical Outcome Prediction. IEEE Rev. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Weng, W.-H. Machine Learning for Clinical Predictive Analytics. In Leveraging Data Science for Global Health; Celi, L.A., Majumder, M.S., Ordóñez, P., Osorio, J.S., Paik, K.E., Somai, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 199–217. ISBN 978-3-030-47994-7. [Google Scholar]
- Fisher, C.K.; Smith, A.M.; Walsh, J.R. Machine Learning for Comprehensive Forecasting of Alzheimer’s Disease Progression. Sci. Rep. 2019, 9, 13622. [Google Scholar] [CrossRef] [Green Version]







| Criteria | Description | |
|---|---|---|
| R | Real-time connectivity | Tests are connected, and/or a reader or mobile phone is used to power the reaction and/or read the test results to give appropriate data to decision-makers |
| E | Ease of specimen collection | Tests should be designed for use with non-invasive specimens |
| A | Affordable | Tests are affordable to end-users and health systems |
| S | Sensitive | Avoid false-negatives |
| S | Specific | Avoid false-positives |
| U | User-friendly | The procedure of testing is simple with few steps and little training |
| R | Rapid and robust | Results are available for giving treatment within the first visit (15 min to 2 h); Tests can survive as stock without additional transport or storage like refrigeration |
| E | Equipment-free or simple environment | The test does not require any special equipment |
| D | Deliverable to end-users | Accessible to those who need the tests |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeCastro, J.; Littig, J.; Chou, P.P.; Mack-Onyeike, J.; Srinivasan, A.; Conboy, M.J.; Conboy, I.M.; Aran, K. The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging. Molecules 2021, 26, 535. https://doi.org/10.3390/molecules26030535
DeCastro J, Littig J, Chou PP, Mack-Onyeike J, Srinivasan A, Conboy MJ, Conboy IM, Aran K. The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging. Molecules. 2021; 26(3):535. https://doi.org/10.3390/molecules26030535
Chicago/Turabian StyleDeCastro, Jonalyn, Joshua Littig, Peichi Peggy Chou, Jada Mack-Onyeike, Amrita Srinivasan, Michael J. Conboy, Irina M. Conboy, and Kiana Aran. 2021. "The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging" Molecules 26, no. 3: 535. https://doi.org/10.3390/molecules26030535
APA StyleDeCastro, J., Littig, J., Chou, P. P., Mack-Onyeike, J., Srinivasan, A., Conboy, M. J., Conboy, I. M., & Aran, K. (2021). The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging. Molecules, 26(3), 535. https://doi.org/10.3390/molecules26030535








