Chemical and Physical Implications of the Use of Alternative Vessels to Oak Barrels during the Production of White Wines
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of the Vessel on the Wine Acidity and Elemental Composition
2.2. Impact of the Vessel on the Wine Color and Phenolic Composition
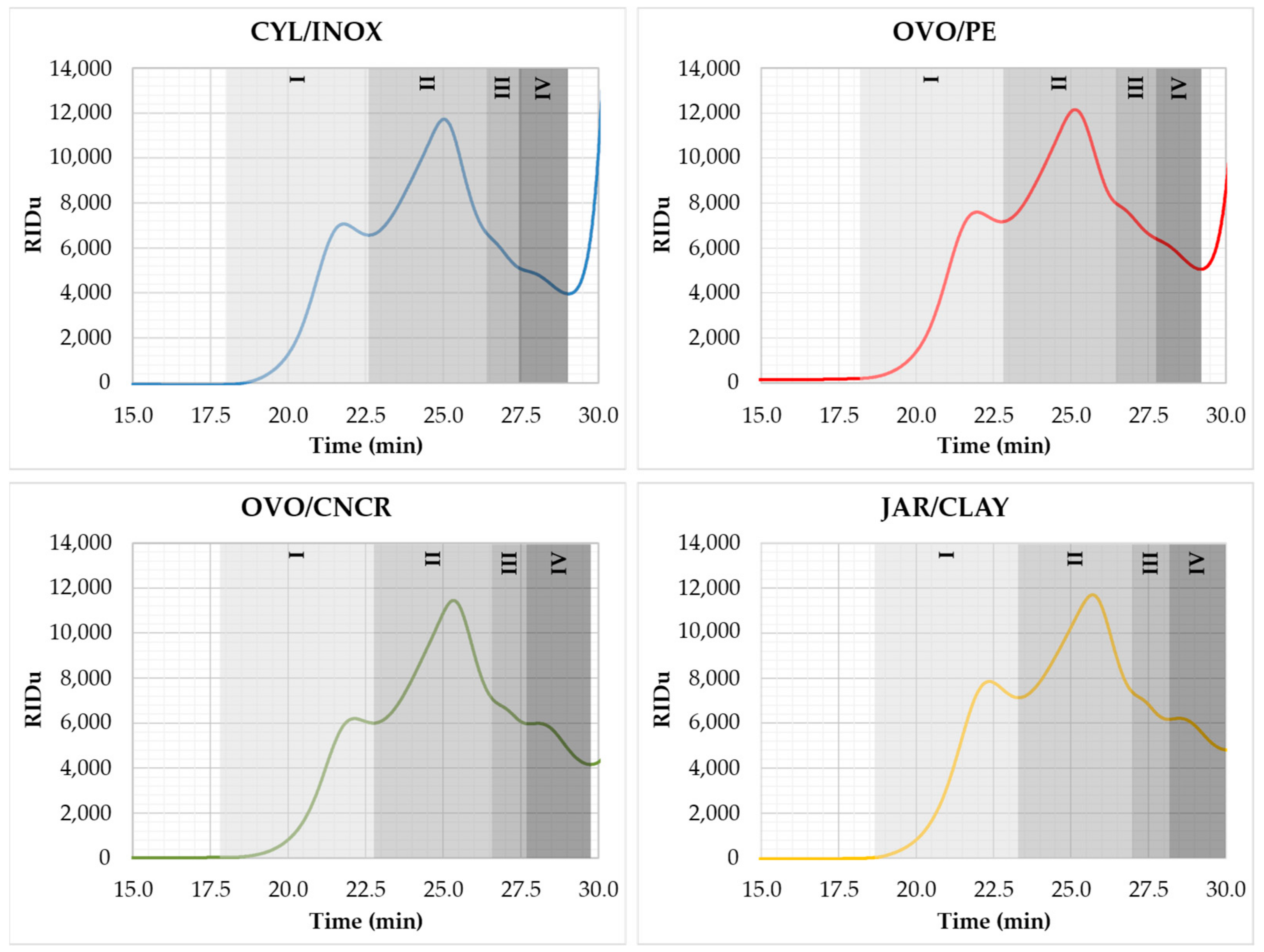
2.3. Impact of the Vessel on the Wine Turbidity and Polysaccharide Content
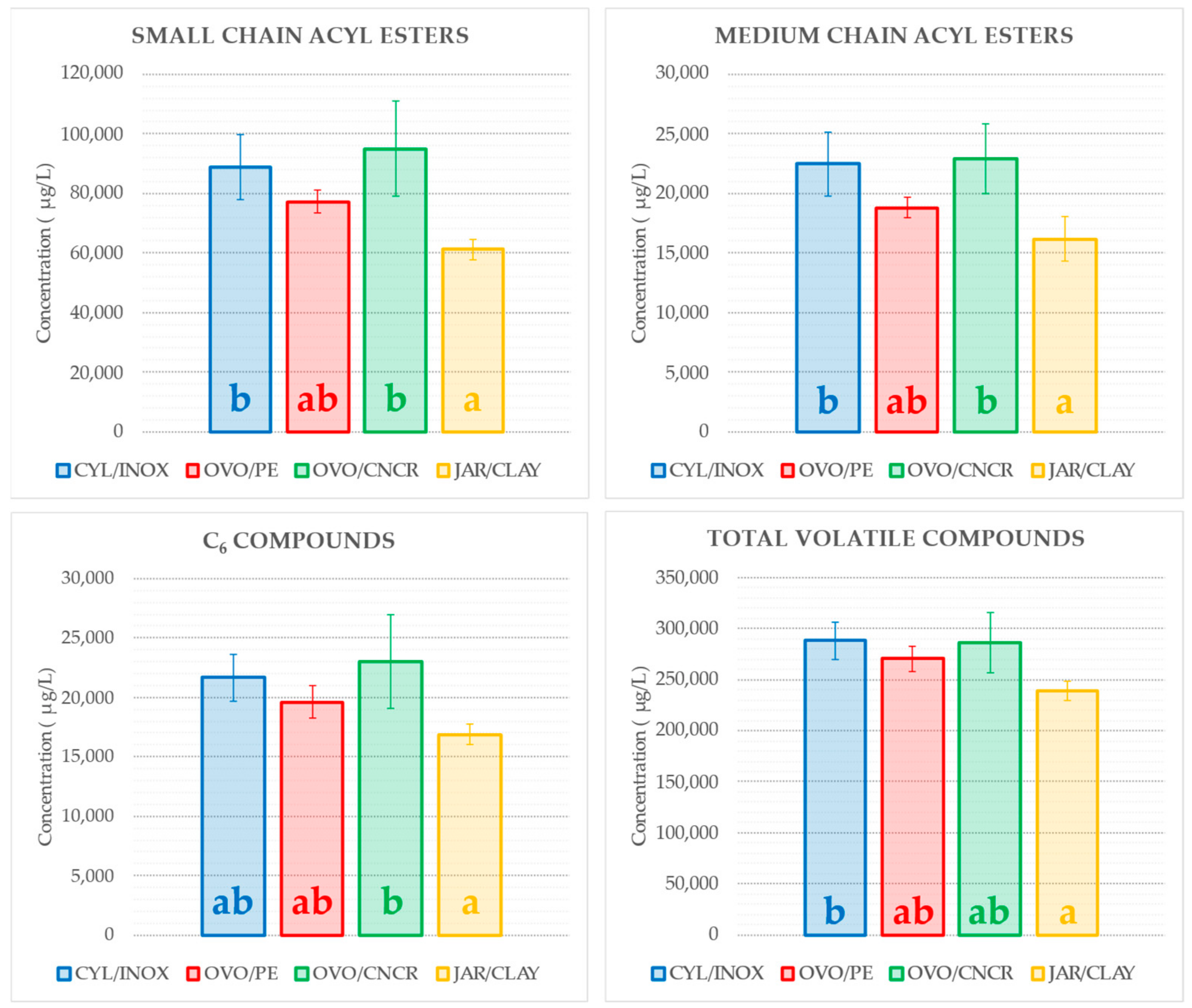
2.4. Impact of the Vessel on the Volatile Composition of Wine
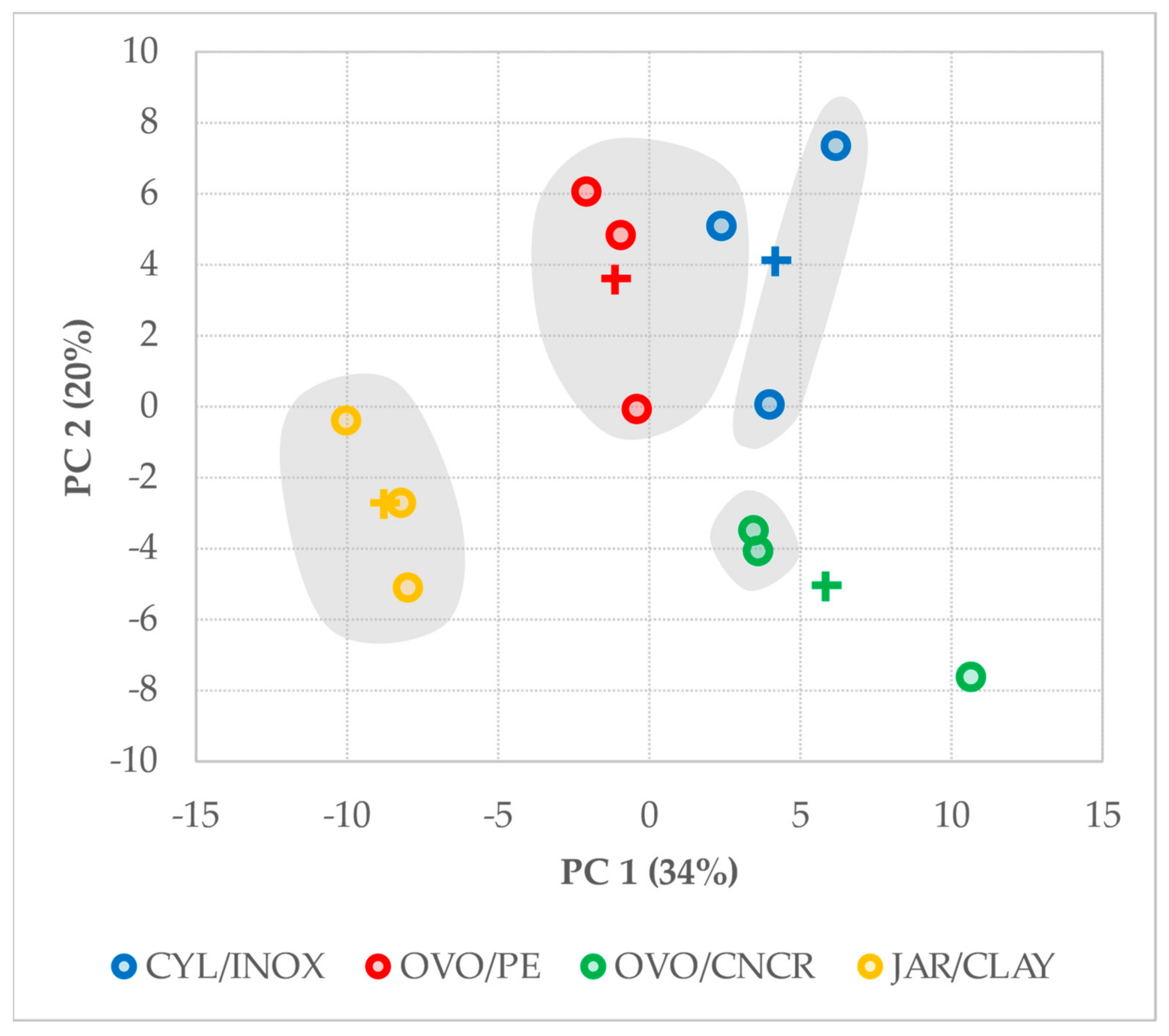
2.5. Multivariate Analysis
3. Materials and Methods
3.1. Experimental Design
3.2. General Analyses
3.3. Organic Acids Analysis
3.4. Elemental Analysis
3.5. Individual Low-Molecular-Mass Phenolic Compound Determination
3.6. Volatile Compounds
3.7. Soluble Polysaccharide Content of Wines
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nevares, I.; del Alamo-Sanza, M. New Materials for the Aging of Wines and Beverages: Evaluation and Comparison. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 375–407. ISBN 978-0-12-811516-9. [Google Scholar]
- Del Alamo-Sanza, M.; Laurie, V.F.; Nevares, I. Wine evolution and spatial distribution of oxygen during storage in high-density polyethylene tanks. J. Sci. Food Agric. 2015, 95, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J.; Gonzalo-Diago, A.; Pérez-Álvarez, E.P.; Bordiga, M. Wine Aging and Spoilage. In Post-Fermentation and -Distillation Technology; Taylor & Francis: Abingdon-on-Thames, UK, 2018; pp. 113–158. [Google Scholar]
- Montalvo, F.F.; García-Alcaraz, J.L.; Cámara, E.M.; Jiménez-Macías, E.; Blanco-Fernández, J. Environmental impact of wine fermentation in steel and concrete tanks. J. Clean. Prod. 2021, 278. [Google Scholar] [CrossRef]
- Del Alamo-Sanza, M.; Nevares, I. Oak wine barrel as an active vessel: A critical review of past and current knowledge. Crit. Rev. Food Sci. Nutr. 2018, 58, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Nevares, I.; del Alamo-Sanza, M. Characterization of the Oxygen Transmission Rate of New—Ancient Natural Materials for Wine Maturation Containers. Foods 2021, 10, 140. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Red Winemaking. In Handbook of Enology; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 327–395. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Clarification and Stabilization Treatments: Fining Wine. In Handbook of Enology; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 301–331. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Chemical Nature, Origins and Consequences of the Main Organoleptic Defects. In Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 233–284. ISBN 9780323609845. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Aging Red Wines in Vat and Barrel: Phenomena Occurring During Aging. In Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 388–428. [Google Scholar]
- Plank, C.M.; Trela, B.C. A review of plastics use in winemaking: Haccp considerations. Am. J. Enol. Vitic. 2018, 69, 307–320. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of wines and spirits by phthalates: Types of contaminants present, contamination sources and means of prevention. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 1605–1615. [Google Scholar] [CrossRef]
- Martins, N.; Garcia, R.; Mendes, D.; Costa Freitas, A.M.; da Silva, M.G.; Cabrita, M.J. An ancient winemaking technology: Exploring the volatile composition of amphora wines. Lwt 2018, 96, 288–295. [Google Scholar] [CrossRef]
- Díaz, C.; Laurie, V.F.; Molina, A.M.; Bücking, M.; Fischer, R. Characterization of selected organic and mineral components of qvevri wines. Am. J. Enol. Vitic. 2013, 64, 532–537. [Google Scholar] [CrossRef]
- Cabrita, M.J.; Martins, N.; Barrulas, P.; Garcia, R.; Dias, C.B.; Pérez-Álvarez, E.P.; Costa Freitas, A.M.; Garde-Cerdán, T. Multi-element composition of red, white and palhete amphora wines from Alentejo by ICPMS. Food Control 2018, 92, 80–85. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef]
- Pati, S.; Liberatore, M.T.; Lamacchia, C.; La Notte, E. Influence of ageing on lees on polysaccharide glycosyl-residue composition of Chardonnay wine. Carbohydr. Polym. 2010, 80, 332–336. [Google Scholar] [CrossRef]
- Doco, T.; Vuchot, P.; Cheynier, V.; Moutounet, M. Structural modification of wine arabinogalactans during aging on lees. Am. J. Enol. Vitic. 2003, 54, 150–157. [Google Scholar]
- Gil i Cortiella, M.; Úbeda, C.; Covarrubias, J.I.; Peña-Neira, Á. Chemical, physical, and sensory attributes of Sauvignon blanc wine fermented in different kinds of vessels. Innov. Food Sci. Emerg. Technol. 2020, 66, 102521. [Google Scholar] [CrossRef]
- Berg, H.W.; Keefer, R.M. Analytical Determination of Tartrate Stability in Wine. I. Potassium Bitartrate. Am. J. Enol. Vitic. 1958, 9, 180–193. [Google Scholar]
- Berg, H.W.; Keefer, R.M. Analytical Determination of Tartrate Stability in Wine. II. Calcium Tartrate. Am. J. Enol. Vitic. 1959, 10, 105–109. [Google Scholar]
- Pilone, B.F.; Berg, H.W. Some Factors Affecting Tartrate Stability in Wine. Am. J. Enol. Vitic. 1965, 16, 195–211. [Google Scholar]
- Allahverdi, A.; Skvara, F. Acidic corrosion of hydrated cement based materials—Part 1. Ceram. Silikáty 2000, 3, 114–120. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. The Maturaion and Aging of Wines. In Principles and Practices of Winemaking; Chapman & Hall: London, UK, 1996; pp. 382–473. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. The Physical and Chemical Stability of Wine. In Principles and Practices of Winemaking; Chapman & Hall: London, UK, 1996; pp. 320–351. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Dry Extract And Minerals. In Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 91–108. [Google Scholar]
- Boulton, R. The General Relationship Between Potassium, Sodium and pH in Grape Juice and Wine. Am. J. Enol. Vitic 1980, 31, 182–186. [Google Scholar]
- Berovič, M.; Košmerl, T. Monitoring of potassium hydrogen tartrate stabilization by conductivity measurement. Acta Chim. Slov. 2008, 55, 535–540. [Google Scholar]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Danilewicz, J.C. Mechanism of Autoxidation of Polyphenols and Participation of Sulfite in Wine: Key Role of Iron. Am. J. Enol. Vitic. 2011, 62, 319–328. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Bowanko, G. Effect of acid and alkali treatments on surface areas and adsorption energies of selected minerals. Clays Clay Miner. 2002, 50, 771–783. [Google Scholar] [CrossRef]
- Soroka, I. Chemical and mineralogical composition. In Portland, Cement, Paste & Concrete; The Macmillan Press Ltd.: London, UK, 1979; pp. 1–27. [Google Scholar]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Ortega, A.F.; Lopez-Toledano, A.; Mayen, M.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during Oxidative Aging of Sherry White Wine. J. Food Sci. 2003, 68, 2461–2468. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 141–203. [Google Scholar]
- Somers, T.C.; Ziemelis, G. Spectral evaluation of total phenolic components in Vitis vinifera: Grapes and wines. J. Sci. Food Agric. 1985, 36, 1275–1284. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; González, M.C.; Suárez-Lepe, J.A. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007, 105, 838–846. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Guerreau, J.; Feuillat, M. Influence of Initial Colloid Content on Yeast Macromolecule Production and on the Metabolism of Wine Microorganisms. Am. J. Enol. Vitic. 1995, 46, 486–492. [Google Scholar]
- Clarke, R.J.; Bakker, J. Volatile components. In Wine Flavour Chemistry; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 120–188. [Google Scholar]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Pueyo, E.; Martínez-Rodríguez, A.; Polo, M.C.; Santa-María, G.; Bartolomé, B. Release of lipids during yeast autolysis in a model wine system. J. Agric. Food Chem. 2000, 48, 116–122. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-Da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between yeast autolysates and volatile compounds in wine and model solution. Food Chem. 2011, 127, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Voilley, A.; Feuillat, M.; Charpentier, C. Influence of mannaproteins from yeast on the aroma intensity of a model wine. LWT Food Sci. Technol. 1994, 27, 108–114. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast derivatives (extracts and autolysates) in winemaking: Release of volatile compounds and effects on wine aroma volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and Phenols. In Handbook of Enology Vol 2: The Chemistry of Wine and Stabilization and Treatments; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 51–64. [Google Scholar]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and must Analysis; International Organisation of Vine and Wine, Ed.; International Organisation of Vine and Wine: Paris, France, 2016; ISBN 979-10-91799-46-1. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Organic Acids in Wine. In Handbook of Enology; John Wiley & Sons, Ltd.: Chinchester, UK, 2006; pp. 1–49. [Google Scholar]
| Parameter (1) | CYL/INOX | OVO/PE | OVO/CNCR | JAR/CLAY |
|---|---|---|---|---|
| Ethanol (% vol.) | 13.4 ± 0.1 | 13.3 ± 0.1 | 13.4 ± 0.2 | 13.3 ± 0.1 |
| pH | 3.32 ± 0.01 a | 3.31 ± 0.01 a | 3.38 ± 0.01 b | 3.28 ± 0.01 a |
| Titratable acidity (2) | 7.06 ± 0.25 b | 7.20 ± 0.04 b | 6.42 ± 0.21 a | 6.90 ± 0.06 b |
| Tartaric acid (g/L) | 3.40 ± 0.16 | 3.49 ± 0.05 | 3.49 ± 0.41 | 3.50 ± 0.03 |
| Malic acid (g/L) | 3.27 ± 0.11 | 3.40 ± 0.01 | 3.29 ± 0.31 | 3.49 ± 0.05 |
| Citric acid (g/L) | 0.68 ± 0.14 | 0.79 ± 0.01 | 0.70 ± 0.04 | 0.72 ± 0.01 |
| Conductivity loss (%) (3) | 7.6 ± 0.7 | 7.6 ± 1.0 | 6.6 ± 0.6 | 7.3 ± 1.1 |
| Conductivity (µS cm−1) | 1.77 ± 0.03 | 1.81 ± 0.05 | 1.81 ± 0.05 | 1.80 ± 0.03 |
| Turbidity (NTUs) | 3.88 ± 0.87 a | 5.58 ± 0.18 b | 6.35 ± 0.90 bc | 7.48 ± 0.76 c |
| Potassium (K, mg/L) | 605 ± 30 | 598 ± 21 | 607 ± 19 | 582 ± 36 |
| Phosphorous (P, mg/L) | 115 ± 2 b | 124 ± 3 c | 112 ± 2 b | 106 ± 5 a |
| Calcium (Ca, mg/L) | 61 ± 3 b | 63 ± 2 b | 38 ± 5 a | 57 ± 7 b |
| Silicon (Si, mg/L) | 32 ± 7 a | 27 ± 1 a | 61 ± 11 b | 26 ± 1 a |
| Sodium (Na, mg/L) | 14.9 ± 2.0 a | 17.1 ± 0.8 a | 22.4 ± 4.5 b | 16.1 ± 0.5 a |
| Magnesium (Mg, mg/L) | 10.8 ± 1.9 a | 8.9 ± 4.0 a | 60.6 ± 27.5 b | 19.3 ± 9.5 a |
| Boron (B, mg/L) | 5.24 ± 0.29 | 5.07 ± 0.37 | 4.59 ± 0.38 | 4.56 ± 0.20 |
| Iron (Fe, mg/L) | 0.520 ± 0.061 a | 0.433 ± 0.021 a | 2.447 ± 0.341 b | 0.577 ± 0.110 a |
| Manganese (Mn, mg/L) | 0.623 ± 0.021 a | 0.627 ± 0.006 a | 0.737 ± 0.049 b | 0.617 ± 0.032 a |
| Zinc (Zn, mg/L) | 0.590 ± 0.010 | 0.550 ± 0.020 | 0.690 ± 0.150 | 0.767 ± 0.106 |
| Copper (Cu, mg/L) | 0.107 ± 0.012 a | 0.110 ± 0.010 a | 0.093 ± 0.015 a | 0.170 ± 0.040 b |
| I280 (4) | 9.03 ± 0.15 a | 9.57 ± 0.14 b | 9.06 ± 0.19 a | 9.99 ± 0.30 c |
| Color Intensity | 0.124 ± 0.020 | 0.124 ± 0.011 | 0.144 ± 0.014 | 0.142 ± 0.009 |
| L* (5) | 98.7 ± 0.3 | 98.6 ± 0.2 | 98.2 ± 0.2 | 98.3 ± 0.1 |
| a* (6) | −1.52 ± 0.06 ab | −1.45 ± 0.06 b | −1.47 ± 0.01 b | −1.62 ± 0.08 a |
| b* (7) | 8.11 ± 1.17 | 7.80 ± 0.62 | 8.61 ± 0.94 | 8.59 ± 0.69 |
| Structure (1) | CYL/INOX | OVO/PE | OVO/CNCR | JAR/CLAY |
|---|---|---|---|---|
| Caffeic acid and derivatives | 145.1 ± 4.2 | 143.3 ± 2.98 | 144.3 ± 2.4 | 151.2 ± 2.2 |
| Coumaric acid and derivatives | 74.1 ± 4.3 | 79.0 ± 2.67 | 72.8 ± 2.2 | 75.0 ± 2.7 |
| Ferulic acid and derivatives | 4.30 ± 0.49 | 3.85 ± 0.44 | 4.50 ± 0.88 | 4.19 ± 0.62 |
| Sinapic acid and derivatives | 2.26 ± 0.26 | 2.53 ± 0.16 | 2.53 ± 0.21 | 2.34 ± 0.03 |
| Free cinnamic acids | 54.5 ± 2.5 | 57.1 ± 0.5 | 57.5 ± 1.0 | 54.8 ± 0.7 |
| Tartaric esters of cinnamic acids | 159.6 ± 4.1 ab | 157.2 ± 4.7 ab | 150.7 ± 2.9 a | 164.0 ± 1.0 b |
| Ethyl esters of cinnamic acids | 14.3 ± 0.9 a | 14.4 ± 0.2 a | 16.0 ± 0.7 b | 13.9 ± 0.6 a |
| Total cinnamic acids and derivatives | 225.8 ± 6.9 | 228.7 ± 5.0 | 224.2 ± 1.8 | 232.7 ± 1.6 |
| FRACTION | Parameter (1) | CYL/INOX | OVO/PE | OVO/CNCR | JAR CLY |
|---|---|---|---|---|---|
| I | mg/L | 64.94 ± 6.59 b | 70.53 ± 3.84 bc | 52.61 ± 4.05 a | 79.28 ± 3.54 c |
| Range (KDa) | 600.42–60.33 | 577.82–55.84 | 694.23–56.12 | 515.64–42.46 | |
| Mn (KDa) (2) | 92.22 ± 6.53 b | 86.49 ± 8.92 b | 77.81 ± 4.42 ab | 69.40 ± 5.33 a | |
| II | mg/L | 148.67 ± 7.78 a | 155.67 ± 3.99 a | 146.71 ± 2.23 a | 154.13 ± 5.37 a |
| Range (KDa) | 60.33–9.69 | 55.84–8.95 | 56.12–8.39 | 42.46–6.63 | |
| Mn (KDa) (2) | 17.65 ± 1.22 b | 16.51 ± 2.06 b | 15.13 ± 1.09 ab | 12.64 ± 1.17 a | |
| III | mg/L | 32.91 ± 6.82 a | 36.78 ± 2.12 a | 34.97 ± 6.19 a | 33.01 ± 2.78 a |
| Range (KDa) | 9.69–5.25 | 8.95–5.02 | 8.39–4.55 | 6.63–3.78 | |
| Mn (KDa) (2) | 9.69 ± 0.60 b | 8.53 ± 1.47 ab | 8.01± 1.54 ab | 6.63 ± 0.50 a | |
| IV | mg/L | 33.52 ± 4.20 a | 43.42 ± 3.99 b | 47.33 ± 4.22 b | 50.12 ± 3.53 b |
| Range (KDa) | 5.25–2.29 | 5.02–2.10 | 4.55–1.61 | 3.78–1.38 | |
| Mn (KDa) (2) | 5.25 ± 0.44 b | 5.01 ± 0.73 b | 3.76 ± 0.27 a | 3.24 ± 0.44 a | |
| Total polysaccharides (mg/L) | 280.05 ± 18.80 a | 306.41 ± 7.88 ab | 281.62 ± 9.20 a | 316.55 ± 11.41 b | |
| F I | F II | F III | F IV | Total Polysaccharides | Small Chain Esters | Medium Chain Esters | |
|---|---|---|---|---|---|---|---|
| F II | 0.6481 * | - | |||||
| F III | −0.0072 | 0.4465 | - | ||||
| F IV | 0.0834 | 0.2698 | 0.1974 | - | |||
| Total polysaccharides | 0.7790 ** | 0.8652 *** | 0.4355 | 0.5500 | - | ||
| Small chain esters | −0.8147 ** | −0.6745 * | −0.3052 | −0.3441 | −0.8546 *** | - | |
| Medium chain esters | −0.8166 ** | −0.6170 * | −0.1536 | −0.2987 | −0.7862 ** | 0.9300 *** | - |
| Turbidity | 0.2614 | 0.2795 | −0.0561 | 0.8216 ** | 0.5259 | −0.3853 | −0.3760 |
| Volatile Compound | LRI (a) | ID (b) | CYL/INOX | OVO/PE | OVO/CNCR | JAR/CLAY |
|---|---|---|---|---|---|---|
| Ethyl butyrate | 1055 | A | 808.03 ± 96.81 b | 699.60 ± 52.31 ab | 832.61 ± 96.15 b | 556.19 ± 110.41 a |
| Ethyl 2-methylbutyrate | 1084 | A | 22.40 ± 2.98 | 21.74 ± 0.64 | 19.19 ± 1.03 | 20.59 ± 5.18 |
| Ethyl 2-ethylbutyrate | 1095 | C | 4.60 ± 0.35 | 5.01 ± 0.35 | 5.06 ± 1.42 | 3.35 ± 0.46 |
| Isoamyl acetate | 1122 | A | 85,474 ± 10,851 b | 74,822 ± 3799 ab | 91,334 ± 16,115 b | 59,224 ± 3638 a |
| Ethyl hexanoate | 1245 | A | 10,750 ± 1555 | 9707 ± 446 | 10,669 ± 2055 | 8912 ± 1261 |
| Hexyl acetate | 1285 | A | 5394.54 ± 763.22 b | 4597.29 ± 106.79 ab | 5566.26 ± 1322.53 b | 3382.21 ± 400.09 a |
| trans-4-Hexenyl acetate | 1295 | C | 52.80 ± 2.26 b | 42.86 ± 4.39 b | 57.56 ± 14.02 b | 20.51 ± 5.77 a |
| cis-3-Hexenyl acetate | 1320 | C | 196.48 ± 28.14 | 176.40 ± 12.31 | 201.47 ± 49.47 | 135.47 ± 17.64 |
| Ethyl 2-hexenoate | 1339 | B | 3.89 ± 0.18 | 3.45 ± 0.14 | 3.99 ± 0.56 | 3.28 ± 0.38 |
| Ethyl heptanoate | 1334 | A | 1.98 ± 0.08 a | 1.82 ± 0.13 a | 1.74 ± 0.26 a | 2.87 ± 0.44 b |
| Ethyl octanoate | 1437 | A | 2261.88 ± 393.31 | 1931.85 ± 233.56 | 2276.79 ± 308.22 | 1697.13 ± 277.35 |
| Isoamyl hexanoate | 1495 | C | 0.86 ± 0.44 | 0.75 ± 0.12 | 1.14 ± 0.41 | 0.68 ± 0.41 |
| Methyl octanoate | 1465 | B | 18.97 ± 2.29 | 17.81 ± 4.00 | 26.86 ± 2.97 | 16.84 ± 5.376 |
| Ethyl nonanoate | 1541 | B | 4.46 ± 1.06 | 3.32 ± 0.94 | 3.57 ± 0.99 | 4.02 ± 0.68 |
| Methyl decanoate | 1600 | A | 7.17 ± 0.79 | 5.98 ± 1.41 | 9.12 ± 2.03 | 5.94 ± 0.81 |
| Ethyl decanoate | 1647 | A | 810.80 ± 113.50 b | 457.27 ± 40.36 a | 705.49 ± 166.62 b | 341.61 ± 62.49 a |
| Diethyl succinate | 1675 | A | 40.39 ± 0.58 | 36.79 ± 8.81 | 30.97 ± 5.62 | 33.27 ± 3.44 |
| Isoamyl octanoate | 1680 | A | 1384.67 ± 248.97 | 1033.59 ± 89.61 | 1456.29 ± 95.94 | 985.56 ± 249.45 |
| β-Phenylethyl acetate | 1851 | A | 4947.58 ± 703.78 | 3844.20 ± 1042.93 | 4164.25 ± 1036.14 | 2823.48 ± 571.46 |
| Ethyl dodecanoate | 1864 | B | 536.12 ± 89.61 | 314.78 ± 32.71 | 624.74 ± 257.51 | 326.99 ± 23.53 |
| Methyl dodecanoate | 1825 | B | 2.83 ± 0.31 b | 1.79 ± 0.16 a | 2.25 ± 0.17 a | 1.98 ± 0.45 a |
| Isoamyl decanoate | 1888 | A | 1041.45 ± 182.94 b | 508.70 ± 149.70 a | 1329.18 ± 254.95 b | 329.49 ± 94.03 a |
| Ethyl tetradecanoate | 2041 | B | 20.85 ± 6.27 ab | 20.05 ± 5.23 ab | 28.79 ± 5.88 b | 13.71 ± 1.48 a |
| Isobutanol | 1074 | A | 32,457 ± 1164 | 33,883 ± 2188 | 27,862 ± 6915 | 31,451 ± 1515 |
| Isoamyl alcohol | 1200 | A | 110,845 ± 6122 | 111,657 ± 7618 | 113,832 ± 16572 | 109,864 ± 5907 |
| Hexanol | 1375 | A | 1275.30 ± 120.00 | 1050.00 ± 135.59 | 1084.08 ± 145.98 | 990.41 ± 40.46 |
| 2,3-Butanediol | 1557 | A | 68.30 ± 4.37 | 67.32 ± 24.83 | 75.66 ± 41.31 | 54.08 ± 35.74 |
| Decanol | 1773 | A | 3.69 ± 0.31 b | 3.02 ± 0.67 b | 3.36 ± 0.65 b | 1.43 ± 0.47 a |
| 2-phenylethanol | 1940 | A | 17664 ± 2227 | 15665 ± 3686 | 10779 ± 3165 | 10931 ± 5288 |
| Dodecanol | 1986 | A | 2.04 ± 0.26 b | 1.03 ± 0.21 a | 0.96 ± 0.07 a | 1.15 ± 0.22 a |
| Benzaldehyde | 1565 | A | 28.18 ± 13.27 | 7.98 ± 3.15 | 32.31 ± 12.83 | 16.80 ± 5.00 |
| Hexanoic acid | 1880 | A | 3710.25 ± 657.96 | 3780.41 ± 1061.81 | 5162.74 ± 836.42 | 3211.51 ± 955.41 |
| Octanoic acid | 2076 | A | 5808.19 ± 468.00 b | 5448.26 ± 596.99 b | 5852.82 ± 872.22 b | 2800.26 ± 785.91 a |
| Decanoic acid | 2339 | A | 2141.84 ± 303.72 b | 628.51 ± 243.61 a | 1793.36 ± 383.54 b | 500.48 ± 51.18 a |
| TDN (c) | 1745 | B | 8.22 ± 1.02 | 6.69 ± 0.84 | 7.54 ± 2.18 | 6.06 ± 1.75 |
| Nerol oxide | 1462 | B | 44.05 ± 3.19 | 43.52 ± 4.98 | 41.57 ± 1.26 | 41.57 ± 7.49 |
| Geranyl ethyl ether | 1481 | C | 7.98 ± 1.40 | 8.01 ± 0.66 | 6.78 ± 0.32 | 6.49 ± 0.90 |
| Linalool formate | 1539 | C | 5.28 ± 0.61 b | 4.66 ± 0.26 ab | 4.80 ± 0.43 ab | 3.77 ± 0.60 a |
| Linalool | 1555 | A | 5.60 ± 1.08 | 5.61 ± 1.92 | 6.10 ± 1.53 | 5.90 ± 0.93 |
| Hotrienol | 1660 | B | 13.02 ± 0.61 | 12.68 ± 2.90 | 14.65 ± 1.53 | 14.94 ± 0.71 |
| cis-β-Farnesene | 1664 | B | 2.80 ± 0.31 b | 2.37 ± 0.11 b | 2.63 ± 0.32 b | 1.67 ± 0.06 a |
| α-Terpineol | 1693 | A | 7.35 ± 1.89 | 7.66 ± 0.93 | 7.27 ± 1.00 | 7.11 ± 1.05 |
| trans-Nerolidol | 2047 | A | 8.10 ± 0.86 b | 6.81 ± 1.70 b | 7.70 ± 1.34 b | 3.41 ± 0.51 a |
| TOTAL ETHYL ESTERS | 15,266 ± 2067 | 13,203 ± 801 | 15,203 ± 2025 | 11,915 ± 1649 | ||
| TOTAL ACETATE ESTERS | 96,065 ± 11,614 b | 83,483 ± 2771 ab | 101,323 ± 16,194 b | 65,585 ± 4002 a | ||
| TOTAL OTHER ESTERS | 2456 ± 149 b | 1569 ± 75 a | 2825 ± 287 b | 1340 ± 218 a | ||
| TOTAL ALCOHOLS | 162,588 ± 5896 | 162,553 ± 9513 | 153,910 ± 10,703 | 153,530 ± 11,813 | ||
| TOTAL TERPENES | 86.08 ± 4.89 | 84.52 ± 8.34 | 83.81 ± 2.70 | 81.47 ± 10.20 | ||
| TOTAL ACIDS | 11,660 ± 1016 b | 9857 ± 1344 b | 12,809 ± 1838 b | 6512 ± 1530 a |
| Variable | PC 1 | PC 2 |
|---|---|---|
| trans-4-Hexenyl acetate | 0.156 | −0.006 |
| TOTAL ESTERS | 0.154 | −0.004 |
| TOTAL ACETATE ESTERS | 0.153 | 0.000 |
| Isoamyl acetate | 0.151 | −0.009 |
| Ethyl butyrate | 0.147 | −0.005 |
| TOTAL ALIPHATIC C6 COMPOUNDS | 0.145 | −0.020 |
| TOTAL VOLATILE COMPOUNDS | 0.143 | 0.021 |
| Hexyl acetate | 0.142 | 0.013 |
| TOTAL ACIDS | 0.140 | 0.005 |
| TOTAL ETHYL ESTERS | 0.136 | −0.022 |
| TOTAL ALIPHATIC C8 ESTERS | 0.135 | −0.041 |
| Octanoic acid | 0.135 | 0.034 |
| Ethyl 2-hexenoate | 0.135 | −0.039 |
| Decanol | 0.135 | 0.042 |
| Farnesene | 0.134 | 0.035 |
| Ethyl octanoate | 0.133 | −0.030 |
| TOTAL OTHER ESTERS | 0.129 | −0.043 |
| cis-3-Hexenyl acetate | 0.126 | 0.026 |
| Decanoic acid | 0.125 | 0.023 |
| M0 FII | 0.125 | 0.102 |
| Mf FI | 0.125 | 0.102 |
| Ethyl 2-ethylbutyrate | 0.124 | −0.004 |
| Isoamyl decanoate | 0.123 | −0.033 |
| Ethyl decanoate | 0.120 | 0.033 |
| Ethyl hexanoate | 0.119 | −0.025 |
| trans-Nerolidol | 0.117 | 0.036 |
| pH | 0.117 | −0.100 |
| Linalool formate | 0.116 | 0.059 |
| Isoamyl octanoate | 0.109 | −0.048 |
| Si | 0.107 | −0.109 |
| TDN | 0.107 | −0.027 |
| M0 FI | 0.103 | −0.030 |
| Ethyl esters of cinnamic acids | 0.100 | −0.129 |
| Isoamyl hexanoate | 0.099 | −0.056 |
| M0 FIII | 0.098 | 0.128 |
| Mf FII | 0.098 | 0.128 |
| cis-3-Hexen-1-ol | 0.095 | 0.002 |
| Mn FII | 0.095 | 0.143 |
| M0 FIV | 0.094 | 0.132 |
| Mf FIII | 0.094 | 0.132 |
| Ethyl caffeoate | 0.093 | −0.148 |
| Hexanol | 0.093 | 0.087 |
| a* | 0.091 | 0.043 |
| Mn | 0.090 | −0.113 |
| Methyl octanoate | 0.089 | −0.114 |
| Benzaldehyde | 0.088 | −0.100 |
| Hexanoic acid | 0.088 | −0.050 |
| Ethyl tetradecanoate | 0.088 | −0.053 |
| Mn FIII | 0.087 | 0.114 |
| Fe | 0.084 | −0.134 |
| Ethyl coumarate | 0.080 | −0.030 |
| Mn FI | 0.080 | 0.159 |
| β-Phenylethyl acetate | 0.078 | 0.120 |
| Methyl decanoate | 0.077 | −0.077 |
| Na | 0.073 | −0.112 |
| Mf FIV | 0.069 | 0.168 |
| K | 0.068 | 0.081 |
| Methyl dodecanoate | 0.068 | 0.046 |
| Ethyl dodecanoate | 0.068 | −0.027 |
| Mn FIV | 0.067 | 0.167 |
| Isoamyl alcohol | 0.061 | −0.054 |
| Geranyl ethyl ether | 0.057 | 0.125 |
| 2,3-Butanediol | 0.057 | −0.019 |
| Ferulic acid and derivatives | 0.054 | −0.124 |
| Caffeic acid | 0.052 | −0.166 |
| P | 0.048 | 0.124 |
| B | 0.047 | 0.134 |
| TOTAL ALCOHOLS | 0.046 | 0.063 |
| Free cinnamic acids | 0.044 | −0.018 |
| Dodecanol | 0.044 | 0.107 |
| TOTAL TERPENES | 0.042 | 0.019 |
| Fertaric acid | 0.040 | −0.146 |
| Diethyl succinate | 0.038 | 0.051 |
| Phenylethanol | 0.033 | 0.147 |
| Sinapic acid | 0.031 | −0.035 |
| Sinapic acid and derivatives | 0.031 | −0.035 |
| Ethyl coumarate | 0.029 | −0.062 |
| Nerol oxide | 0.027 | 0.021 |
| Mg | 0.027 | −0.157 |
| Tartaric Acid | 0.026 | −0.028 |
| α Terpineol | 0.017 | 0.032 |
| Ferulic acid | 0.010 | 0.175 |
| b* | 0.010 | −0.157 |
| C* | 0.008 | −0.157 |
| Ethyl 2-methylbutyrate | 0.008 | 0.055 |
| trans-coumaric acid | 0.000 | 0.118 |
| L* | 0.000 | 0.191 |
| Color Intensity | −0.006 | −0.186 |
| Conductivity | −0.009 | 0.023 |
| Conductivity Loss (%) | −0.010 | 0.131 |
| Ethyl nonanoate | −0.014 | 0.077 |
| FIII | −0.020 | −0.027 |
| Hotrienol | −0.025 | −0.129 |
| Linalol | −0.026 | 0.028 |
| Citric Acid | −0.026 | 0.030 |
| Zn | −0.032 | −0.131 |
| cis-coumaric acid | −0.043 | −0.090 |
| h* | −0.051 | 0.131 |
| Coumaric acid and derivatives | −0.057 | 0.139 |
| FIV | −0.063 | −0.155 |
| Titratable Acidity | −0.066 | 0.150 |
| Ca | −0.067 | 0.159 |
| Isobutanol | −0.067 | 0.101 |
| Turbidity | −0.080 | −0.121 |
| FII | −0.091 | 0.012 |
| Coutaric acid | −0.100 | 0.141 |
| Caffeic acid and derivatives | −0.100 | −0.053 |
| Ethyl heptanoate | −0.110 | −0.054 |
| Malic Acid | −0.114 | 0.032 |
| TOTAL cinnamic acids | −0.114 | 0.039 |
| I280 | −0.129 | −0.047 |
| Cu | −0.130 | −0.007 |
| Caftaric acid | −0.132 | 0.036 |
| TOTAL polysaccharides | −0.132 | −0.033 |
| Tartaric esters of cinnamic acids | −0.133 | 0.064 |
| FI | −0.138 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil i Cortiella, M.; Ubeda, C.; Covarrubias, J.I.; Laurie, V.F.; Peña-Neira, Á. Chemical and Physical Implications of the Use of Alternative Vessels to Oak Barrels during the Production of White Wines. Molecules 2021, 26, 554. https://doi.org/10.3390/molecules26030554
Gil i Cortiella M, Ubeda C, Covarrubias JI, Laurie VF, Peña-Neira Á. Chemical and Physical Implications of the Use of Alternative Vessels to Oak Barrels during the Production of White Wines. Molecules. 2021; 26(3):554. https://doi.org/10.3390/molecules26030554
Chicago/Turabian StyleGil i Cortiella, Mariona, Cristina Ubeda, José Ignacio Covarrubias, V. Felipe Laurie, and Álvaro Peña-Neira. 2021. "Chemical and Physical Implications of the Use of Alternative Vessels to Oak Barrels during the Production of White Wines" Molecules 26, no. 3: 554. https://doi.org/10.3390/molecules26030554
APA StyleGil i Cortiella, M., Ubeda, C., Covarrubias, J. I., Laurie, V. F., & Peña-Neira, Á. (2021). Chemical and Physical Implications of the Use of Alternative Vessels to Oak Barrels during the Production of White Wines. Molecules, 26(3), 554. https://doi.org/10.3390/molecules26030554






