Bicyclic Basic Merbarone Analogues as Antiproliferative Agents
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
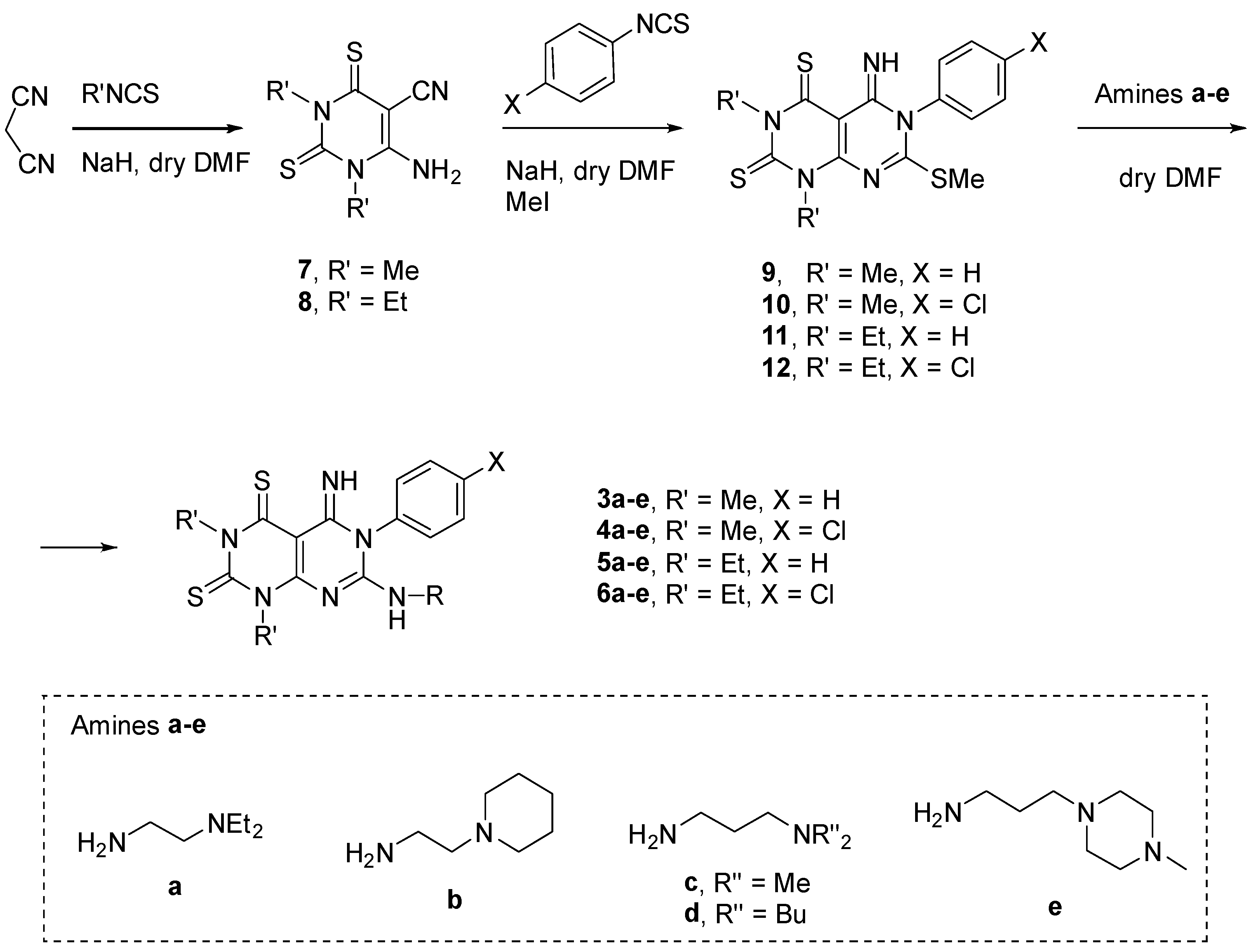
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Compounds 7 and 8
3.1.2. General Procedure for the Synthesis of Compounds 9–12
3.1.3. General Procedure for the Synthesis of Compounds 3–6
3.2. Biology
3.2.1. MTT Assay
3.2.2. Annexin V & Dead Cell Assay
3.3. Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, J.C. DNA topoisomerases. Annu. Rev. Biochem. 1996, 65, 635–692. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, L.; Redinbo, M.R.; Qiu, X.; Hol, W.G.J.; Champoux, J.J. A model for the mechanism of human topoisomerase I. Science 1998, 279, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Moving one DNA double helix through another by a type II DNA topoisomerase: The story of a simple molecular machine. Q. Rev. Biophys. 1998, 31, 107–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Huang, X.S.; Wu, J.F.; Yang, L.; Zheng, Y.T.; Shen, Y.M.; Li, Z.Y.; Li, X. Discovery of Novel Topoisomerase II Inhibitors by Medicinal Chemistry Approaches. J. Med. Chem. 2018, 61, 8947–8980. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wu, Q.; Luan, S.; Yin, Z.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. A Comprehensive Review of Topoisomerase Inhibitors as Anticancer Agents in the Past Decade. Eur. J. Med. Chem. 2019, 171, 129–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tse-Dinh, Y.C. Recent Advances in Use of Topoisomerase Inhibitors in Combination Cancer Therapy. Curr. Top. Med. Chem. 2019, 19, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Pogorelcnik, B.; Perdih, A.; Solmajer, T. Recent Advances in the Development of Catalytic Inhibitors of Human DNA Topoisomerase IIalpha as Novel Anticancer Agents. Curr. Med. Chem. 2013, 20, 694–709. [Google Scholar] [CrossRef]
- Riddell, I.A.; Agama, K.; Park, G.Y.; Pommier, Y.; Lippard, S.J. Phenanthriplatin Acts as a Covalent Poison of Topoisomerase II Cleavage Complexes. ACS Chem. Biol. 2016, 11, 2996–3001. [Google Scholar] [CrossRef]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Catalytic Topoisomerase II Inhibitors in Cancer Therapy. Pharmacol. Ther. 2003, 99, 167–181. [Google Scholar] [CrossRef]
- Andoh, T.; Ishida, R. Catalytic inhibitors of DNA topoisomerase II. Biochim. Biophys. Acta 1998, 1400, 155–171. [Google Scholar] [CrossRef]
- Fortune, J.M.; Osheroff, N. Merbarone Inhibits the Catalytic Activity of Human Topoisomerase IIalpha by Blocking DNA Cleavage. J. Biol. Chem. 1998, 273, 17643–17650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimaggio, J.J.; Warrell, R.P., Jr.; Muindi, J.; Stevens, Y.W.; Lee, S.J.; Lowenthal, D.A.; Haines, I.; Walsh, T.D.; Baltzer, L.; Yaldaei, S.; et al. Phase I Clinical and Pharmacological Study of Merbarone. Cancer Res. 1990, 50, 1151–1155. [Google Scholar] [PubMed]
- Malik, U.R.; Dutcher, J.P.; Caliendo, G.; Lasala, P.; Mitnick, R.; Wiernik, P.H. Phase II Trial of Merbarone in Patients with Malignant Brain Tumors. Med. Oncol. 1997, 14, 159–162. [Google Scholar] [CrossRef]
- Ranise, A.; Spallarossa, A.; Schenone, S.; Bruno, O.; Bondavalli, F.; Pani, A.; Marongiu, M.E.; Mascia, V.; La Colla, P.; Loddo, R. Synthesis and antiproliferative activity of basic thioanalogues of merbarone. Bioorg. Med. Chem. 2003, 11, 2575–2589. [Google Scholar] [CrossRef]
- Spallarossa, A.; Rotolo, C.; Sissi, C.; Marson, G.; Greco, M.L.; Ranise, A.; La Colla, P.; Busonera, B.; Loddo, R. Further SAR studies on bicyclic basic merbarone analogues as potent antiproliferative agents. Bioorg. Med. Chem. 2013, 21, 6328–6336. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, J.M.; Brindani, N.; Franco-Ulloa, S.; Nigro, M.; Kuriappan, J.A.; Ottonello, G.; Bertozzi, S.M.; Summa, M.; Girotto, S.; Bertorelli, R.; et al. Design, synthesis, dynamic docking, biochemical characterization, and in vivo pharmacokinetics studies of novel topoisomerase II poisons with promising antiproliferative activity. J. Med. Chem. 2020, 63, 3508–3521. [Google Scholar] [CrossRef]
- Ortega, J.A.; Riccardi, L.; Minniti, E.; Borgogno, M.; Arencibia, J.M.; Greco, M.L.; Minarini, A.; Sissi, C.; De Vivo, M. Pharmacophore Hybridization to Discover Novel Topoisomerase II Poisons with Promising Antiproliferative Activity. J. Med. Chem. 2018, 61, 1375–1379. [Google Scholar] [CrossRef]
- Negri, C.; Bemardi, R.; Donzelli, M.; Scovassi, A.I. Induction of apoptotic cell death by DNA topoisomerase II inhibitors. Biochimie 1995, 77, 893–899. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Tshepelevitsh, S.; Kütt, A.; Lõkov, M.; Kaljurand, I.; Saame, J.; Heering, A.; Plieger, P.G.; Vianello, R.; Leito, I. On the basicity of organic bases in different media. Eur. J. Org. Chem. 2019, 40, 6735–6748. [Google Scholar] [CrossRef]
- Wendorff, T.J.; Schmidt, B.H.; Heslop, P.; Austin, C.A.; Berger, J.M. The Structure of DNA-Bound Human Topoisomerase II Alpha: Conformational Mechanisms for Coordinating Inter-Subunit Interactions with DNA Cleavage. J. Mol. Biol. 2012, 424, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.C.; Li, T.K.; Farh, L.; Lin, L.Y.; Lin, T.S.; Yu, Y.J.; Yen, T.J.; Chiang, C.W.; Chan, N.L. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 2011, 333, 459–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Cryst. 2011, D67, 235–242. [Google Scholar]





 | ||||||
|---|---|---|---|---|---|---|
| Cpd | R′ | X | R | Reaction Conditions | Yields (%) | mp (°C) |
| 3a | Me | H | –CH2CH2NEt2 | 80 °C, 4 h | 86 | 204–205 |
| 3b | Me | H | –CH2CH2-Pip | 80 °C, 4 h | 54 | 211–213 |
| 3c | Me | H | –CH2CH2CH2NMe2 | rt, 16 h | 90 | 214–215 |
| 3d | Me | H | –CH2CH2CH2N(nBu)2 | rt, 16 h | 51 | 184–185 |
| 3e | Me | H | –CH2CH2CH2N(CH2CH2)2NMe | rt, 16 h | 51 | 175–177 |
| 4a | Me | Cl | –CH2CH2NEt2 | 80 °C, 4 h | 54 | 229–231 |
| 4b | Me | Cl | –CH2CH2-Pip | 80 °C, 4 h | 38 | 237–239 |
| 4c | Me | Cl | –CH2CH2CH2NMe2 | rt, 16 h | 69 | 232–234 |
| 4d | Me | Cl | –CH2CH2CH2N(nBu)2 | rt, 16 h | 53 | 184–185 |
| 4e | Me | Cl | –CH2CH2CH2N(CH2CH2)2NMe | 80 °C, 4 h | 38 | 125–126 |
| 5a | Et | H | –CH2CH2NEt2 | rt, 16 h | 66 | 186–188 |
| 5b | Et | H | –CH2CH2-Pip | 80 °C, 2 h | 79 | 196–198 |
| 5c | Et | H | –CH2CH2CH2NMe2 | rt, 16 h | 59 | 191–193 |
| 5d | Et | H | –CH2CH2CH2N(nBu)2 | rt, 16 h | 23 | 88–90 |
| 5e | Et | H | –CH2CH2CH2N(CH2CH2)2NMe | 60 °C, 2 h | 28 | 163–165 |
| 6a | Et | Cl | –CH2CH2NEt2 | 100 °C, 5 min μwave | 51 | 167–169 |
| 6b | Et | Cl | –CH2CH2-Pip | 80 °C, 4 h | 60 | 210–212 |
| 6c | Et | Cl | –CH2CH2CH2NMe2 | rt, 16 h | 90 | 209–211 |
| 6d | Et | Cl | –CH2CH2CH2N(nBu)2 | rt, 16 h | 68 | 150–152 |
| 6e | Et | Cl | –CH2CH2CH2N(CH2CH2)2NMe | 100 °C, 5 min μwave | 70 | 183–184 |
| Growth Percent | Growth Percent | ||||
|---|---|---|---|---|---|
| Cpd | SKOV-3 | MCF-7 | Cpd | SKOV-3 | MCF-7 |
| 3a | 60.65 | 14.08 | 5a | 4.15 | 3.13 |
| 3b | 63.28 | 13.58 | 5b | 24.89 | −0.40 |
| 3c | −37.72 | −7.26 | 5c | −7.94 | 2.40 |
| 3d | −32.17 | −12.78 | 5d | −76.50 | −33.72 |
| 3e | 8.84 | 12.45 | 5e | −32.58 | −55.88 |
| 4a | 48.52 | 19.77 | 6a | 39.32 | 8.06 |
| 4b | 58.62 | 21.47 | 6b | 28.02 | 3.26 |
| 4c | 34.70 | 20.94 | 6c | −4.85 | −10.89 |
| 4d | 11.96 | 6.29 | 6d | 1.13 | −24.00 |
| 4e | 17.12 | 10.25 | 6e | −39.38 | −27.93 |
| 1d | −86.70 | −83.51 | 1d | −86.70 | −83.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spallarossa, A.; Lusardi, M.; Caneva, C.; Profumo, A.; Rosano, C.; Ponassi, M. Bicyclic Basic Merbarone Analogues as Antiproliferative Agents. Molecules 2021, 26, 557. https://doi.org/10.3390/molecules26030557
Spallarossa A, Lusardi M, Caneva C, Profumo A, Rosano C, Ponassi M. Bicyclic Basic Merbarone Analogues as Antiproliferative Agents. Molecules. 2021; 26(3):557. https://doi.org/10.3390/molecules26030557
Chicago/Turabian StyleSpallarossa, Andrea, Matteo Lusardi, Chiara Caneva, Aldo Profumo, Camillo Rosano, and Marco Ponassi. 2021. "Bicyclic Basic Merbarone Analogues as Antiproliferative Agents" Molecules 26, no. 3: 557. https://doi.org/10.3390/molecules26030557
APA StyleSpallarossa, A., Lusardi, M., Caneva, C., Profumo, A., Rosano, C., & Ponassi, M. (2021). Bicyclic Basic Merbarone Analogues as Antiproliferative Agents. Molecules, 26(3), 557. https://doi.org/10.3390/molecules26030557








