Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family
Abstract
1. Introduction
2. Results and Discussion
2.1. Color Analysis
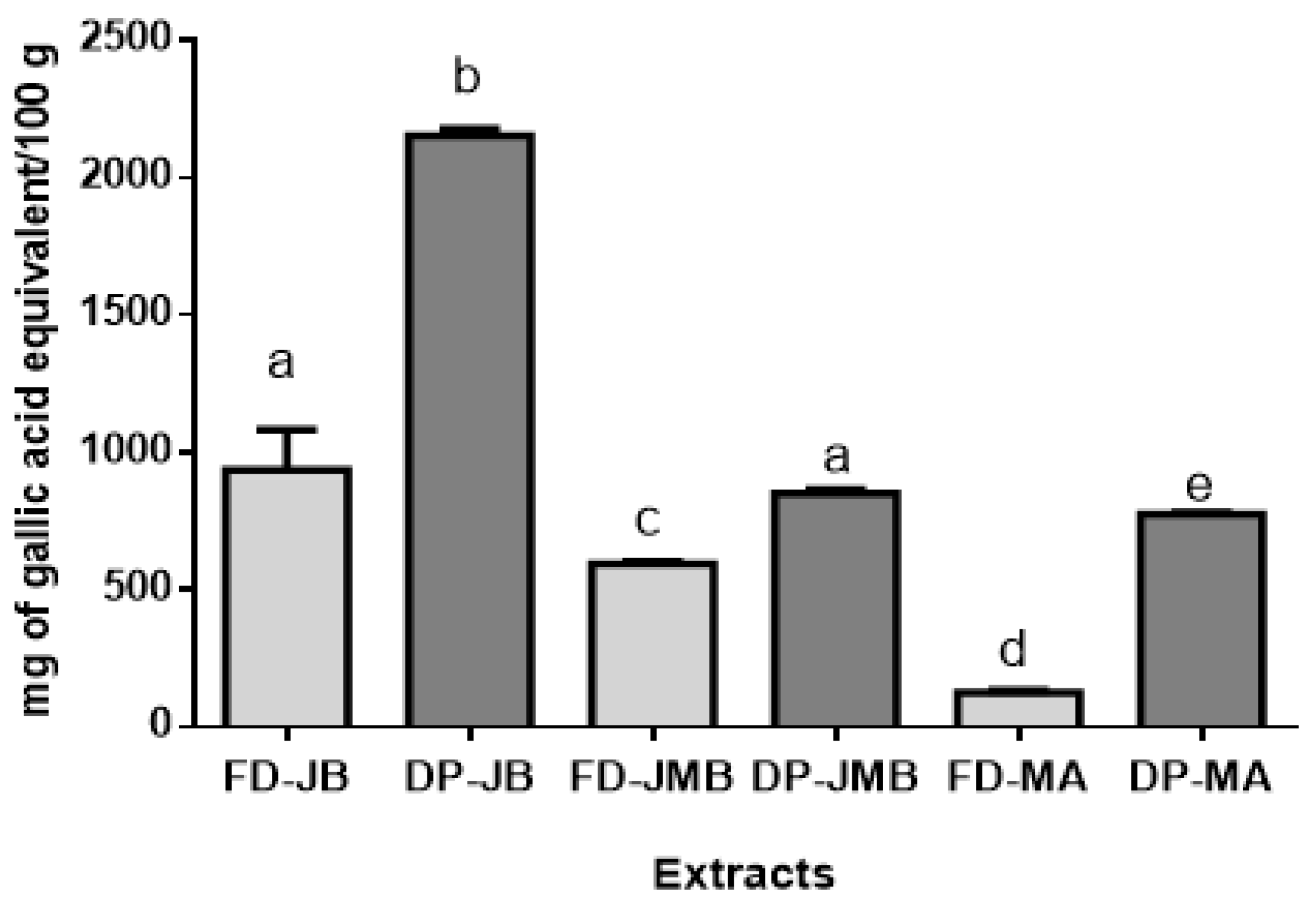
2.2. Phenolic Compound Content
2.3. Anthocyanin Quantification
2.4. Antioxidant Activity
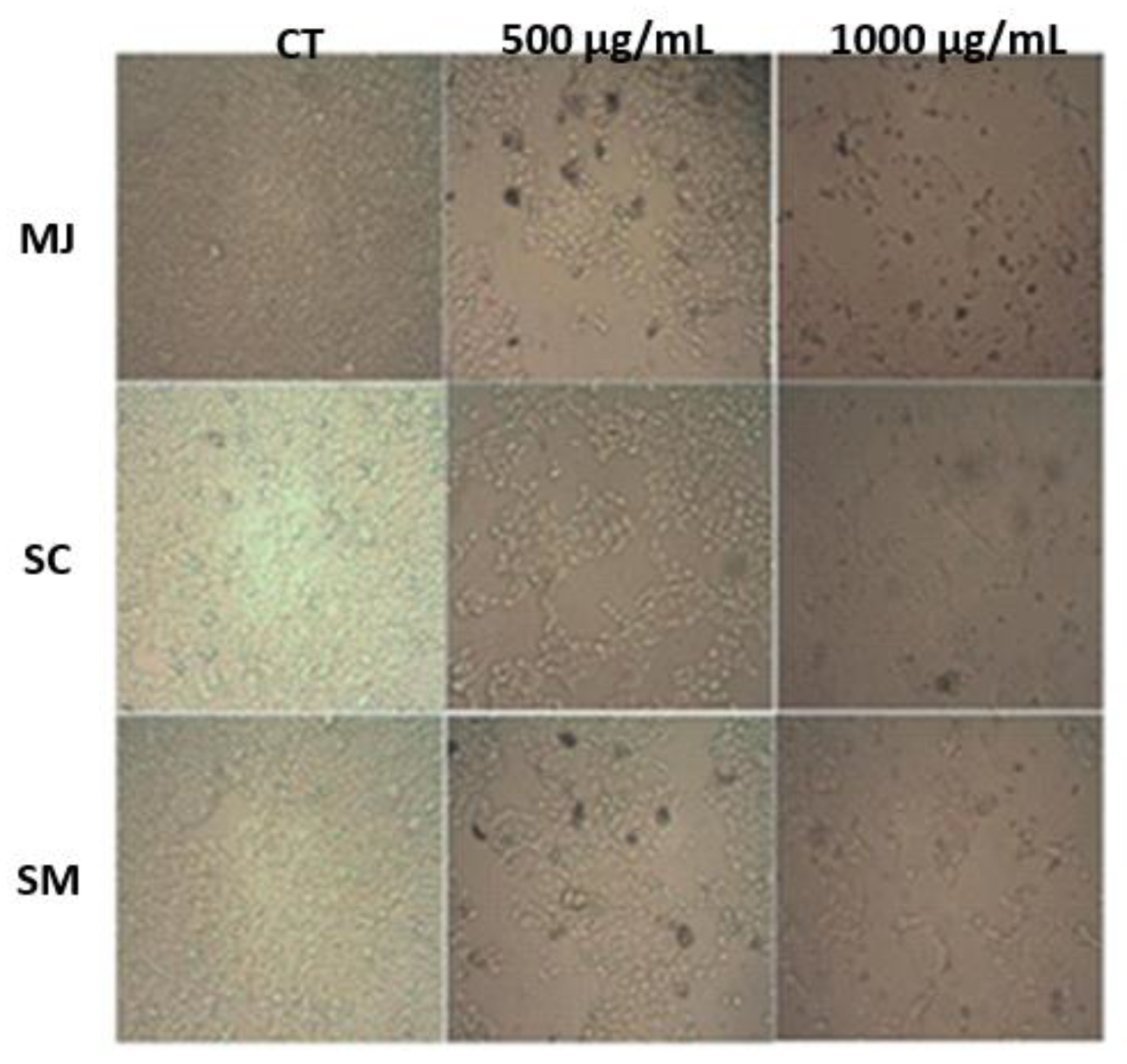
2.5. Cell Assays Results
2.5.1. Effect of M. jaboticaba (FD-MJ and DP-MJ), S. cumini (FD-SC and DP-SC), and S. malaccense (FD-SM and DP-SM) Extracts on Cell Viability
2.5.2. Effect of M. jaboticaba (DP-MJ), S. cumini (DP-SC), and S. malaccense (DP-SM) Extracts on the Cell Cycle
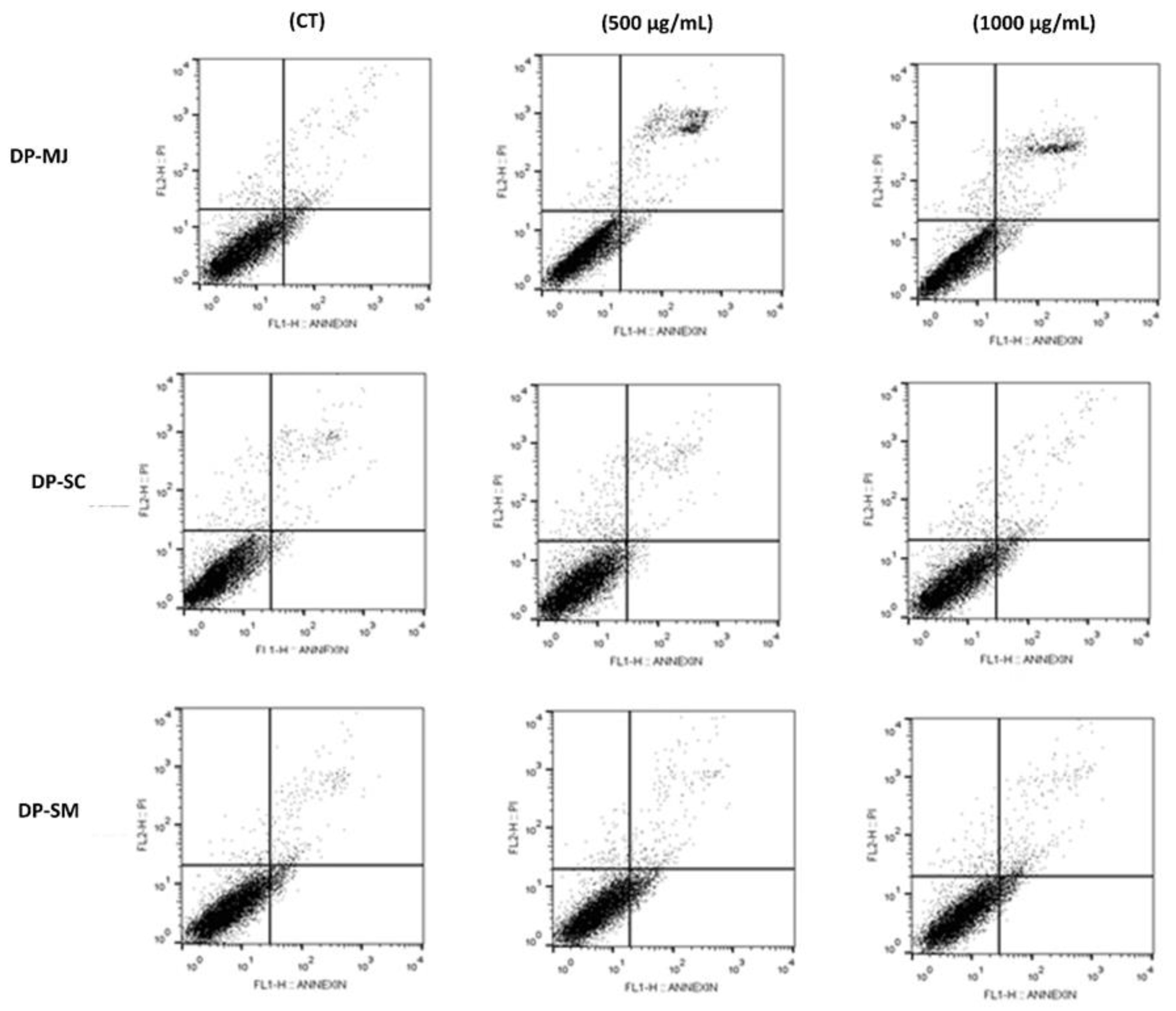
2.5.3. Apoptosis
3. Materials and Methods
3.1. Chemicals
3.2. Anthocyanin-Rich extracts
3.2.1. Samples
3.2.2. Dried Peel Powder and Freeze-Dried Extracts
3.3. Color Analysis
3.4. Total Phenolics Content
3.5. Anthocyanins Analysis
3.5.1. Sample Extraction
3.5.2. HPLC with MS
3.6. Antioxidant Activity
3.6.1. DPPH Assay
3.6.2. Trolox Equivalent Antioxidant Capacity (TEAC)
3.6.3. Ferric Reducing Ability (FRAP)
3.6.4. ORAC Assay
3.7. Cell Culture and Treatment Protocol
3.8. Cell Viability
3.9. Cell Cycle Analysis
3.10. Apoptosis Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Andrade, R.G., Jr.; Dalvi, L.T.; Silva, J.M.C., Jr.; Lopes, G.K.; Alonso, A.; Hermes-Lima, M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch. Biochem. Biophys. 2005, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Leite-Legatti, A.V.; Batista, Â.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; de Carvalho, J.E. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef] [PubMed]
- Frauches, N.S.; do Amaral, T.O.; Largueza, C.B.D.; Teodoro, A.J. Brazilian Myrtaceae fruits: A review of anticancer proprieties. J. Pharm. Res. Int. 2016, 12, 1–15. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; DeSantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Shi, Y. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017, 152, 1944–1953. [Google Scholar] [CrossRef]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models–An indispensable tool in food science and nutrition. Trends Food Sci. Technol. 2011, 22, S11–S20. [Google Scholar] [CrossRef]
- Hodin, R.A.; Meng, S.; Archer, S.; Tang, R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 1996, 7, 647–653. [Google Scholar]
- Lenaerts, K.; Bouwman, F.G.; Lamers, W.H.; Renes, J.; Mariman, E.C. Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genom. 2007, 8, 1–14. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Gupta, R.C. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L.(Jamun, the Indian Blackberry). Nutr Cancer. 2012, 64, 428–438. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Brown, E.; Gill, C.I.R.; Gordon, J.M.; Derek, S. Mechanisms Underlying the Anti-Proliferative Effects of Berry Components in In vitro Models of Colon Cancer. Curr. Pharm. Biotechnol. 2012, 13, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hunter Lab Applications Note; Hunter Associates Laboratories: Reston, VA, USA, 1996; Volume 8, pp. 1–15.
- Lawless, H.T.; Heymann, H. Color and Appearance. In Sensory Evaluation of Food: Principles and Practices, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2013; pp. 406–429. [Google Scholar]
- Montes, C.; Vicario, I.M.; Raymundo, M.; Fett, R.; Heredia, F.J. Application of tristimulus colorimetry to optimize the extraction of anthocyanins from Jaboticaba (Myricia Jaboticaba Berg.). Food Res. Int. 2005, 38, 983–988. [Google Scholar] [CrossRef]
- Batista, Â.G.; da Silva, J.K.; Cazarin, C.B.B.; Biasoto, A.C.T.; Sawaya, A.C.H.F.; Prado, M.A.; Júnior, M.R.M. Red-jambo (Syzygium malaccense): Bioactive compounds in fruits and leaves. Food Sci. Technol. 2017, 76, 284–291. [Google Scholar] [CrossRef]
- Sari, P.; Wijaya, C.H.; Sajuthi, D.; Supratman, U. Colour properties, stability, and free radical scavenging activity of jambolan (Syzygium cumini) fruit anthocyanins in a beverage model system: Natural and copigmented anthocyanins. Food Chem. 2012, 132, 1908–1914. [Google Scholar] [CrossRef]
- Santiago, M.C.; Gouvêa, A.C.; Peixoto, F.M.; Borguini, R.G.; Godoy, R.L.; Pacheco, S.; Nogueira, R.I. Characterization of jamelão (Syzygium cumini (L.) Skeels) fruit peel powder for use as natural colorant. Fruits 2016, 71, 3–8. [Google Scholar] [CrossRef]
- Shelembe, J.S.; Cromarty, D.; Bester, M.; Minnaar, A.; Duodu, K.G. Effect of acidic condition on phenolic composition and antioxidant potential of aqueous extracts from sorghum (Sorghum bicolor) bran. J. Food Biochem. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; De la Torre, R.; Corella, D.; Fiol, M. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Fiol, M. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The Predimed study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Phenolic compounds in fruit processing. Fruit Phenol. 1990, 1, 295–358. [Google Scholar]
- Figueiredo, F.J.; Lima, V.L.A.G. Antioxidant activity of anthocyanins from quixabeira (Sideroxylon obtusifolium) fruits. Rev. Bras. Plantas Med. 2015, 17, 473–479. [Google Scholar] [CrossRef][Green Version]
- Lenquiste, S.A.; Batista, Â.G.; da Silva Marineli, R.; Dragano, N.R.V.; Maróstica, M.R., Jr. Freeze-dried jaboticaba peel added to high-fat diet increases HDL-cholesterol and improves insulin resistance in obese rats. Food Res. Int. 2012, 49, 153–160. [Google Scholar] [CrossRef]
- Wu, S.B.; Long, C.; Kennelly, E.J. Phytochemistry and health benefits of jaboticaba, an emerging fruit crop from Brazil. Food Res. Int. 2013, 54, 148–159. [Google Scholar] [CrossRef]
- De Jesús Ornelas-Paz, J.; Cira-Chávez, L.A.; Gardea-Béjar, A.A.; Guevara-Arauza, J.C.; Sepúlveda, D.R.; Reyes-Hernández, J.; Ruiz-Cruz, S. Effect of heat treatment on the content of some bioactive compounds and free radical-scavenging activity in pungent and non-pungent peppers. Food Res. Int. 2013, 50, 519–525. [Google Scholar]
- Rezaire, A.; Robinson, J.C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Fils-Lycaon, B. Amazonian palm Oenocarpus bataua (“patawa”): Chemical and biological antioxidant activity–Phytochemical composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.J. Antioxidants in food: The significance of characterisation, identification, chemical and biological assays in determining the role of antioxidants in food. Foods 2017, 6, 68. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Sarfaraz, S.; Khan, N.; Kedlaya, R.; Setaluri, V.; Mukhtar, H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol. Appl. Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Wallace, A.M.; Adachi, S.; Gil, R.R.; Yang, H.; Basile, M.J.; Kennelly, E.J. Bioactive Depsides and Anthocyanins from Jaboticaba (Myrciaria cauliflora). J. Nat. Prod. 2006, 69, 1228–1230. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Vadde, R.; Walia, S.; Radhakrishnan, S.; Vanamala, J.K. Eugenia jambolana (Java plum) fruit extract exhibits anti-cancer activity against early stage human HCT-116 colon cancer cells and colon cancer stem cells. Cancers 2016, 8, 29. [Google Scholar] [CrossRef]
- Rabeta, M.S.; Chan, S.; Neda, G.D.; Lam, K.L.; Ong, M.T. Anticancer effect of underutilized fruits. Int. Food Res. J. 2013, 20, 551–556. [Google Scholar]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; Springer: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar]
- Correia, C.R. Evaluation of Pathogenic Potential of Aeromonas Spp. Strains Using In Vitro Methodologies. Doctoral Dissertation, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Lisboa, Portugal, 2013. [Google Scholar]
- Tsai, T.C.; Huang, H.P.; Chang, K.T.; Wang, C.J.; Chang, Y.C. Anthocyanins from roselle extract arrest cell cycle G2/M phase transition via ATM/Chk pathway in p53-deficient leukemia HL-60 cells. Environ. Toxicol. 2017, 32, 1290–1304. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- NSyed, D.; Chamcheu, J.C.; MAdhami, V.; Mukhtar, H. Pomegranate extracts and cancer prevention: Molecular and cellular activities. Curr. Med. Chem. Anticancer Agents 2013, 13, 1149–1161. [Google Scholar]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef]
- Wang, W.H.; Tyan, Y.C.; Chen, Z.S.; Lin, C.G.; Yang, M.H.; Yuan, S.S.; Tsai, W.C. Evaluation of the antioxidant activity and antiproliferative effect of the jaboticaba (Myrciaria cauliflora) seed extracts in oral carcinoma cells. BioMed Res. Int. 2014, 2014, 185946. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Mosele, J.I.; Macià, A.; Ludwig, I.A.; Motilva, M.J. Exploring the colonic metabolism of grape and strawberry anthocyanins and their in vitro apoptotic effects in HT-29 colon cancer cells. J. Agric. Food Chem. 2017, 65, 6477–6487. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Booth, L.A.; Tavallai, S.; Hamed, H.A.; Cruickshanks, N.; Dent, P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 2014, 26, 549–555. [Google Scholar] [CrossRef]
- Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brito, E.S.; Araújo, M.C.P.; Alves, R.E.; Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanins Present in Selected Tropical Fruits: Acerola, Jambolão, Jussara, and Guajiru. J. Agric. Food Chem. 2007, 55, 9389–9394. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, A.C.M.; Melo, A.; Santiago, M.C.; Peixoto, F.M.; Freitas, V.; Godoy, R.L.; Ferreira, I.M. Identification and quantification of anthocyanins in fruits from Neomitranthes obscura (DC.) N. Silveira an endemic specie from Brazil by comparison of chromatographic methodologies. Food Chem. 2015, 185, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, A.C.M.S.; Araujo, M.C.P.D.; Schulz, D.F.; Pacheco, S.; Godoy, R.L.D.O.; Cabral, L.M.C. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci. Technol. 2012, 32, 43–46. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; de Azevedo Maia, G.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef]

| Parameters | Freeze-Dried Extracts | Dried Peel Powder Extracts | ||||
|---|---|---|---|---|---|---|
| FD-MJ | FD-SC | FD-SM | DP-MJ | DP-SC | DP-SM | |
| L* | 32.46 ± 0.01 a | 43.91 ± 0.04 b | 53.21 ± 0.01 c | 19.12 ± 0.02 d | 21.93 ± 0.03 e | 34.21 ± 0.03 f |
| a* | 14.67 ± 0.02 a | 18.32 ± 0.01 b | 20.84 ± 0.01 c | 8.15 ± 0.01 d | 11.19 ± 0.02 e | 15.91 ± 0.01 f |
| b* | 4.98 ± 0.02 a | 2.56 ± 0.03 b | 9.65 ± 0.02 c | 3.03 ± 0.01 d | 1.59 ± 0.01 e | 7.59 ± 0.01 f |
| Anthocyanins (mg/100 g) | Freeze-Dried Extracts | Dried Peel Powder Extracts | ||||
|---|---|---|---|---|---|---|
| FD-MJ | FD-SC | FD-SM | DP-MJ | DP-SC | DP-SM | |
| Cyanidin-3,5-O-diglucoside | ND | 9.75 ± 2.37 a | 8.46 ± 2.06 b | ND | 11.18 ± 2.10 a | 11.37 ± 2.12 a |
| Cyanidin-3-O-glucoside | 171.39 ± 4.22 a | ND | 61.87 ± 5.78 b | 789.48 ± 3.98 c | ND | 144.68 ± 5.52 d |
| Delphinidin-3-O-glucoside | 9.30 ± 1.26 a | ND | ND | 13.42 ± 1.93 b | 206.26 ± 1.83 c | ND |
| Delphinidin-3,5-O-diglucoside | ND | 51.37 ± 6.35 | ND | ND | ND | ND |
| Petunidin-3,5-O-diglucoside | ND | 86.90 ± 9.24 a | ND | ND | 208.26 ± 2.70 b | ND |
| Malvidin-3,5-O-diglucoside | ND | 83.01 ± 8.17 a | ND | ND | 149.50 ± 6.44 b | ND |
| Total | 180.69 ± 0.78 a | 231.03 ± 0.32 b | 70.33 ± 0.72c | 802.90 ± 1.93 d | 575.20 ± 0.98 e | 156.05 ± 2.35 f |
| Assays | Freeze-Dried Extracts | Dried Peel Powder Extracts | ||||
|---|---|---|---|---|---|---|
| FD-MJ | FD-SC | FD-MA | DP-MJ | DP-SC | DP-MA | |
| DPPH (µmol trolox/g) | 554.44 ± 2.68 a | 324.93 ± 1.22 b | 61.24 ± 0.27 c | 576.02 ± 3.66 d | 378.46 ± 1.07 e | 100.27 ± 1.22 f |
| FRAP (µmol Fe3+/g) | 338.14 ± 3.15 a | 154.74 ± 3.51 b | 119.03 ± 2.29 c | 708.48 ± 3.40 d | 702.52 ± 2.79 d | 609.23 ± 3.51 e |
| TEAC (µmol trolox/g) | 987.15 ± 0.70 a | 1095.63 ± 3.76 b | 357.85 ± 1.00 c | 1271.91 ± 5.02 d | 1038.50 ± 2.98 e | 492.03 ± 3.99 f |
| ORAC (µmol trolox/g) | 508.81 ± 2.73 a | 241.92 ± 4.51 b | 21.23 ± 3.09 c | 883.94 ± 5.03 d | 610.23 ± 1.29 e | 570.18 ± 3.99 f |
| Cell Cycle Phase | CT | 500 µg/mL | 1000 µg/mL | |
|---|---|---|---|---|
| G0/G1 | 51.32 ± 0.03 | 51.56 ± 2.82 | 56.63 ± 2.31 * | |
| DP-MJ | S | 7.70 ±0.46 | 11.97 ± 1.11 * | 8.70 ± 0.66 |
| G2/M | 40.97 ± 2.73 | 36.45 ± 0.19 * | 32.27 ± 0.38 * | |
| G0/G1 | 41.82 ± 1.63 | 41.62 ± 2.27 | 44.05 ± 2.45 * | |
| DP-SC | S | 9.91 ± 1.89 | 12.34 ± 1.26 * | 11.63 ± 1.74 * |
| G2/M | 48.25 ±2.47 | 45.72 ± 3.54 * | 43.83± 2.45 * | |
| G0/G1 | 40.36 ± 2.45 | 42.36± 1.63 * | 46.31± 2.27 * | |
| DP-SM | S | 11.72 ± 1.74 | 11.00 ± 1.69 | 15.84 ± 1.98 * |
| G2/M | 47.91 ± 0.01 | 46.62 ± 0.53 | 44.91 ± 0.74 * |
| Stages of Cell Death | CT | DP-MJ (µg/mL) | DP-SC (µg/mL) | DP-SM (µg/mL) | |||
|---|---|---|---|---|---|---|---|
| 500 | 1000 | 500 | 1000 | 500 | 1000 | ||
| Viable cells (Annexin V−/PI−) | 95.50 ± 1.25 | 89.52 ± 0.47 * | 87.9 ± 3.00 * | 92.8 ± 2.00 | 91.00 ± 0.80 | 90.60 ± 0.62 | 91.95 ± 0.95 |
| Early apoptosis (Annexin V+/PI−) | 2.51 ± 0.78 | 6.88 ± 0.10 * | 7.25 ± 2.72 * | 2.27 ± 0.23 | 2.58 ± 0.22 | 3.00 ± 0.40 | 2.80 ± 0.86 |
| Late apoptosis (Annexin V+/PI+) | 1.57 ± 0.85 | 3.61 ± 0.09 * | 4.03 ± 0.13 ** | 1.08 ± 1.09 | 0.98 ± 0.40 | 0.82 ± 0.25 | 1.27 ± 0.11 |
| Non-apoptotic cells (Annexin V−/PI+) | 4.04 ± 1.08 | 2.62 ± 0.11 * | 1.77 ± 1.14 * | 1.84 ± 0.66 * | 5.45 ± 1.01 | 5.60 ± 1.02 | 3.97 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simas Frauches, N.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules 2021, 26, 564. https://doi.org/10.3390/molecules26030564
Simas Frauches N, Montenegro J, Amaral T, Abreu JP, Laiber G, Junior J, Borguini R, Santiago M, Pacheco S, Nakajima VM, et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules. 2021; 26(3):564. https://doi.org/10.3390/molecules26030564
Chicago/Turabian StyleSimas Frauches, Nayara, Júlia Montenegro, Thuane Amaral, Joel Pimentel Abreu, Gabriela Laiber, Jorge Junior, Renata Borguini, Manuela Santiago, Sidney Pacheco, Vania Mayumi Nakajima, and et al. 2021. "Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family" Molecules 26, no. 3: 564. https://doi.org/10.3390/molecules26030564
APA StyleSimas Frauches, N., Montenegro, J., Amaral, T., Abreu, J. P., Laiber, G., Junior, J., Borguini, R., Santiago, M., Pacheco, S., Nakajima, V. M., Godoy, R., & Teodoro, A. J. (2021). Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules, 26(3), 564. https://doi.org/10.3390/molecules26030564








