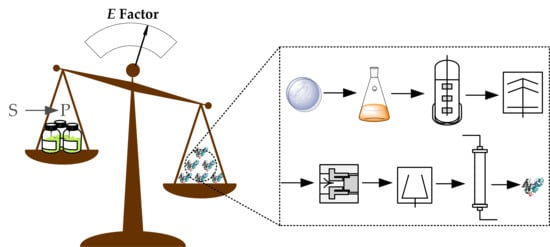
Environmental Assessment of Enzyme Production and Purification
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Recombinant Expression
3.2. Protein Purification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Unit | Plating E. coli | Pre-Culture | Inoculum Pre-Fermenter | Pre-Fermenter | Main- Fermenter | |
|---|---|---|---|---|---|---|
| Water | L | 0.05 | 0.05 | 2.95 | 27 | 245 |
| Tryptone | g | 0.5 | 0.8 | 47.2 | 432 | 3920 |
| Yeast Extract | g | 0.25 | 0.5 | 29.5 | 270 | 2450 |
| NaCl | g | 0.25 | 0.25 | 14.75 | 135 | 1225 |
| Agar | g | 0.75 | - | - | - | - |
| IPTG | g | - | - | - | - | 29.2 |
| Chloramphenicol | mg | 0.6 | 0.6 | 35.4 | 324 | 2940 |
| Kanamycin | mg | 2.5 | 2.5 | 147.5 | 1350 | 12,250 |
| Energy | kWh | 13.68 | 2 | 14.8 | 100.6 | 412.2 |
| Unit | Cell Harvesting | Cell Disruption | Centrifugation | Affinity Chromatography | Buffer Exchange | |
|---|---|---|---|---|---|---|
| Water | L | - | 5 | - | 23 | 63 |
| EtOH | L | - | - | - | 0.5 | 3.2 |
| Tris-HCl | g | - | 39.4 | - | 96.2 | - |
| NaCl | g | - | 87.65 | - | 223.6 | - |
| Imidazole | g | - | 13.65 | - | 139.7 | - |
| TCEP | g | - | 1.435 | - | - | - |
| HEPES | g | - | - | - | - | 173.5 |
| MgCl2 · 6 H2O | g | - | - | - | - | 37 |
| Energy | kWh | 17.55 | 26.03 | 4.6 | 8.8 | 3.2 |
References
- Straathof, A.J.J.; Panke, S.; Schmid, A. The Production of Fine Chemicals by Biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EN ISO. ISO 14044: Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Organisation for Standardisation: Geneva, Switzerland, 2006. [Google Scholar]
- Ni, Y.; Holtmann, D.; Hollmann, F. How Green Is Biocatalysis? To Calculate Is to Know. ChemCatChem 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Woodley, J.M. Bioprocesses: Modeling Needs for Process Evaluation and Sustainability Assessment. Comput. Chem. Eng. 2010, 34, 1009–1017. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen Years On. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Tieves, F.; Tonin, F.; Fernández-Fueyo, E.; Robbins, J.M.; Bommarius, B.; Bommarius, A.S.; Alcalde, M.; Hollmann, F. Energising the E-Factor: The E + -Factor. Tetrahedron 2019, 75, 1311–1314. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménez-González, C.; Preston, C.; Constable, D.J.C.; Woodley, J.M. EHS & LCA Assessment for 7-ACA Synthesis A Case Study for Comparing Biocatalytic and Chemical Synthesis. Ind. Biotechnol. 2008, 4, 180–192. [Google Scholar]
- Ma, S.K.; Gruber, J.; Davis, C.; Newman, L.; Gray, D.; Wang, A.; Grate, J.; Huisman, G.W.; Sheldon, R.A. A Green-by-Design Biocatalytic Process for Atorvastatin Intermediate. Green Chem. 2010, 12, 81–86. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. CGAS Produces a 2′-5′-Linked Cyclic Dinucleotide Second Messenger That Activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubensky, T.W.; Kanne, D.B.; Leong, M.L. Rationale, Progress and Development of Vaccines Utilizing STING-Activating Cyclic Dinucleotide Adjuvants. Ther. Adv. Vaccines 2013, 1, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D.; Bode, M.L. The Hitchhiker’s Guide to Biocatalysis: Recent Advances in the Use of Enzymes in Organic Synthesis. Chem. Sci. 2020, 11, 2587–2605. [Google Scholar] [CrossRef] [Green Version]
- Farid, S.S.; Baron, M.; Stamatis, C.; Nie, W.; Coffman, J. Benchmarking Biopharmaceutical Process Development and Manufacturing Cost Contributions to R&D. MAbs 2020, 12, 1754999. [Google Scholar] [CrossRef]
- Rosenthal, K.; Becker, M.; Rolf, J.; Siedentop, R.; Hillen, M.; Nett, M.; Lütz, S. Catalytic Promiscuity of CGAS: A Facile Enzymatic Synthesis of 2′-3′-Linked Cyclic Dinucleotides. ChemBioChem 2020, 21, 3225–3228. [Google Scholar] [CrossRef]
- Novotná, B.; Vaneková, L.; Zavřel, M.; Buděšínský, M.; Dejmek, M.; Smola, M.; Gutten, O.; Tehrani, Z.A.; Pimková Polidarová, M.; Brázdová, A.; et al. Enzymatic Preparation of 2′–5′,3′–5′-Cyclic Dinucleotides, Their Binding Properties to Stimulator of Interferon Genes Adaptor Protein, and Structure/Activity Correlations. J. Med. Chem. 2019, 62, 10676–10690. [Google Scholar] [CrossRef]
- Waegeman, H.; De Lausnay, S.; Beauprez, J.; Maertens, J.; De Mey, M.; Soetaert, W. Increasing Recombinant Protein Production in Escherichia Coli K12 through Metabolic Engineering. New Biotechnol. 2013, 30, 255–261. [Google Scholar] [CrossRef]
- Restaino, O.F.; Borzacchiello, M.G.; Scognamiglio, I.; Fedele, L.; Alfano, A.; Porzio, E.; Manco, G.; De Rosa, M.; Schiraldi, C. High Yield Production and Purification of Two Recombinant Thermostable Phosphotriesterase-like Lactonases from Sulfolobus Acidocaldarius and Sulfolobus Solfataricus Useful as Bioremediation Tools and Bioscavengers. BMC Biotechnol. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Rosano, G.L.; Morales, E.S.; Ceccarelli, E.A. New Tools for Recombinant Protein Production in Escherichia Coli: A 5-Year Update. Protein Sci. 2019, 28, 1412–1422. [Google Scholar] [CrossRef]
- Hoffman, B.J.; Broadwater, J.A.; Johnson, P.; Harper, J.; Fox, B.G.; Kenealy, W.R. Lactose Fed-Batch Overexpression of Recombinant Metalloproteins in Escherichia Coli BL21(DE3): Process Control Yielding High Levels of Metal-Incorporated, Soluble Protein. Protein Expr. Purif. 1995, 6, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Icha, P.; Kuhs, G. Climate Change: Entwicklung Der Spezifischen Kohlendioxid-Emissionen Des Deutschen Strommix in Den Jahren 1990–2019; Umweltbundesamt: Dessau-Roßlau, Germany, 2020. [Google Scholar]
- Velasco-Lozano, S.; da Silva, E.S.; Llop, J.; López-Gallego, F. Sustainable and Continuous Synthesis of Enantiopure L-Amino Acids by Using a Versatile Immobilised Multienzyme System. ChemBioChem 2018, 19, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Grajales-Hernández, D.; Armendáriz-Ruiz, M.; Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C. Chitosan-Based CLEAs from Aspergillus Niger Type A Feruloyl Esterase: High-Productivity Biocatalyst for Alkyl Ferulate Synthesis. Appl. Microbiol. Biotechnol. 2020, 104, 10033–10045. [Google Scholar] [CrossRef]
- Sheldon, R.A. E Factors, Green Chemistry and Catalysis: An Odyssey. Chem. Commun. 2008, 29, 3352–3365. [Google Scholar] [CrossRef]
- Rosenthal, K.; Lütz, S. Recent Developments and Challenges of Biocatalytic Processes in the Pharmaceutical Industry. Curr. Opin. Green Sustain. Chem. 2018, 11, 58–64. [Google Scholar] [CrossRef]
- Serra, I.; Daly, S.; Alcantara, A.R.; Bianchi, D.; Terreni, M.; Ubiali, D. Redesigning the Synthesis of Vidarabine via a Multienzymatic Reaction Catalyzed by Immobilized Nucleoside Phosphorylases. RSC Adv. 2015, 5, 23569–23577. [Google Scholar] [CrossRef]
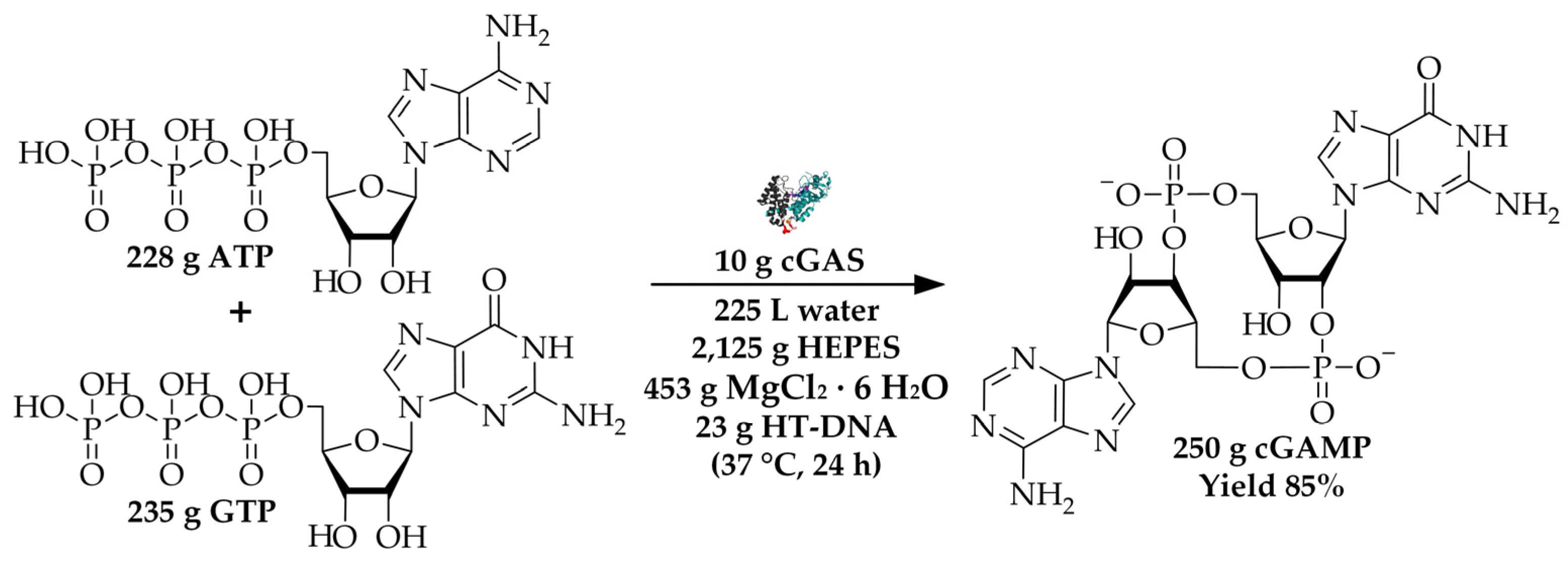



| Chemicals | [g] |
|---|---|
| Tryptone | 4401 |
| Yeast Extract | 2750 |
| NaCl | 1687 |
| Agar | 2 |
| Kanamycin | 14 |
| Chloramphenicol | 3 |
| IPTG 1 | 29 |
| Imidazole | 153 |
| TCEP 2 | 2 |
| Tris-HCl | 136 |
| HEPES 3 | 174 |
| MgCl2 · 6 H2O | 37 |
| Ethanol | 2919 |
| Water | 366,150 |
| cGAS [g·g−1] (This Study) | AoFOx [g·g−1] [8] | rAaeUPO [g·g−1] [8] | |
|---|---|---|---|
| simple E factor | 938 | 4300 | 18,500 |
| complete E factor | 37,835 | 106,100 | 209,000 |
| E+ factor | 62,033 | 157,800 | 566,800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, M.; Lütz, S.; Rosenthal, K. Environmental Assessment of Enzyme Production and Purification. Molecules 2021, 26, 573. https://doi.org/10.3390/molecules26030573
Becker M, Lütz S, Rosenthal K. Environmental Assessment of Enzyme Production and Purification. Molecules. 2021; 26(3):573. https://doi.org/10.3390/molecules26030573
Chicago/Turabian StyleBecker, Martin, Stephan Lütz, and Katrin Rosenthal. 2021. "Environmental Assessment of Enzyme Production and Purification" Molecules 26, no. 3: 573. https://doi.org/10.3390/molecules26030573
APA StyleBecker, M., Lütz, S., & Rosenthal, K. (2021). Environmental Assessment of Enzyme Production and Purification. Molecules, 26(3), 573. https://doi.org/10.3390/molecules26030573