The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crude Extract, Alkaloid-Enriched Fraction and Their Antitrypanosomal Activities
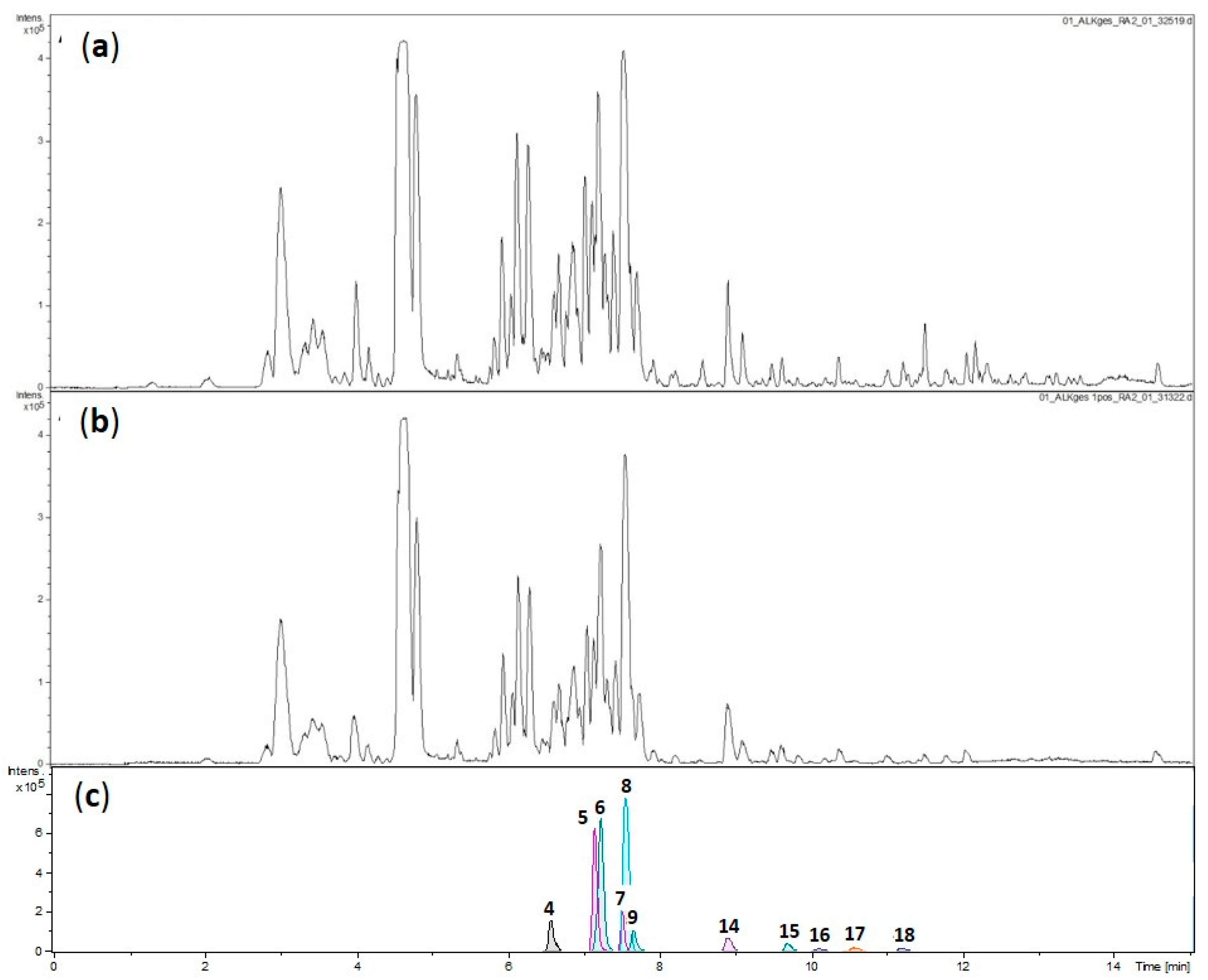
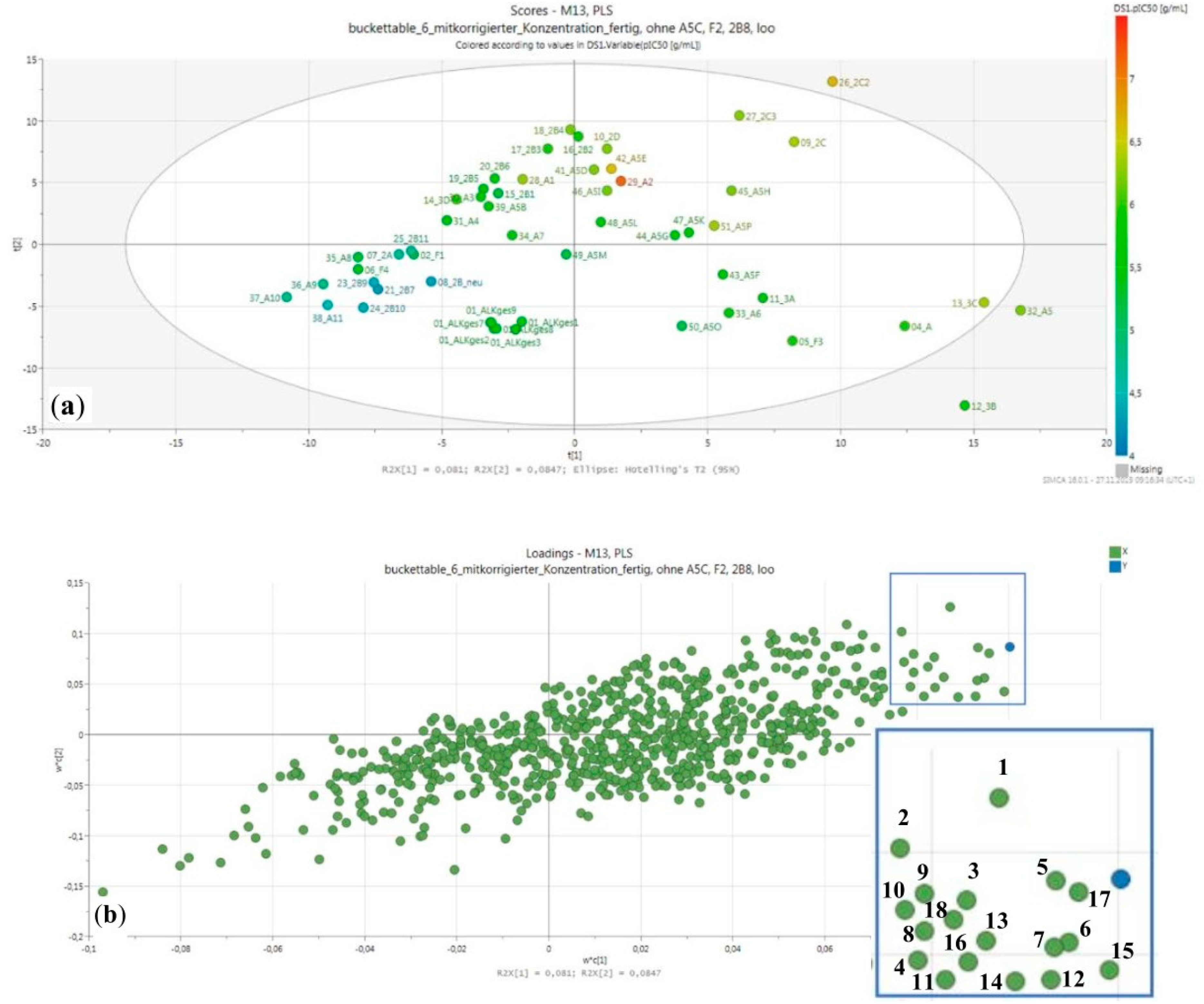
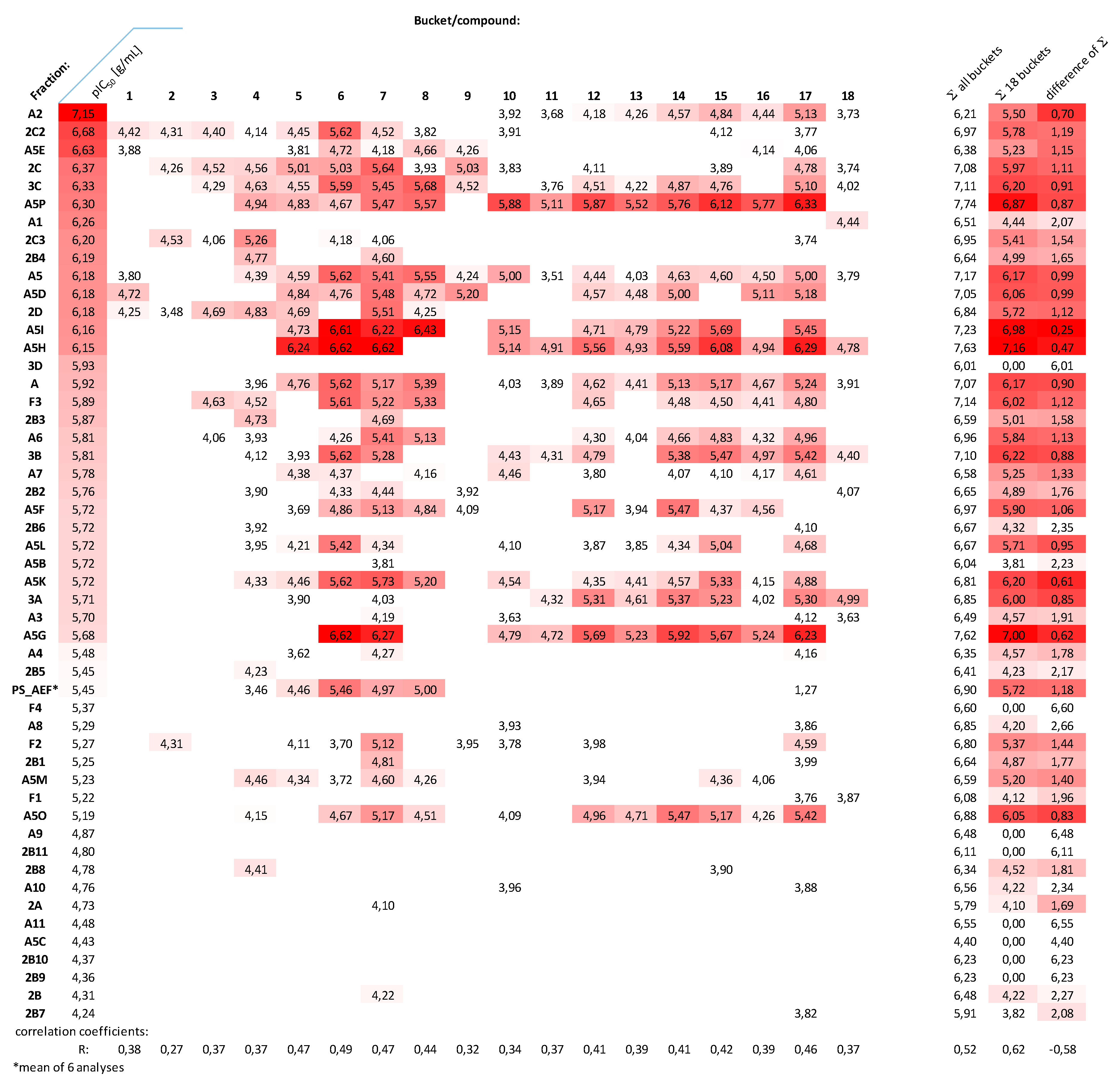
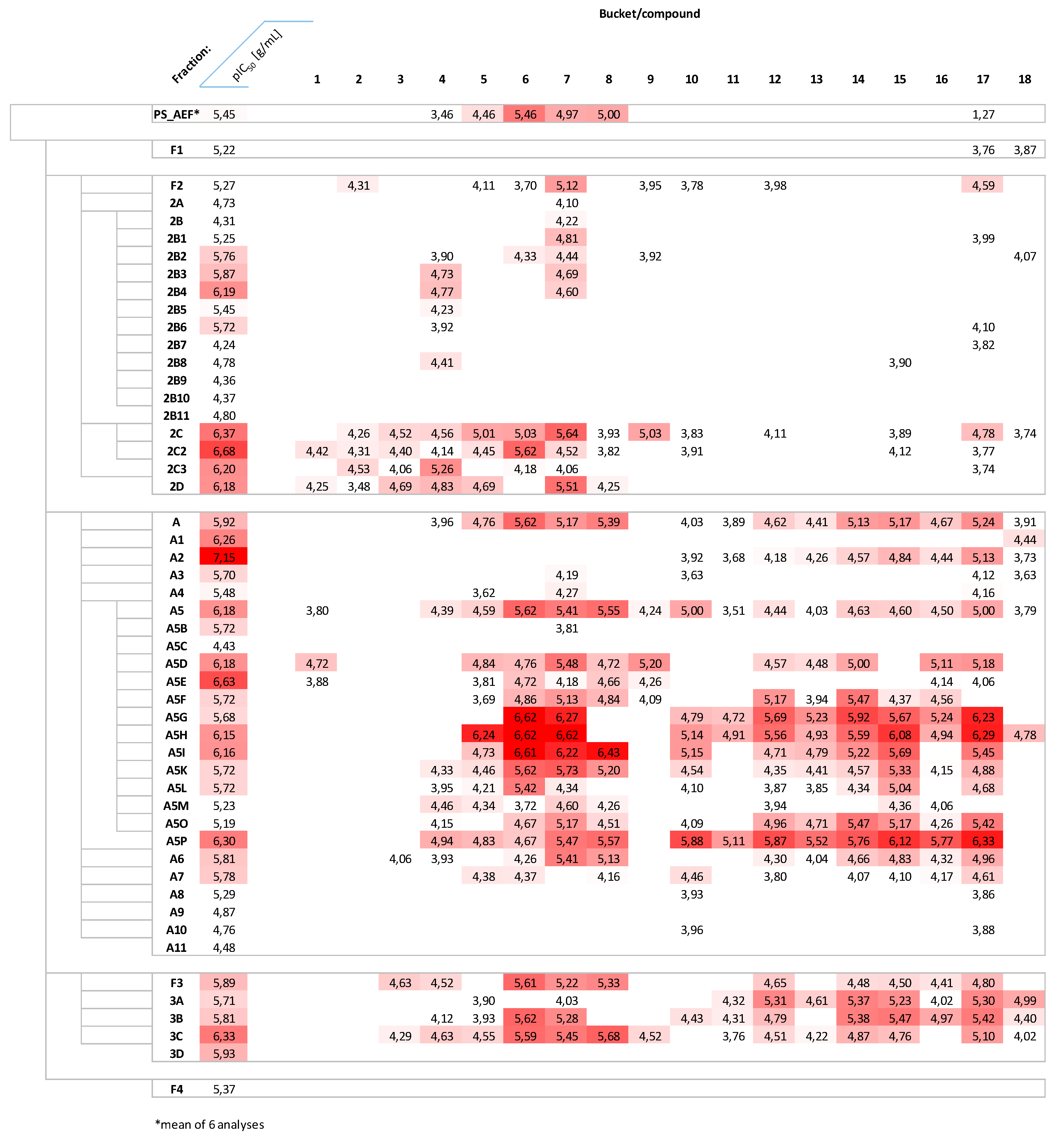
2.2. Fractionation, Bioactivity Testing and Localization of Presumably Active Constituents by UHPLC–MS Profiling and PLS Regression Modelling
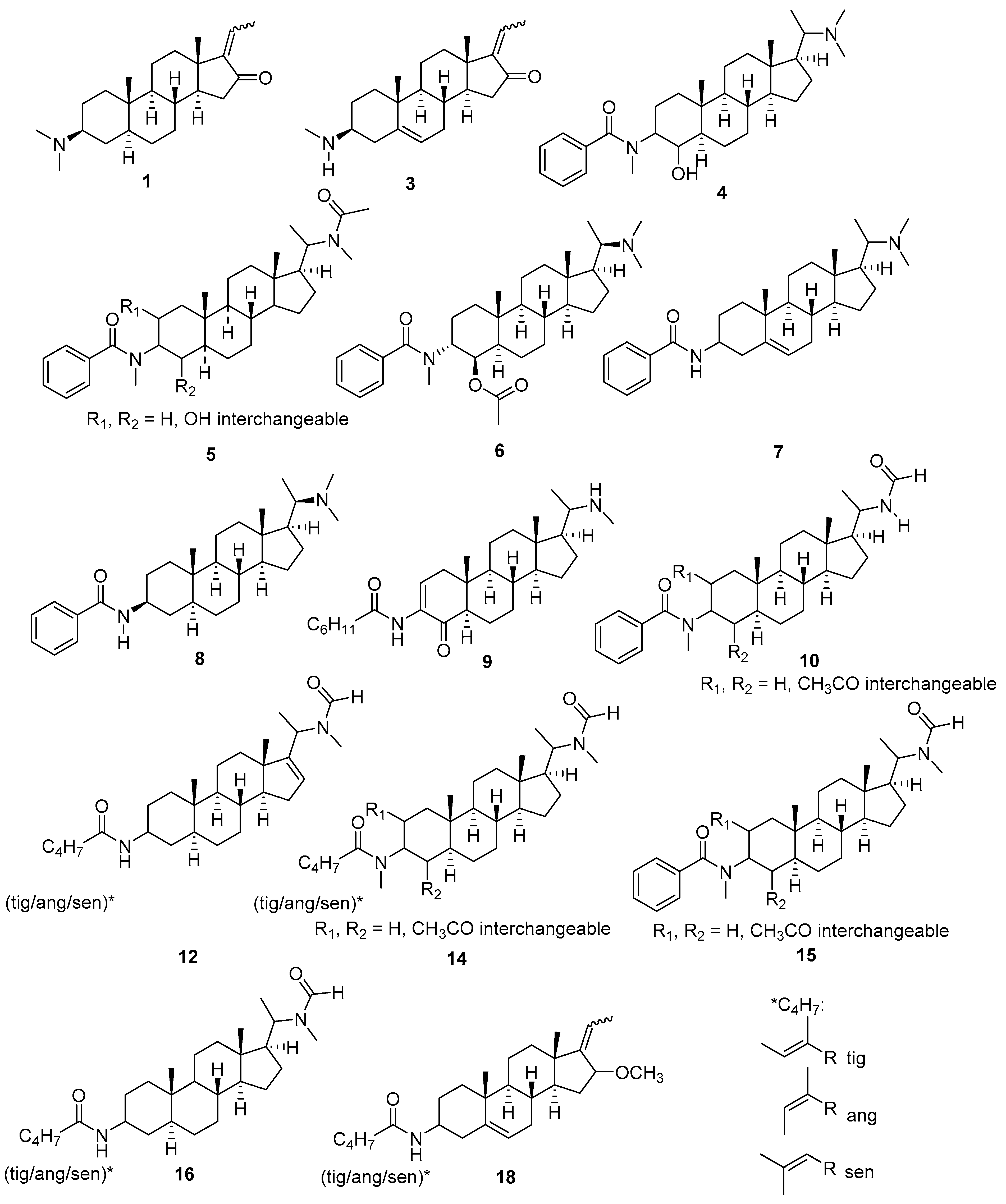
2.3. Structural Characterization of Presumably Active Constituents Based on Their +ESI QTOF Mass Spectra
| Comp. | Bucket [tR:m/z] | [M + H]+ Elem. Formula | Prominent Fragments | Identity Assignment |
|---|---|---|---|---|
| 1 | 6.21:344.289 | C23H38NO | 299;281;107 | E/Z-Salignone 1 |
| 2 | 6.63:222.151 | n.a. | n.a. | |
| 3 | 6.72:328.263 | C22H34NO | 297;279;119;105 | 3β-Methylamino-16-oxo-5,17(20)-cis/trans-pregnadiene 1 |
| 4 | 6.82:481.355 | C31H49N2O2 | 436;314;283;105 | see Figure 3 3 |
| 5 | 7.15:509.375 | C32H49N2O3 | 418;283;314;105 | see Figure 3 3 |
| 6 | 7.24:523.392 | C33H51N2O3 | 478;436;418;283;136;105 | Pachysandrin A 1 |
| 7 | 7.57:449.340 | C30H45N2O | 418;387;122;105 | see Figure 3 3 |
| 8 | 7.58:451.368 | C30H47N2O | 406;285;122;105 | Epipachysamine D 2 |
| 9 | 8.02:455.385 | C29H47N2O2 | 424;406;314;279;136;122;111 | see Figure 3 3 |
| 10 | 9.22:523.356 | C32H47N2O4 | 463;441;283,136;105 | see Figure 3 3 |
| 11 | 9.72:469.345 | C29H45N2O3 | 387;359;328;300 | n.a. |
| 12 | 9.79:441.346 | C28H45N2O2 | 359;328;283;121;100;86;83 | see Figure 3 3 |
| 13 | 9.80:471.360 | C29H47N2O3 | 387;359;328;300 | n.a. |
| 14 | 9.88:515.386 | C31H51N2O4 | 455;433;373;314;283;114;86 | see Figure 3 3 |
| 15 | 9.99:537.370 | C33H49N2O4 | 477;455;342;283;136,105;86 | see Figure 3 3 |
| 16 | 10.10:443.365 | C28H47N2O2 | 443;384;285;100;86;83 | see Figure 3 3 |
| 17 | 10.62:477.336 | C31H45N2O2 | 477;418;392;172;86 | n.a. |
| 18 | 11.18:412.319 | C27H42NO2 | 386;313;281;157;131;100;83 | see Figure 3 3 |
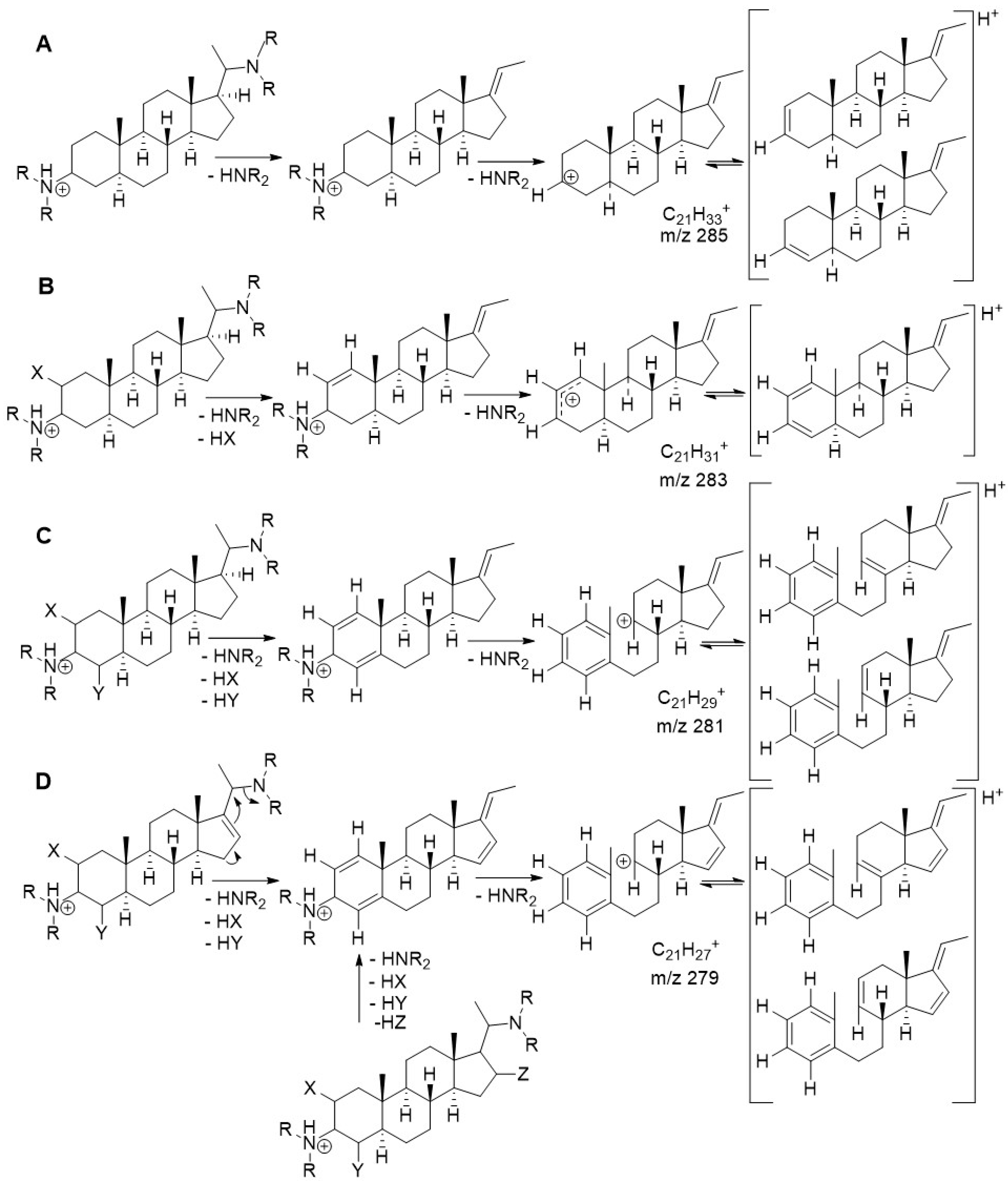
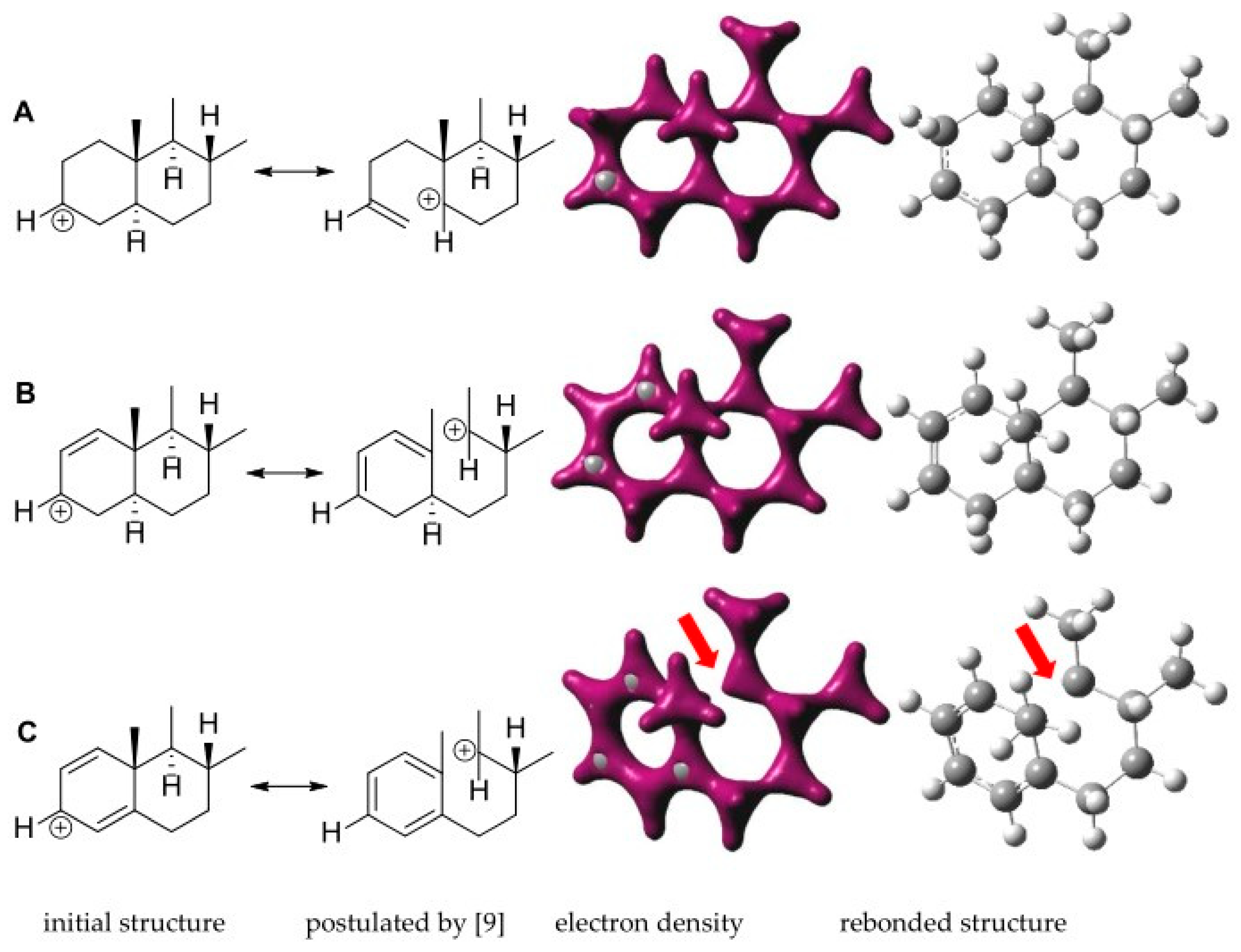
2.4. Quantum Mechanical Assessment of the (+)ESI MS Fragmentation
2.5. Distribution of Presumably Active Compounds in the Various Fractions of PS_AEF
3. Materials and Methods
3.1. Plant Material
3.2. General Methods
3.3. Extraction and Fractionation
3.4. UHPLC-(+)ESI QTOF MS and MS/MS Analysis
3.5. Data Pretreatment of LC–MS-Data
3.6. Multivariate Data Analysis—PLS Regression Modeling
3.7. Biological Testing
3.8. Quantum Mechanical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Trypanosomiasis, Human African (Sleeping-Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 5 January 2021).
- Chapuis, F. Oral Fexinidazole for Human African Trypanosomiasis. Lancet 2018, 391, 100–102. [Google Scholar] [CrossRef]
- Nnadi, C.O.; Nwodo, N.J.; Kaiser, M.; Brun, R.; Schmidt, T.J. Steroid Alkaloids from Holarrhena africana with Strong Activity against Trypanosoma brucei rhodesiense. Molecules 2017, 22, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nnadi, C.O.; Althaus, J.B.; Nwodo, N.J.; Schmidt, T.J. A 3D-QSAR Study on the Antitrypanosomal and Cytotoxic Activities of Steroid Alkaloids by Comparative Molecular Field Analysis. Molecules 2018, 23, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nnadi, C.O.; Ebiloma, G.U.; Black, J.A.; Nwodo, N.J.; Lemgruber, L.; Schmidt, T.J.; de Koning, H.P. Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs. Molecules 2019, 24, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funayama, S.; Noshita, T.; Shinoda, K.; Haga, N.; Nozoe, S.; Hayashi, M.; Komiyama, K. Cytotoxic Alkaloids of Pachysandra terminalis. Biol. Pharm. Bull. 2000, 23, 262–264. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Y.; Yu, Y.; Jia, M.; Jin, M.-N.; Qin, N.; Zhao, C.; Duan, H.-Q. Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules 2016, 21, 1283. [Google Scholar] [CrossRef]
- Althaus, J.B.; Jerz, G.; Winterhalter, P.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal Activity of Buxus Sempervirens and Activity-Guided Isolation of O-Tigloyl-Cyclovirobuxeine-B as Main Constituent Active against Plasmodium falciparum. Molecules 2014, 19, 6184–6201. [Google Scholar] [CrossRef] [Green Version]
- Musharraf, S.G.; Goher, M.; Ali, A.; Adhikari, A.; Choudhary, M.I.; Rahman, A. Rapid Characterization and Identification of Steroidal Alkaloids in Sarcococca coriacea Using Liquid Chromatography Coupled with Electrospray Ionization Quadropole Time-of-Flight Mass Spectrometry. Steroids 2012, 77, 138–148. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ueo, S.; Nishinaga, T. Pachysandra Alkaloids. V. Structure of Epipachysamine-A, -B, -C, -D, -E, and -F. Chem. Pharm. Bull. 1967, 15, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Gazolla, M.C.; Marques, L.M.M.; e Silva, M.G.; Araújo, M.T.M.F.; Mendes, R.L.; da Silva Almeida, J.R.G.; Vessecchi, R.; Lopes, N.P. Characterization of 3—Aminospirostane Alkaloids from Roots of Solanum paniculatum L. with Hepatoprotective Activity. Rapid Commun Mass Spectrom. 2020, 34, e8705. [Google Scholar] [CrossRef]
- Zhai, H.-Y.; Zhao, C.; Zhang, N.; Jin, M.-N.; Tang, S.-A.; Qin, N.; Kong, D.-X.; Duan, H.-Q. Alkaloids from Pachysandra terminalis Inhibit Breast Cancer Invasion and Have Potential for Development as Antimetastasis Therapeutic Agents. J. Nat. Prod. 2012, 75, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Uyeo, S., Jr.; Ando, M.; Yamamoto, A. Studies on the Alkaloids of Pachysandra terminalis Sieb. et Zucc. (3): Systematic Separation and Characterization of Alkaloids. Tetrahedron Lett. 1964, 5, 1817–1823. [Google Scholar] [CrossRef]
- Ali, A.; Jan, N.U.; Ali, S.; Ahmad, B.; Ali, A.; Samrana, S.; Jahan, A.; Ali, H.; Khan, I.A.; Rahim, H.; et al. Steroidal Alkaloids Efficient Aromatase Inhibitors with Potential for the Treatment of Postmenopausal Breast Cancer. Chem. Biol. Drug Des. 2020, 95, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.-U.; Haq, Z.-U.; Feroz, F.; Khalid, A.; Nawaz, S.A.; Khan, M.R.; Choudhary, M.I. New Cholinesterase-Inhibiting Steroidal Alkaloids from Sarcococca saligna. Helv. Chim. Acta 2004, 87, 439–448. [Google Scholar] [CrossRef]
- Zou, Z.-M.; Li, L.-J.; Yang, M.; Yu, S.-S.; Cong, P.-Z.; Yu, D.-Q. Steroidal Alkaloids from Roots of Sarcococca vagans. Phytochemistry 1997, 46, 1091–1093. [Google Scholar] [CrossRef]
- Wu, J.-C.; Huo, S.-J.; Du, J. 4-Dehydroxyepisarcovagine a, a New Steroidal Alkaloid from Sarcococca pruniformis Lindl. Nat. Prod. Res. 2019, 33, 169–173. [Google Scholar] [CrossRef]
- Kalauni, S.K.; Choudhary, M.I.; Shaheen, F.; Manandhar, M.D.; Rahman, A.-U.; Gewali, M.B.; Khalid, A. Steroidal Alkaloids from the Leaves of Sarcococca coriacea of Nepalese Origin. J. Nat. Prod. 2001, 64, 842–844. [Google Scholar] [CrossRef]
- Naeem, I.; Khan, T.M.; Anwar, R. A New Pregnane-Type Alkaloid from Sarcococca saligna. J. Appl. Sci. 2005, 5, 1182–1184. [Google Scholar] [CrossRef]
- Zhang, P.-Z.; Wang, F.; Yang, L.-J.; Zhang, G.-L. Pregnane Alkaloids from Sarcococca hookeriana Var. digyna. Fitoterapia 2013, 89, 143–148. [Google Scholar] [CrossRef]
- Babar, Z.U.; Ata, A.; Meshkatalsadat, M.H. New Bioactive Steroidal Alkaloids from Buxus hyrcana. Steroids 2006, 71, 1045–1051. [Google Scholar] [CrossRef]
- Szabó, L.U.; Schmidt, T.J. Target-Guided Isolation of O-Tigloylcyclovirobuxeine-B from Buxus Sempervirens L. by Centrifugal Partition Chromatography. Molecules 2020, 25, 4804. [Google Scholar] [CrossRef] [PubMed]
- Bernal, F.A.; Kaiser, M.; Wünsch, B.; Schmidt, T.J. Structure—Activity Relationships of Cinnamate Ester Analogs as Potent Antiprotozoal Agents. ChemMedChem 2020, 15, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Tbr | Tc | Ld | Pf | Cytotox (L6) |
|---|---|---|---|---|---|
| PS_DCM | 4.2 1 | 93 1 | 41 1 | >50 1 | n.t. |
| PS_AEF | 0.39 ± 0.08 2 | 8.5 1 | 34 1 | 1.8 1 | 9.5 ± 2.8 2 |
| PS_NF | 7.0 1 | 60 1 | >100 1 | 28 1 | >100 1 |
| Pos. control 2 | 0.004 ± 0.001 3 | 0.57 ± 0.04 4 | 0.09 ± 0.03 5 | 0.002 ± 0.001 6 | 0.004 ± 0.001 7 |
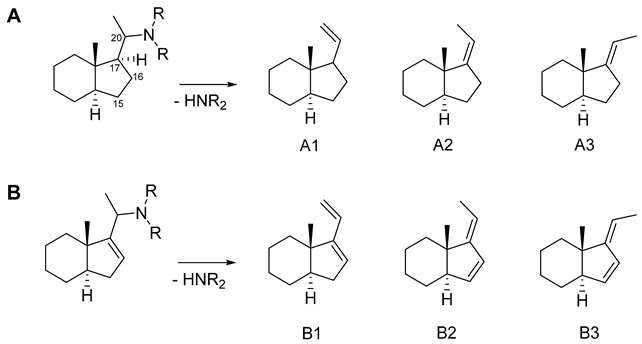
| ΔE 1 (kcal/mol) | % 2 | |
|---|---|---|
| Product | B3LYP\ 6-31G+ (d,p) | |
| A1 3 | 4.76 | 0.03 |
| A2 | 3.64 | 0.22 |
| A3 | 0.00 | 99.8 |
| B1 | 1.23 | 10.7 |
| B2 | 1.81 | 4.0 |
| B3 | 0.00 | 85.3 |
| N Components | R2 | Q2 * |
|---|---|---|
| 1 | 0.442 | 0.137 |
| 2 | 0.688 | 0.166 |
| 3 | 0.877 | 0.473 |
| 4 | 0.949 | 0.662 |
| 5 | 0.982 | 0.628 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flittner, D.; Kaiser, M.; Mäser, P.; Lopes, N.P.; Schmidt, T.J. The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense. Molecules 2021, 26, 591. https://doi.org/10.3390/molecules26030591
Flittner D, Kaiser M, Mäser P, Lopes NP, Schmidt TJ. The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense. Molecules. 2021; 26(3):591. https://doi.org/10.3390/molecules26030591
Chicago/Turabian StyleFlittner, Dagmar, Marcel Kaiser, Pascal Mäser, Norberto P. Lopes, and Thomas J. Schmidt. 2021. "The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense" Molecules 26, no. 3: 591. https://doi.org/10.3390/molecules26030591








