Interplay between Oxo and Fluoro in Vanadium Oxyfluorides for Centrosymmetric and Non-Centrosymmetric Structure Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure Description
2.3. IR Spectra
2.4. Magnetic Measurements
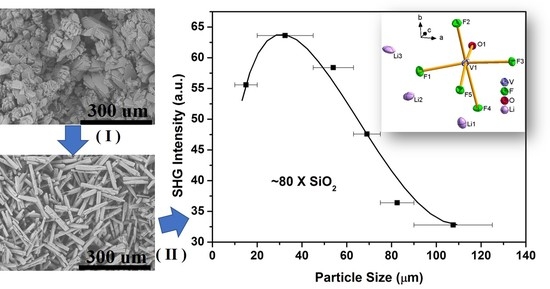
2.5. SHG Measurements
3. Materials and Methods
3.1. Caution
3.2. Materials
3.3. Synthesis
4. Material Characterization
4.1. Single-Crystal X-ray Diffraction
4.2. Powder X-ray Diffraction (PXRD)
4.3. IR Spectroscopy
4.4. Magnetic Measurements
4.5. SHG Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Harada, J.K.; Charles, N.; Poeppelmeier, K.R.; Rondinelli, J.M. Heteroanionic Materials by Design: Progress Toward Targeted Properties. Adv. Mater. 2019, 31, 1805295. [Google Scholar] [CrossRef]
- Kubo, A.; Giorgi, G.; Yamashita, K. Anion Ordering in CaTaO2N: Structural Impact on the Photocatalytic Activity. Insights from First-Principles. Chem. Mater. 2017, 29, 539–545. [Google Scholar] [CrossRef]
- Zhang, R.; Read, G.; Lang, F.; Lancaster, T.; Blundell, S.J.; Hayward, M.A. La2SrCr2O7F2: A Ruddlesden–Popper Oxyfluoride Containing Octahedrally Coordinated Cr4+ Centers. Inorg. Chem. 2016, 55, 3169–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Senn, M.S.; Hayward, M.A. Directed Lifting of Inversion Symmetry in Ruddlesden–Popper Oxide–Fluorides: Toward Ferroelectric and Multiferroic Behavior. Chem. Mater. 2016, 28, 8399–8406. [Google Scholar] [CrossRef]
- Zhao, L.-D.; He, J.; Berardan, D.; Lin, Y.; Li, J.-F.; Nan, C.-W.; Dragoe, N. BiCuSeO Oxyselenides: New Promising Thermoelectric Materials. Energy Environ. Sci. 2014, 7, 2900–2924. [Google Scholar] [CrossRef]
- Slater, P.R.; Gover, R.K.B. Synthesis and Structure of the New Oxide Fluoride Sr2TiO3F2 from the Low Temperature Fluorination of Sr2TiO4: An Example of a Staged Fluorine Substitution/Insertion Reaction. J. Mater. Chem. 2002, 12, 291–294. [Google Scholar] [CrossRef]
- Xu, X.; Randorn, C.; Efstathiou, P.; Irvine, J.T.S. A Red Metallic Oxide Photocatalyst. Nat. Mater. 2012, 11, 595–598. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Choudhury, A. Phosphorous Acid Route Synthesis of Iron Tavorite Phases, LiFePO4(OH)xF1−x [0 ≤ x ≤ 1] and Comparative Study of Their Electrochemical Activities. RSC Adv. 2014, 4, 37691–37700. [Google Scholar] [CrossRef]
- Park, Y.U.; Seo, D.H.; Kim, H.; Kim, J.; Lee, S.; Kim, B.; Kang, K. A Family of High-Performance Cathode Materials for Na-Ion Batteries, Na3(VO1-xPO4)2 F1+2x (0 ≤ x ≤ 1): Combined First-Principles and Experimental Study. Adv. Funct. Mater. 2014, 24, 4603–4614. [Google Scholar] [CrossRef]
- Broux, T.; Bamine, T.; Fauth, F.; Simonelli, L.; Olszewski, W.; Marini, C.; Ménétrier, M.; Carlier, D.; Masquelier, C.; Croguennec, L. Strong Impact of the Oxygen Content in Na3V2(PO4)2F3-yOy (0 ≤ y ≤ 2) on Its Structural and Electrochemical Properties. Chem. Mater. 2016, 28, 7683–7692. [Google Scholar] [CrossRef] [Green Version]
- Maggard, P.A.; Nault, T.S.; Stern, C.L.; Poeppelmeier, K.R. Alignment of Acentric MoO3F33− Anions in a Polar Material: (Ag3MoO3F3)(Ag3MoO4)Cl. J. Solid State Chem. 2003, 175, 27–33. [Google Scholar] [CrossRef]
- Kikkawa, S.; Sun, S.; Masubuchi, Y.; Nagamine, Y.; Shibahara, T. Ferroelectric Response Induced in Cis-Type Anion Ordered SrTaO2N Oxynitride Perovskite. Chem. Mater. 2016, 28, 1312–1317. [Google Scholar] [CrossRef]
- Brink, F.J.; Withers, R.L.; Norén, L. An Electron Diffraction and Crystal Chemical Investigation of Oxygen/Fluorine Ordering in Niobium Oxyfluoride, NbO2F. J. Solid State Chem. 2002, 166, 73–80. [Google Scholar] [CrossRef]
- Dabachi, J.; Body, M.; Galven, C.; Boucher, F.; Legein, C. Preparation-Dependent Composition and O/F Ordering in NbO2F and TaO2F. Inorg. Chem. 2017, 56, 5219–5232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Jo, H.; Yoon, S.W.; Choi, K.-Y.; Chen, W.-T.; Chou, F.; Ok, K.M. Mixed Transition Metal (Oxy)fluoride Paramagnet Chains: Synthesis, Structure, and Characterization. Eur. J. Inorg. Chem. 2019, 2019, 3112–3119. [Google Scholar] [CrossRef]
- Ahmed, B.; Jo, H.; Lee, S.; Choi, K.Y.; Ok, K.M. Influence of structure-directing polyhedra and heterocyclic ligands on the chain structures and O/F ordering in a series of zinc vanadium oxyfluorides. CrystEngComm 2020, 22, 3206–3214. [Google Scholar] [CrossRef]
- Donakowski, M.D.; Görne, A.; Vaughey, J.T.; Poeppelmeier, K.R. AgNa(VO2F2)2: A Trioxovanadium Fluoride with Unconventional Electrochemical Properties. J. Am. Chem. Soc. 2013, 135, 9898–9906. [Google Scholar] [CrossRef]
- Donakowski, M.D.; Gautier, R.; Yeon, J.; Moore, D.T.; Nino, J.C.; Halasyamani, P.S.; Poeppelmeier, K.R. The Role of Polar, Lamdba (Λ)-Shaped Building Units in Noncentrosymmetric Inorganic Structures. J. Am. Chem. Soc. 2012, 134, 7679–7689. [Google Scholar] [CrossRef] [Green Version]
- Donakowski, M.D.; Gautier, R.; Lu, H.; Tran, T.T.; Cantwell, J.R.; Halasyamani, P.S.; Poeppelmeier, K.R. Syntheses of Two Vanadium Oxide–Fluoride Materials That Differ in Phase Matchability. Inorg. Chem. 2015, 54, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Tulsky, E.G.; Long, J.R. Dimensional reduction: A practical formalism for manipulating solid structures. Chem. Mater. 2001, 13, 1149–1166. [Google Scholar] [CrossRef]
- Burns, J.H.; Tennissen, A.C.; Brunton, G.D. The crystal structure of α-lithium hexafluoroaluminate. Acta Cryst. B 1968, 24, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Garton, G.; Wanklyn, B.M. Polymorphism in Li3AIF6. J. Inorg. Nuel. Chem. 1965, 27, 2466–2469. [Google Scholar] [CrossRef]
- Holm, J.L.; Jenssen, B. A note on the polymorphy and structure of Li3AlF6, Acta. Chem. Scand. 1969, 23, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Massa, W.; Rüdorff, W. On α- and β-Li3MeF6 Compounds. Z. Naturforsch. 1971, 26b, 1216–1218. [Google Scholar] [CrossRef]
- Wilkening, M.; Romanova, E.E.; Nakhal, S.; Weber, D.; Lerch, M.; Heitjans, P. Time-resolved and site-specific insights into migration pathways of Li + in α-Li3VF6 by 6Li 2D exchange MAS NMR. J. Phys. Chem. C. 2010, 114, 19083–19088. [Google Scholar] [CrossRef]
- Ok, K.M.; Chi, E.O.; Halasyamani, P.S. Bulk characterization methods for non-centrosymmetric materials: Second-harmonic generation, piezoelectricity, pyroelectricity, and ferroelectricity. Chem. Soc. Rev. 2006, 35, 710–717. [Google Scholar] [CrossRef]
- Zyss, J.; Oudar, J.L. Relations between Microscopic and Macroscopic Lowest-Order Optical Nonlinearities of Molecular Crystals with One—or Two-Dimensional Units. Phys. Rev. A 1982, 26, 2028–2048. [Google Scholar] [CrossRef]
- Yamamoto, H.; Katogi, S.; Watanabe, T.; Sato, H.; Miyata, S.; Hosomi, T. New Molecular Design Approach for Noncentrosymmetric Crystal Structures: Lambda (Λ)-shaped Molecules for Frequency Doubling. Appl. Phys. Lett. 1992, 60, 935–937. [Google Scholar] [CrossRef]
- Bruker’s SMART, Bruker AXS Inc.: Madison, WI, USA, 2002.
- Bruker’s SAINT; SADABS; SHELXTL, Bruker AXS Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M.; Hubshle, C.B.; Dittrich, B. Shelxle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281. [Google Scholar]






| Empirical Formula | Li2VO0.55(H2O)0.45F5⋅2H2O | Li3VOF5 |
|---|---|---|
| Formula weight | 212.76 | 182.76 |
| Temperature | 200 (2) K | 298 (2) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | I2/a | Pna21 |
| a | 6.052(3) Å | 5.1173(2) Å |
| b | 7.928(4) Å | 8.612(3) Å |
| c | 12.461(6) Å | 9.346(3) Å |
| α | 90° | 90° |
| β | 103.99(2)° | 90° |
| γ | 90° | 90° |
| Volume | 580.1 (5) Å3 | 411.9 (2) Å3 |
| Z | 4 | 4 |
| Density (calculated) | 2.436 g/cm3 | 2.947 g/cm3 |
| F(000) | 412 | 340 |
| Reflections collected | 10964 | 3537 |
| Independent reflections | 1273 [R(int) = 0.0966] | 770 [R(int) = 0.0414] |
| Goodness-of-fit on F2 | 1.033 | 1.059 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0668, wR2 = 0.1733 | R1 = 0.0286, wR2 = 0.0635 |
| R indices (all data) | R1 = 0.0878, wR2 = 0.1955 | R1 = 0.0329, wR2 = 0.0659 |
| Largest diff. peak and hole | 1.017 and −1.198 e·Å−3 | 0.415 and −0.345 e·Å−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandineni, P.; Yaghoobnejad Asl, H.; Zhang, W.; Halasyamani, P.S.; Ghosh, K.; Choudhury, A. Interplay between Oxo and Fluoro in Vanadium Oxyfluorides for Centrosymmetric and Non-Centrosymmetric Structure Formation. Molecules 2021, 26, 603. https://doi.org/10.3390/molecules26030603
Sandineni P, Yaghoobnejad Asl H, Zhang W, Halasyamani PS, Ghosh K, Choudhury A. Interplay between Oxo and Fluoro in Vanadium Oxyfluorides for Centrosymmetric and Non-Centrosymmetric Structure Formation. Molecules. 2021; 26(3):603. https://doi.org/10.3390/molecules26030603
Chicago/Turabian StyleSandineni, Prashanth, Hooman Yaghoobnejad Asl, Weiguo Zhang, P. Shiv Halasyamani, Kartik Ghosh, and Amitava Choudhury. 2021. "Interplay between Oxo and Fluoro in Vanadium Oxyfluorides for Centrosymmetric and Non-Centrosymmetric Structure Formation" Molecules 26, no. 3: 603. https://doi.org/10.3390/molecules26030603
APA StyleSandineni, P., Yaghoobnejad Asl, H., Zhang, W., Halasyamani, P. S., Ghosh, K., & Choudhury, A. (2021). Interplay between Oxo and Fluoro in Vanadium Oxyfluorides for Centrosymmetric and Non-Centrosymmetric Structure Formation. Molecules, 26(3), 603. https://doi.org/10.3390/molecules26030603