Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Bioactive Compounds by HPLC-ESI-MS/MS
2.2. Total Phenolic Compounds and Flavonoids
2.3. Assessment of Antioxidant Properties
2.3.1. DPPH Radical Scavenging Assay
2.3.2. ABTS Radical Scavenging Assay
2.3.3. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
2.3.4. Determination of Superoxide Dismutase (SOD) Activity
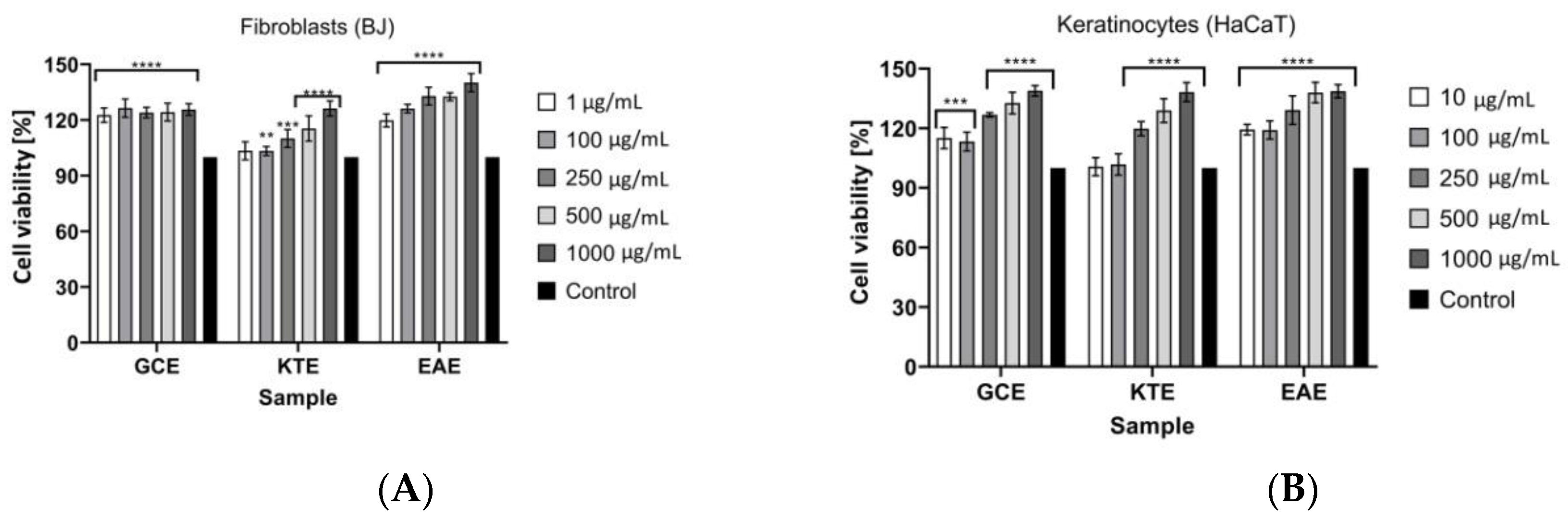
2.4. Cell Viability Assays
2.5. Determination of Anti-Collagenase Activity
2.6. Assessment of Anti-Inflammatory Potential
2.7. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3. Materials and Methods
3.1. Plant Material and Extraction Procedure
3.2. Determination of Biologically Active Compounds
3.2.1. Determination of Bioactive Compounds by HPLC-ESI-MS/MS
3.2.2. The Determination of the Total Phenolic Content (TPC)
3.2.3. The Determination of the Total Flavonoids Content (TFC)
3.3. Assessment of Antioxidant Properties
3.3.1. DPPH Radical Scavenging Assay
3.3.2. ABTS+ Scavenging Assay
- As—absorbance of the sample;
- Ac—absorbance of the control sample.
- Measurements were carried out in triplicate for each extract sample.
3.3.3. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.3.4. Determination of Superoxide Dismutase (SOD) Activity
3.4. Cytotoxicity Analyses
3.4.1. Cell Culture
3.4.2. Cell Viability Assay
3.4.3. Neutral Red Uptake Assay
3.4.4. Alamar Blue Assay
3.4.5. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
3.5. Determination of Anti-Collagenase Activity
3.6. Determination of Anti-Inflammatory Properties
3.6.1. Inhibition of Protein Denaturation
- As is the absorbance of the tested sample,
- Ac is the absorbance of negative control.
- The final result was the arithmetic mean of threeindependent measurements.
3.6.2. Inhibition of Lipoxygenase Activity
- As is the absorbance of the tested sample,
- Ac is the absorbance of negative control.
- The final result was the arithmetic mean of fiveindependent measurements.
3.7. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Compund | Precursor Ion m/z [M-H]− | Product Ion, m/z [M-H]− | DP, V | EP, V | CE, V | CXP, V |
|---|---|---|---|---|---|---|
| Quinic acid | 190.96 | 92.9 * | −70 | −10 | −32 | −5 |
| 126.8 | −70 | −10 | −26 | −7 | ||
| Gallic acid | 168.919 | 124.7 * | −60 | −10 | −22 | −5 |
| 78.8 | −60 | −10 | −32 | −11 | ||
| 5-Caffeoylquinic acid | 353.023 | 190.8 * | −60 | −10 | −24 | −9 |
| 84.7 | −60 | −10 | −66 | −11 | ||
| 3-Caffeoylquinic acid | 353.023 | 190.8 * | −70 | −10 | −26 | −13 |
| 178.9 | −70 | −10 | −28 | −13 | ||
| Caffeic acid | 178.952 | 134.7 * | −60 | −10 | −24 | −7 |
| 134.2 | −60 | −10 | −36 | −1 | ||
| Quercetin | 300.903 | 150.8 * | −115 | −10 | −30 | −7 |
| 178.6 | −115 | −10 | −26 | −7 |
References
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease.Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallyathan, V. Oxidative stress/antioxidant status in health and disease. Lung Biol. Health Dis. 2004, 187, 35–58. [Google Scholar]
- Talhouk, R.S.; Karam, C.; Fostok, S.; El-Jouni, W.; Barbour, E.K. Anti-inflammatory bioactivities in plant extracts. J. Med. Food 2007, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, K.H.; Han, C.S.; Yang, H.C.; Park, S.H.; Jang, H.I.; Kim, J.W.; Choi, Y.S.; Lee, N.H. Anti-wrinkle activity of Platycarya strobilacea extract and its application as a cosmeceutical ingredient. J. Cosmet. Sci. 2010, 61, 211–224. [Google Scholar]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kosmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef]
- Štajner, D.; Popović, B.M.; Boža, P. Evalution of willowherb’s (Epilobium angustifolium L.) antioxidant and radical scavenging capacities. Phytother. Res. 2007, 21, 1242–1245. [Google Scholar] [CrossRef]
- Kosalec, I.; Kopjar, N.; Kremer, D. Antimicrobial activity of willowherb (Epilobium angustifolium L.) leaves and flowers. Curr. Drug Targets 2013, 14, 986–991. [Google Scholar] [CrossRef]
- Tita, B.; Abdel-Haq, H.; Vitalone, A.; Mazzanti, G.; Saso, L. Analgesic properties of Epilobium angustifolium evaluated by the hot plate test and the writhingtest. Il Farm. 2001, 56, 341–343. [Google Scholar] [CrossRef]
- Hiermann, A.; Bucar, F. Studies of Epilobium angustifolium extracts on growth of accessory sexual organs in rats. J. Ethnopharmacol. 1997, 55, 179–183. [Google Scholar] [CrossRef]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial activity of Epilobium spp. extracts. Il Farm. 2001, 56, 345–348. [Google Scholar] [CrossRef]
- Hiermann, A.; Juan, H.; Sametz, W. Influence of Epilobium extracts on prostaglandin biosyntesis and carrageenin induced oedema of theratpaw. J. Ethnopharmacol. 1986, 17, 161–169. [Google Scholar] [CrossRef]
- Hiermann, A.; Reidlinger, M.; Juan, H.; Sametz, W. Isolation of the antiphlogistic principle from Epilobium angustifolium. Planta Med. 1991, 57, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Hiermann, A.; Schramm, H.W.; Laufer, S. Anti-inflammatory activity of myricetin-3-O-beta-d-glucuronide and related compounds. Inflamm. Res. 1998, 47, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bazylko, A.; Kiss, A.K.; Kowalski, J. High-performance thin-layer chromatography method for quantitative determination of oenothein Band quercetin glucuronide in aqueous extract of Epilobii angustifoliiherba. J. Chromatogr. A 2007, 1173, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Kumar, R.; Agarwal, A.; Reetaa, K.H.; Guptaa, Y.K. Comparison of three differen textracts of Centella asiatica for anti-amnesic, antioxidant and anticholinergic activities: In vitro and in vivo study. Biomed. Pharmacother. 2018, 105, 1344–1352. [Google Scholar] [CrossRef]
- Sushma, T.; Sangeeta, G.; Gambir, I.S. Centella asiatica: A concise drug review with probable clinical uses. J. Stress Physiol. Biochem. 2011, 7, 39–44. [Google Scholar]
- Ling, A.P.K.; Marziah, M.; Tan, S.E. Triterpenoids Distribution in Whole Plant and Callus Cultures of Centella Asiatica Accessions. In Proceedings of the 16th National Seminar on Natural Products, Serdang, Malaysia, 24–25 October 2000; pp. 165–168. [Google Scholar]
- Bhavana, D.; Jyoti, K. Centella asiatica: The elixir of life. Int. J. Res. Ayurveda Pharm. 2011, 2, 431–438. [Google Scholar]
- Zheng, C.J.; Qin, L.P. Chemical components of Centella asiatica and their bioactive. Chin. J. Integr. Med. 2007, 5, 348–351. [Google Scholar] [CrossRef]
- Chandrika, U.G.; PrasadKumarab, P.A.A.S. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Adv. Food Nutr. Res. 2015, 76, 125–157. [Google Scholar]
- Sayasinha, P.; Warnasuriya, D.; Dissanayake, H. History, Medicinal and Aromatic Plant Series; Information Services Centre Industrial Technology Institute: Colombo, Sri Lanka, 1999; Volume 1. [Google Scholar]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop Med. 2013, 6, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.K.; Chahal, J.; Bhatia, M. Clitoria ternatea (L.): Old and new aspects. J. Pharm Res. 2010, 3, 2610–2614. [Google Scholar]
- Chayaratanasin, P.; Barbieri, M.A.; Suanpairintr, N.; Adisakwattana, S. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complementary Altern. Med. 2015, 15, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daisy, P.; Santosh, K.; Rajathi, M. Antihyperglycemic and antihyperlipidemic effects of Clitoria ternatea Linn. in alloxan-induced diabetic rats. Afr. J. Microbiol. Res. 2009, 3, 287–291. [Google Scholar]
- Kamilla, L.; Mnsor, S.M.; Ramanathan, S.; Sasidharan, S. Antimicrobial activity of Clitoria ternatea (L.) extracts. Pharmacologyonline 2009, 1, 731–738. [Google Scholar]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five new anthocyanins, ternatinsA3, B4 B3, B2, and D2, from Clitoria ternate aflowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Lijon, M.B.; Meghla, N.S.; Jahedi, E.; Rahman, M.A.; Hossain, I. Phytochemistry and pharmacological activities of Clitoria ternatea. Int. J. Nat. Soc. Sci. 2017, 4, 1–10. [Google Scholar]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. 2009, 14, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Rad. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Asad, S.F.; Singh, S.; Ahmad, A.; Hadi, S.M. Flavonoids: Antioxidants in diet and potential anticancer agents. Med. Sci. Res. 1998, 26, 723–772. [Google Scholar]
- Aburjai, T.; Natsheh, F.M. Plants used as cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.T.; Paye, M.; Maibach, H. Handbook of Cosmetic Science and Technology, 3rd ed.; Taylor & Francis Group: BocaRaton, FL, USA, 2014; pp. 301–311. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pittella, F.; Dutra, R.C.; Junior, D.D.; Lopes, M.T.P.; Barbosa, N.R. Antioxidant and Cytotoxic Activities of Centella asiatica (L) Urb. Int. J. Mol. Sci. 2009, 10, 3713–3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol. Med. 1996, 20, 933–995. [Google Scholar] [CrossRef]
- Kiss, A.K.; Kowalski, J.; Melzig, M.F. Compounds from Epilobium angustifolium inhibit the specific metallopeptidases ACE, NEPandAPN. Planta Med. 2004, 70, 919–923. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwinska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different species belonging to the genus of Epilobium (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Bonfill, M.; Mangas, S.; Cusidó, R.M.; Osuna, L.; Piñol, M.T.; Palazón, J. Identification of triterpenoid compounds of Centella asiatica by thin-layer chromatography and masss pectrometry. Biom. Chromatogr. 2006, 20, 151–153. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awizen, P.; Studzinska-Sroka, E.; Danczak-Pazdrowska, A.; Brzezinska, M. Centella asiatica in dermatology: An overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Vargeshe, N.; Simon, A. An investigation on cytotoxic and antioxidant properties of Clitoria ternatea L. Int. J. Drug Discov. 2011, 3, 74–77. [Google Scholar] [CrossRef]
- Kamkaen, N.; Wilkinson, J.M. The Antioxidant Activity of Clitoria ternatea Flower Petal Extracts and Eye Gel. Phytother. Res. 2009, 23, 1624–1625. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, A.; Cornick, S.M.; Chominski, V.; RibeirodeNoronha, S.M.; Correade Noronha, S.A.A.; Ferreira, L.M. Review of major theories of skin aging. Adv. Aging Res. 2014, 3, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Howes, M.J.R.; Simmonds, M.S.J. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Moolsap, F.; Tanasawet, S.; Tantisira, M.H.; Pilaiwanwadee, H.; Tipmanee, V.; Sukketsiri, W. Standardized extract of Centella asiatica EC a 233 inhibits lipopolysaccharide-induced cytokine release in skin keratinocytes by suppressing ERK1/2 pathways. Asian Pac. J. Trop. Biomed. 2020, 10, 273–280. [Google Scholar]
- Wanasuntronwong, A.; Tantisira, M.H.; Tantisira, B.; Watanabe, H. Anxiolytic effects of standardized extract of Centella asiatica (ECa233) after chronic immobilization stress in mice. J. Ethnopharmacol. 2012, 143, 579–585. [Google Scholar] [CrossRef]
- Bian, D.; Liu, M.; Li, Y.; Xia, Y.; Gong, Z.; Dai, Y. Madecassoside, atriterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J. Biochem. Mol. Toxicol. 2012, 26, 399–405. [Google Scholar] [CrossRef]
- Hussin, M.; Hamid, A.A.; Mohamad, S.; Saari, N.; Ismail, M.; Bejo, M.H. Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem. 2007, 100, 535–541. [Google Scholar] [CrossRef]
- Zakaria, N.N.A.; Okello, E.J.; Howes, M.J.; Birch-Machin, M.A.; Bowman, A. In vitro protective effects of an aqueous extract of Clitoria ternatea L. Flower agains thydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother. Res. 2018, 32, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.G. Oxidative stress and superoxide dismutase in development, aging and gene regulation. Age 1998, 21, 47–76. [Google Scholar] [CrossRef] [Green Version]
- Petruk, G.; DelGiudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Talekar, Y.P.; Apte, K.G.; Paygude, S.V.; Tondare, P.R.; Parab, P.B. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J. Ayurveda Integr. Med. 2017, 8, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelli, U. Antioxidant use in nutraceuticals. Clin. Dermatol. 2009, 27, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella asiatica in cosmetology. Postepy Dermatol. Alergol. 2013, 30, 46–49. [Google Scholar] [CrossRef]
- Sampson, J.H.; Raman, A.; Karlsen, G.; Navsaria, H.; Leigh, I.M. In vitro keratinocyte antiproliferant effect of Centella asiatica extract and triterpenoid saponins. Phytomedicine 2001, 8, 230–235. [Google Scholar] [CrossRef]
- Vasantharuba, S.; Banumathi, P.; Premalatha, M.R.; Sundaram, S.P.; Arumugam, T. Functional properties of Centella asiatica (L.): A review. Int. J. Pharm. Pharm. 2012, 4, 8–14. [Google Scholar]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison betweens un-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and antielastase activities of Phyllanthusemblica, Manilkarazapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarveswaran, R.; Jayasuriya, W.J.A.B.; Suresh, T.S. In vitro assays to investigate the anti-inflammatory activity of herbal extracts: A Review. World J. Pharm. Res. 2017, 6, 131–141. [Google Scholar]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase [sol]cyclooxygenase. Phytother. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef]
- Darlenski, R.; Sassning, S.; Tsankov, N.; Fluhr, J.W. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur. J. Pharm Biopharm. 2009, 72, 295–303. [Google Scholar] [CrossRef]
- Naveed, A.; Ahmad, Z.; Atif, A.; Mahmood, T.; ShoaibKhan, H.M.; Khan, B.A.; Rasul, A.; Mustafa, R.; Madni, A. Effects of Emblica Officinalis Extract Cream on Human Skin Trans-epidermal Water Loss Measured with Non Invasive Probe. J. Pharm. Alternat. Med. 2012, 1, 32–38. [Google Scholar]
- Visscher, M.O.; Said, D.; Wickett, R. Transepidermal water loss: Effect of hand hygiene. Skin Res. Technol. 2010, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Occlusion vs. skin barrier function. Skin Res. Technol. 2002, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sator, P.G.; Schmidt, J.B.; Hönigsmann, H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with a topic dermatitis. J. Am. Acad. Derm. 2003, 48, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T. Antioxidant Activity and Cytotoxicity of Medicago sativa L. Seeds and Her bExtract on Skin Cells. BioResearch Open Access 2020, 9, 229–242. [Google Scholar] [CrossRef]
- Matejić, J.; Dzamic, A.; Mihajilov-Krstev, T.; Randjelovic, V.; Krivošej, Z.; Marin, P. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. taxa. Cent. Eur. J. Biol. 2012, 7, 1116–1122. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell. Longev. 2020, 2020, 5307614. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [Green Version]

















| No. | Retention Time (min) | Molecular Formula | Molar Mass (Da) | Precursor Ion m/z | Main Product Ions MS2 m/z | Identification | EAE | GCE | KTE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.6 | C7H12O6 | 192.2 | 191 [M-H]− | 127 [M-H-H2O-HCOOH]−, 85 [M-C3H7O4]−, 59 [M-C5H9O4]− | Quinic acid | x | x | x |
| 2 | 2.1 | C7H6O5 | 170.1 | 169 [M-H]− | 151 [M-H-H2O]−, 125 [M-H-CO2]−, 107 [M-H-CO2-H2O]−, 83 [M-C3H3O3]− | Gallic acid | x | - | - |
| 3 | 2.8 | C16H18O9 | 354.3 | 353 [M-H]− | 191 [M-3H2O-C6H5O2]−, 179 [M-3H2O-C6H4-COOH]− | 5-Caffeoylquinic acid | x | x | - |
| 4 | 4.2 | C16H18O9 | 354.3 | 353 [M-H]− | 191 [M-3H2O-C6H5O2]−, 179 [M-3H2O-C6H4-COOH]− | 3-Caffeoylquinic acid | x | x | - |
| 5 | 5.1 | C9H8O4 | 180.2 | 179 [M-H]− | 135 [M-COOH]−, 107 [M-C3H5O2]−, 71 [M-C6H5O2]−, 59 [M-C7H5O2]− | Caffeic acid | x | x | x |
| 6 | 6.8 | C27H30O16 | 610.5 | 609 [M-H]− | 300 [M-H-C12H21O9]−, | Rutin | - | - | x |
| 7 | 7.5 | C27H30O15 | 594.5 | 593 [M-H]− | 383 [M-C8H19O6]−, 352 [M-C9H21O7]−, 284 [M-C12H22O9]− | Kaempferol-3-O-rutinoside | - | - | x |
| 8 | 7.5 | C25H24O12 | 516.4 | 515 [M-H]− | 353 [M-C9H7O3]−, 335 [M-C9H9O4]−, 179 [M-C16H17O8]− | 3,4-Dicaffeoylquinic acid | x | - | - |
| 9 | 8.0 | C15H10O7 | 302.2 | 301 [M-H]− | 272 [M-CHO]−, 255 [M-H-CO-H2O]−, 151 [M-C8H7O3]−, 121 [M-C8H5O5]−, 107 [M-C9H5O5]− | Quercetin | x | x | x |
| 10 | 9.9 | C23H22O13 | 506.4 | 505 [M-H]− | 300 [M-H2O-C6H8O4-COCH3]−, 271 [M-H2O-C6H9O4-COCH3-CHO]−, 255 [M-C6H15O8], 179 [M-C15H7O6-COCH3] | Quercetin-acetyl-glucoside | x | x | x |
| 11 | 11.3 | C21H20O11 | 448.3 | 447 [M-H]− | 284 [M-H2O-C6H10O4]−, 255 [M-C6H9O7]−, 179 [M-C15H9O5]− | Kaempferol-3-O-glucoside | x | x | x |
| Compound | Content [µg/mL] | ||
|---|---|---|---|
| EAE | GCE | KTE | |
| Quinic acid | 220.7 ± 11.7 | 218.0 ± 12.5 | 43.1 ± 3.1 |
| Gallic acid | 93.7 ± 14.0 | <LOD | <LOD |
| Caffeic acid | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 5-CQA | 55.1 ± 6.0 | 26.3 ± 1.4 | 1.2 ± 0.0 |
| 3-CQA | 47.7 ± 6.7 | 10.9 ± 0.4 | 3.4 ± 0.6 |
| Quercetin | 50.0 ± 1.4 | 1.1 ± 0.0 | 0.6 ± 0.0 |
| Sum of Quantified Compounds | 467.6 | 256.6 | 75.6 |
| Chemical Compound | TPC [mg GAE/g DW] | TFC [mg QE/g DW] |
|---|---|---|
| Centella asiatica L. | 2.96 ± 0.08 | 0.82 ± 0.04 |
| Clitoria ternatea L. | 15.62 ± 0.14 | 7.26 ± 0.12 |
| Epilobium angustifolium L. | 12.03 ± 0.16 | 3.21 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. https://doi.org/10.3390/molecules26030614
Zagórska-Dziok M, Ziemlewska A, Bujak T, Nizioł-Łukaszewska Z, Hordyjewicz-Baran Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules. 2021; 26(3):614. https://doi.org/10.3390/molecules26030614
Chicago/Turabian StyleZagórska-Dziok, Martyna, Aleksandra Ziemlewska, Tomasz Bujak, Zofia Nizioł-Łukaszewska, and Zofia Hordyjewicz-Baran. 2021. "Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts" Molecules 26, no. 3: 614. https://doi.org/10.3390/molecules26030614
APA StyleZagórska-Dziok, M., Ziemlewska, A., Bujak, T., Nizioł-Łukaszewska, Z., & Hordyjewicz-Baran, Z. (2021). Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules, 26(3), 614. https://doi.org/10.3390/molecules26030614






