Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors–In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Profiling
2.2. Antioxidant Assays
2.2.1. ONOO−-Scavenging Ability in the Evans Blue Solution
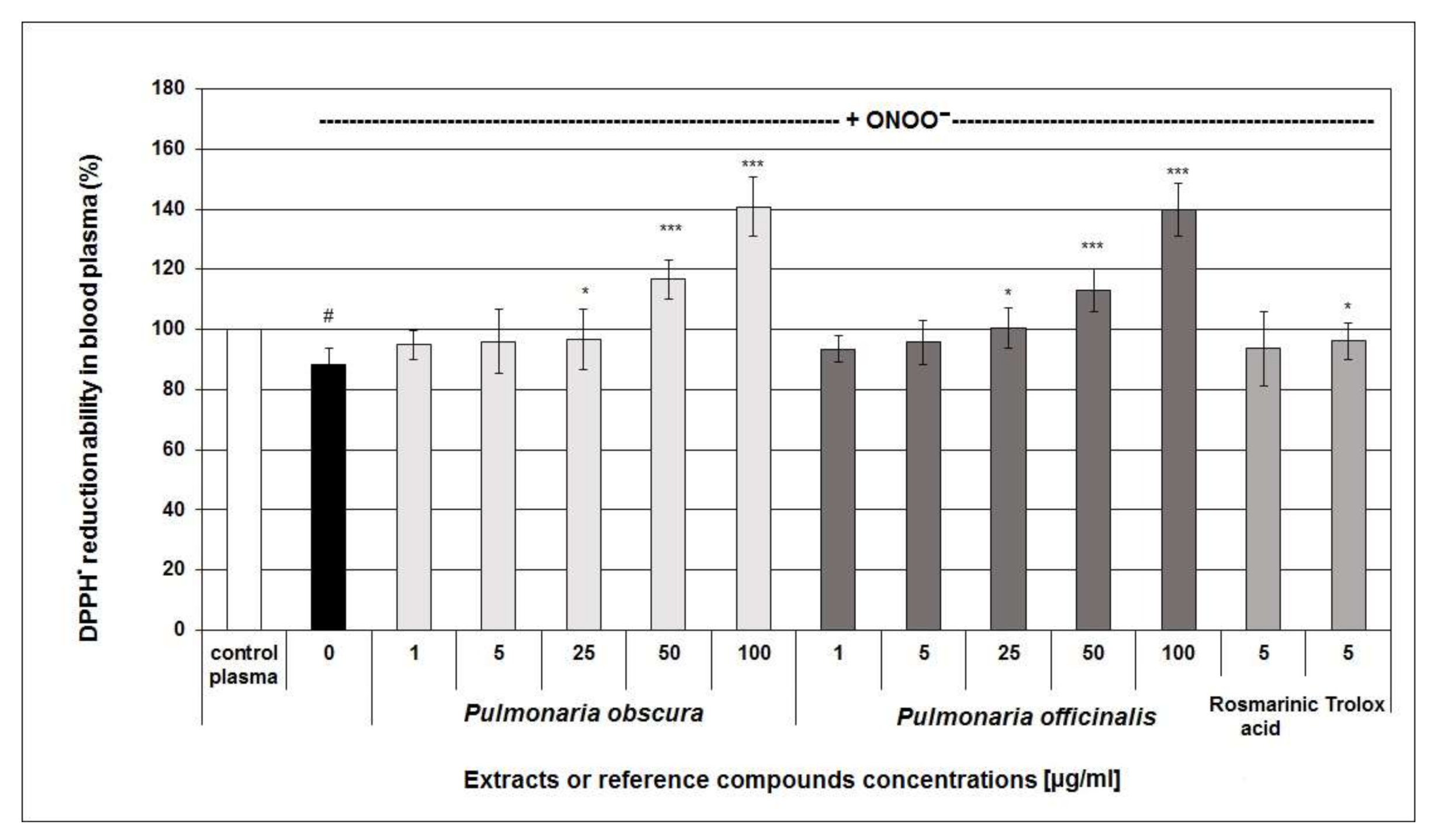
2.2.2. Antioxidant Activity in Human Blood Plasma
2.3. COX-2 Inhibitor Screening Tests
2.4. Assessments of Cellular Safety
2.5. Prediction of Protease Inhibitors, Molecular Docking and Drug-Likeness
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material
4.3. Preparation and Quantitative Analysis of P. obscura and P. officinalis Phenolic–Rich Fractions
4.4. Peroxynitrite (ONOO−) Scavenging Assay
4.5. Preparation of Blood Plasma Samples
4.6. Determination of Antioxidant Capacity of Blood Plasma Using DPPH• Assay
4.7. Determination of Lipid Peroxidation Biomarkers in Blood Plasma Exposed to 100 µM ONOO−
4.8. Immunodetection of 3–NT in Blood Plasma and Experimental System of the Isolated Fibrinogen Preparation
4.9. Evaluation of COX–2 Inhibitory Effects
4.10. Cytotoxicity Assays
4.11. In Silico Study: Prediction of Bioactivity and Docking
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Łuczaj, Ł.; Szymański, W.M. Wild vascular plants gathered for consumption in the Polish countryside: A review. J. Ethnobiol. Ethnomed. 2007, 3, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczaj, Ł. Wild food plants used in Poland from the mid-19th century to the present. Etnobiol. Pol. 2011, 1, 57–125. [Google Scholar]
- National Database of Health Protection Products. Available online: http://kbpoz.gs1.pl/ (accessed on 27 May 2019).
- Tiţă, I.; Mogoşanu, G.D.; Tiţă, M.G. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia 2009, 57, 141–156. [Google Scholar]
- Redžić, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 3, 869–890. [Google Scholar]
- Marković, M.; Ma;tović, M.; Pa;vlović, D.; Zla;tković, B.; Ma;rković, A.; Jotić, B.; Sta;nkov-Jova;nović, V. Resources of medicinal plants and herbs collector’s calendar of Pirot County (Serbia). Biol. Nyssana 2010, 1, 9–21. [Google Scholar]
- Zlatković, B.; Bogosavljević, S. Taxonomic and pharmacological valorization of the medical flora in SvrljiškiTimok Gorge (Eastern Serbia). Facta Univ. Ser. Med. Biol. 2014, 16, 76–86. [Google Scholar]
- Kochmarov, V.; Kozuharova, E.; Naychov, Z.; Momekov, G.; Mincheva, I. Ethnobotany and ethnopharmacology of Arum maculatum L. (Araceae) in Bulgaria with an emphasis on its effects against hemorrhoids. Int. J. Pharm. Chem. Biol. 2015, 5, 394–402. [Google Scholar]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Derm. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Akram, M.; Rashid, A. Anti-coagulant activity of plants: Mini review. J. Thromb. Thrombol. 2017, 44, 406–411. [Google Scholar] [CrossRef]
- Hawrył, M.A.; Waksmundzka-Hajnos, M. Micro 2D-TLC of selected plant extracts in screening of their composition and antioxidative properties. Chromatographia 2013, 76, 1347–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centariumumbellatum extracts. Saudij. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska, B.; Wójcik, U.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Stochmal, A.; Rywaniak, J.; Burzyńska, J.; Różalska, B. The pros and cons of cystic fibrosis (CF) patient use of herbal supplements containing Pulmonaria officinalis L. extract: The evidence from an in vitro study on Staphylococcus aureus CF clinical isolates. Molecules 2019, 24, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel phenolic constituents of Pulmonaria officinalis L. LC-MS/MS comparison of spring and autumn metabolite profiles. Molecules 2018, 23, 2277. [Google Scholar]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary polyphenols in the prevention of stroke. Oxid. Med. Cell Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- García-Redondo, A.B.; Aguado, A.; Briones, A.M.; Salaices, M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharm. Res. 2016, 114, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species: Akeyhallmark of cardiovascular disease. Adv. Med. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, P.; Kossmann, S.; Münzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation–immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gole, M.D.; Souza, J.M.; Choi, I.; Hertkorn, C.; Malcolm, S.; Foust, R.F., III; Finkel, B.; Lanken, P.N.; Ischiropoulos, H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Vadseth, C.; Souza, J.M.; Thomson, L.; Seagraves, A.; Nagaswami, C.; Scheiner, T.; Torbet, J.; Vilaire, G.; Bennett, J.; Murciano, J.-C.; et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. Nitrogen Species. J. Biol. Chem. 2004, 279, 8820–8826. [Google Scholar] [CrossRef] [Green Version]
- Lupidi, G.; Angeletti, M.; Eleuteri, A.M.; Tacconi, L.; Coletta, M.; Fioretti, E. Peroxynitrite-mediated oxidation of fibrinogen inhibits clot formation. FEBS Lett. 1999, 462, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, V.G.; Crow, J.P.; Zhou, F.; Parks, D.C. Peroxynitrite inactivates tissue plasminogen activator. Anesth. Analg. 2004, 98, 1312–1317. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Ponczek, M.B.; Nowak, P. Peroxynitrite and fibrinolytic system—The effects of peroxynitrite on t-PA-induced plasmin activity. Int. J. Biol. Macromol. 2015, 81, 212–219. [Google Scholar] [CrossRef]
- Krzyżanowska-Kowalczyk, J.; Kolodziejczyk-Czepas, J.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Yunnaneic acid B–a component of Pulmonaria officinalis extract prevents the peroxynitrite-induced oxidative stress in vitro. J. Agric. Food Chem. 2017, 65, 3827–3834. [Google Scholar] [CrossRef]
- Habu, J.B.; Ibeh, B.O. In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldialaevis ethanolic leaf extract. Biol. Res. 2015, 48, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Hazra, B.; Mandal, N.; Chaudhuri, T.K. Assessment of the antioxidant and free radical scavenging activities of methanolic extract of Diplaziumesculentum. Int. J. Food Prop. 2013, 16, 1351–1370. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative chemistry of peroxynitrite. Methods Enzym. 1994, 233, 229–240. [Google Scholar]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef]
- Ondua, M.; Adebayo, S.A.; Shai, L.J.; Lebelo, S.L. The anti-inflammatory and anti-nociceptive activities of some medicinal plant species used to treat inflammatory pain conditions in Southern Africa. Int. J. Pharm. Phytochem. Res. 2016, 8, 1571–1575. [Google Scholar]
- Taylor, J.L.S.; van Staden, J. COX-1 and COX-2 inhibitory activity in extracts prepared from Eucomis species, with further reference to extracts from E. autumnalisautumnalis. S. Afr. J. Bot. 2002, 68, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Ursu, T.M.; Zanfirescu, A.; Negres, S.; Chirita, C.; Radu, G.L. Anti-inflammatory and antioxidant activities of the Impatiens noli-tangere and Stachys officinalis polyphenolic-rich extracts. Rev. Bras. Farm. 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Colica, C.; Di Renzo, L.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. Rosmarinicacid as potential anti-inflammatory agent. Rev. Recent Clin. Trials 2018, 13, 240–242. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Murata, T.; Oyama, K.; Fujiyama, M.; Oobayashi, B.; Umehara, K.; Miyase, T.; Yoshizaki, F. Diastereomers of lithospermic acid and lithospermic acid B from Monarda fistulosa and Lithospermumerythrorhizon. Fitoterapia 2013, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Tran, P.T.; Lee, J.H.; Min, B.S.; Kim, J.A. Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza Bunge. Arch. Pharm. Res. 2018, 41, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Sirat, H.M. COX-2 inhibitors from stem bark of Bauhinia rufescens Lam. (Fabaceae). Excli J. 2013, 12, 824–830. [Google Scholar] [PubMed]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Ngu-Schwemlein, M.; Squadrito, G.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med. 1991, 1, 75–83. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Krzyżanowska-Kowalczyk, J.; Sieradzka, M.; Nowak, P.; Stochmal, A. Clovamide and clovamide-rich extracts of three Trifolium species as antioxidants and moderate antiplatelet agents in vitro. Phytochemistry 2017, 143, 54–63. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Gay, C.; Gebicki, J.M. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal. Biochem. 2000, 284, 217–220. [Google Scholar] [CrossRef]
- Wachowicz, B. Adenine nucleotides in thrombocytes of birds. Cell Biochem. Funct. 1984, 2, 167–170. [Google Scholar] [CrossRef]
- Olas, B.; Nowak, P.; Kolodziejczyk, J.; Wachowicz, B. The effects of antioxidants on peroxynitrite-induced changes in platelet proteins. Thromb. Res. 2004, 113, 399–406. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk, J.; Tsirigotis-Maniecka, M.; Komorowska, H.; Wachowicz, B.; Zaczyńska, E.; Czarny, A.; Czechowski, F.; Nowak, P.; Pawlaczyk, I. The influence of conjugatesisolated from Matricariachamomilla L. on plateletsactivity and cytotoxicity. Int. J. Biol. Macromol. 2013, 61, 218–229. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R.J. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halgren, T.A.J. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E.J. UCSF Chimera -a visualization system for exploratory research and analysis. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
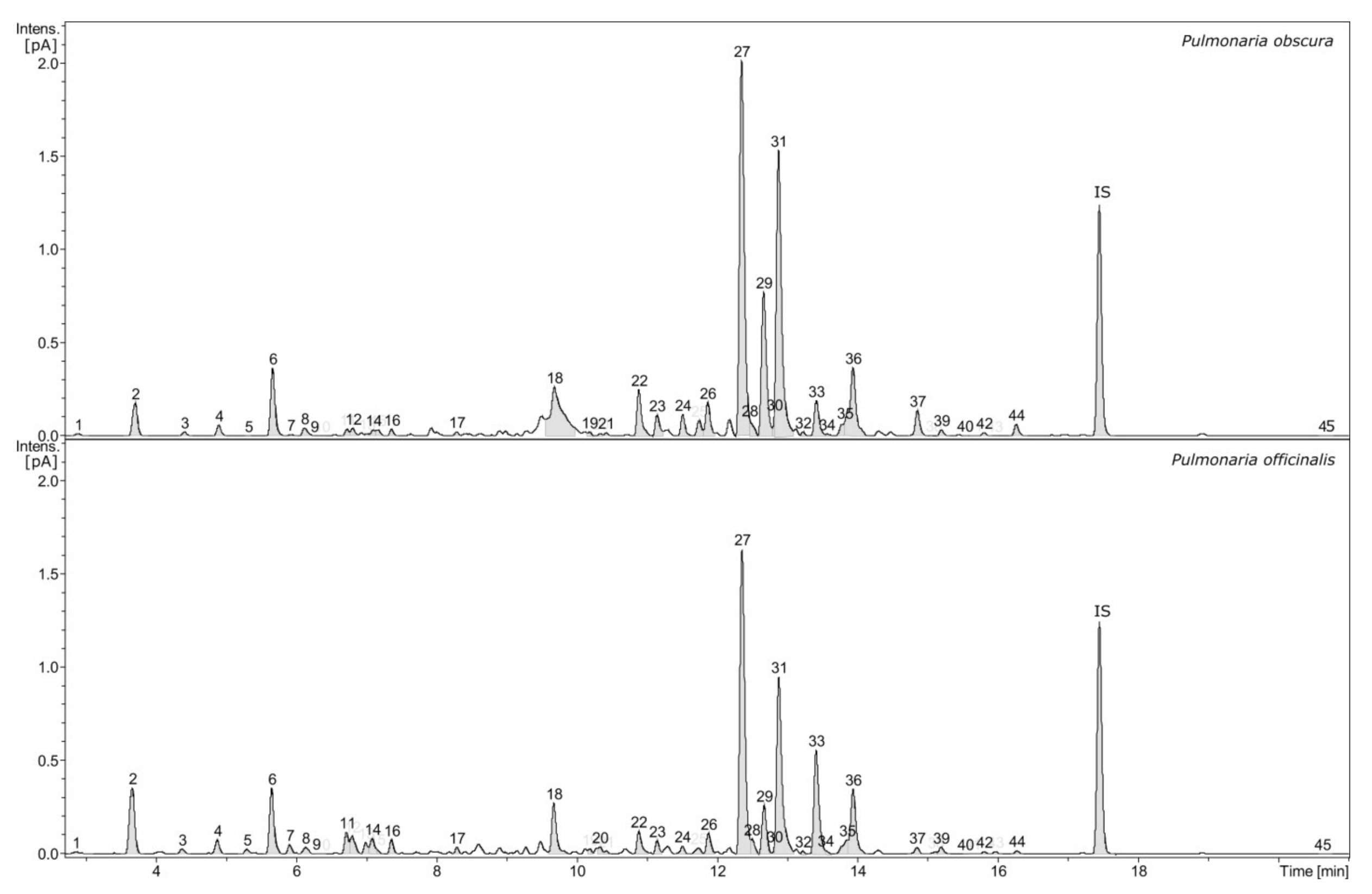

| Content of Phytochemicals (% of the Fraction) | ||
|---|---|---|
| P. obscura | P. officinalis | |
| Phenolic acids | 81.01 | 81.15 |
| Lignans | 11.27 | 6.46 |
| Flavonol glycosides | 4.95 | 5.16 |
| Others | 2.36 | 7.22 |
| No. | Compound Name | Contents [µg/mg of Fraction] (Mean ± SD, n = 3) | |
|---|---|---|---|
| P. obscura | P. officinalis | ||
| 1 | Danshensu | 2.40 ± 0.10 | 1.52 ± 0.06 |
| 2 | Menisdaurin | 16.36 ± 0.43 | 39.08 ± 1.56 |
| 3 | 3-O-(E)-caffeoyl-threonic acid | 1.18 ± 0.10 | 1.53 ± 0.05 |
| 4 | 2-O-(E)-caffeoyl-l-threonic acid | 3.34 ± 0.11 | 4.52 ± 0.14 |
| 5 | Lycoperodine-1 | Traces | 1.20 ± 0.04 |
| 6 | Chlorogenic acid | 4.87 ± 0.13 | 4.32 ± 0.11 |
| 7 | Actinidioionoside | Traces | Traces |
| 8 | Caffeic acid | Traces | 2.31 ± 0.11 |
| 9 | Cryptochlorogenic acid | 0.74 ± 0.03 | 0.22 ± 0.01 |
| 10 | 3’-O-(E)-feruloyl-α-sorbopyranosyl-(2’→1)-α-glucopyranoside | Traces | 0.45 ± 0.01 |
| 11 | 2-O-(E)-caffeoyl-d-glyceric acid | 1.24 ± 0.02 | 5.10 ± 0.14 |
| 12 | 4-O-(E)-caffeoyl-l-threonic acid | 1.50 ± 0.08 | 2.78 ± 0.07 |
| 13 | Neochlorogenic acid | 0.37 ± 0.01 | 0.04 ± 0.00 |
| 14 | 3-O-(E)-caffeoyl-glyceric acid | Traces | 2.08 ± 0.06 |
| 15 | 3-O-p-coumaroylquinic acid | 0.87 ± 0.03 | 2.29 ± 0.04 |
| 16 | 4-O-p-coumaroylquinic acid | Traces | Traces |
| 17 | 5-O-p-coumaroylquinic acid | 0.93 ± 0.04 | 1.88 ± 0.04 |
| 18 | Globoidnan B | 29.79 ± 4.46 | 24.16 ± 3.33 |
| 19 | Rutin | Traces | Traces |
| 20 | Nicotiflorin isomer | Traces | Traces |
| 21 | Quercetin 3-O-β-glucoside | 0.62 ± 0.01 | 0.33 ± 0.02 |
| 22 | Yunnaneic acid E | 27.32 ± 0.23 | 12.07 ± 0.33 |
| 23 | Quercetin 3-O-(6′′-O-malonyl)-β-glucoside | 4.10 ± 0.18 | 2.82 ± 0.15 |
| 24 | Nicotiflorin | Traces | Traces |
| 25 | Astragalin | Traces | Traces |
| 26 | Shimobashiric acid C | 7.46 ± 0.17 | 5.46 ± 0.17 |
| 27 | Rosmarinic acid | 301.46 ± 35.04 | 159.28 ± 17.09 |
| 28 | Kaempferol 3-O-(6′′-O-malonyl)-β-glucoside | 0.40 ± 0.08 | Traces |
| 29 | Monardic acid A | 42.99 ± 5.04 | 17.56 ± 2.77 |
| 30 | Yunnaneic acid E-1 | Traces | Traces |
| 31 | Lithospermic acid A | 80.13 ±6.44 | 44.20 ± 0.05 |
| 32 | Pulmonarioside A | Traces | Traces |
| 33 | Salvianolic acid H | 8.97 ± 0.82 | 26.23 ± 0.75 |
| 34 | Lithospermic acid B | NA | NA |
| 35 | Pulmonarioside B | 0.50 ± 0.03 | Traces |
| 36 | Yunnaneic acid B | 23.65 ± 0.52 | 22.65 ± 1.49 |
| 37 | Globoidnan A | 4.10 ± 0.17 | 0.57 ± 0.02 |
| 38 | Pulmitric acid A | Traces | Traces |
| 39 | Pulmitric acid B | Traces | Traces |
| 40 | Isosalvianolic acid A | Traces | Traces |
| 41 | Isosalvianolic acid A-1 | Traces | Traces |
| 42 | Isosalvianolic acid A isomer | Traces | Traces |
| 43 | Rosmarinic acid methyl ester | Traces | Traces |
| 44 | Salvianolic acid H-9′′-methylester | 3.87 ± 0.11 | 0.79 ± 0.02 |
| 45 | Lycopic acid C | NA | NA |
| Inhibition of the ONOO−-Induced Evans Blue Peroxidation | |
|---|---|
| IC50 [µg/mL] | |
| P. obscura | 36.71 |
| P. officinalis | 32.66 |
| Rosmarinic acid | 13.34 |
| Trolox | 2.76 |
| Pulmonaria Phenolic-Rich Fractions and Reference Compounds [µg/mL] | 3-Nitrotyrosine [nmol 3-NT-Fg/mg of Protein] | |||
|---|---|---|---|---|
| Isolated Fibrinogen | Blood Plasma | |||
| control (untreated) samples | 0 | 0.006 ± 0.003 | 0.026 ± 0.008 | |
| samples treated with ONOO− in the absence of examined substances | 0 | 1.326 ± 0.072 ### | 2.605 ± 0.392 ### | |
| samples treated with ONOO− in the presence of: | P. obscura | 1 | 1.357 ± 0.058 | 2.152 ± 0.640 |
| 5 | 1.200 ± 0.158 | 2.040 ± 0.520 | ||
| 25 | 0.889 ± 0.259 ** | 1.881 ± 0.709 ** | ||
| 50 | 0.785 ± 0.354 * | 1.590 ± 0.562 *** | ||
| 100 | 0.620 ± 0.459 *** | 1.060 ± 0.539 *** | ||
| P. officinalis | 1 | 1.257 ± 0.111 | 2.070 ± 0.207 ** | |
| 5 | 1.106 ± 0.279 | 2.335 ± 0.379 | ||
| 25 | 0.895 ± 0.244 * | 2.301 ± 0.559 | ||
| 50 | 0.917 ± 0.280 * | 1.342 ± 0.401 ** | ||
| 100 | 0.799 ± 0.387 ** | 0.943 ± 0.317 *** | ||
| Rosmarinic acid | 5 | 0.803 ± 0.411 ** | 2.069 ± 0.308 ** | |
| Trolox | 5 | 0.898 ± 0.328 * | 1.788 ± 0.793 ** | |
| Pulmonaria Phenolic-Rich Fractions and Reference Compounds [µg/mL] | Plasma Lipid Peroxidation Biomarkers | |||
|---|---|---|---|---|
| % of Lipid Hydroperoxides Generation | % of TBARS Formation | |||
| plasma treated with ONOO− in the presence of: | P. obscura | 1 | 72.924 ± 6.660 ** | 96.234 ± 6.746 |
| 5 | 65.102 ± 6.242 ** | 83.308 ± 8.987 * | ||
| 25 | 54.075 ± 5.957 *** | 71.977 ± 14.638 * | ||
| 50 | 54.983 ± 6.966 *** | 62.389 ± 10.826 *** | ||
| 100 | 53.805 ± 9.557 *** | 63.759 ± 9.306 *** | ||
| P. officinalis | 1 | 75.492 ± 6.788 ** | 84.685 ± 13.169 | |
| 5 | 70.085 ± 6.394 ** | 79.188 ± 11.122 * | ||
| 25 | 59.695 ± 5.208 ** | 75.782 ± 10.019 * | ||
| 50 | 59.927 ± 8.141 *** | 66.851 ± 12.462 ** | ||
| 100 | 57.126 ± 8.145 *** | 57.746 ± 8.855 *** | ||
| Rosmarinic acid | 5 | 58.246 ± 14.864 ** | 67.552 ± 11.641 ** | |
| Trolox | 5 | 63.986 ± 7.585 *** | 69.212 ± 11.459 *** | |
| The Examined Substances | IC50 [µg/mL] | |
|---|---|---|
| The PGF2αGeneration (ELISA) | The Oxidation of Chromogenic Substrate (Colorimetric Assay) | |
| P. obscura | 51.00 | 58.59 |
| P. officinalis | 7.24 | 13.28 |
| Indomethacin | 0.05 | 2.06 |
| Pulmonaria Phenolic-Rich Fraction Concentration [µg/mL] | % Viability of PBMCs Based on Propidium Iodide Assay | % Viability of PBMCs Based on Resazurin Reduction Assay | % of LDH Leakage from Blood Platelets | |
|---|---|---|---|---|
| P. obscura | 1 | 102.15 ± 13.12 | 96.84 ± 8.70 | 100.55 ± 9.04 |
| 5 | 106.80 ± 6.21 | 94.59 ± 8.14 | 99.67 ± 12.50 | |
| 25 | 100.11 ± 9.78 | 95.25 ± 8.68 | 101.16 ± 11.22 | |
| 50 | 105.45 ± 6.52 | 98.53 ± 11.10 | 97.86 ± 6.98 | |
| 100 | 100.21 ± 12.08 | 93.75 ± 8.69 | 94.92 ± 6.84 | |
| P. officinalis | 1 | 102.97 ± 9.32 | 93.71 ± 8.10 | 103.30 ± 13.33 |
| 5 | 106.66 ± 7.05 | 93.76 ± 10.54 | 105.59 ± 15.16 | |
| 25 | 104.18 ± 10.77 | 93.59 ± 9.32 | 102.32 ± 7.57 | |
| 50 | 104.67 ± 9.15 | 96.70 ± 7.51 | 98.68 ± 13.30 | |
| 100 | 107.05 ± 5.53 | 97.33 ± 9.43 | 99.65 ± 7.67 |
| (No.) | Compound Names, Chemical Formula and SMILES | ~MW (Da) | H.A. | milogP | MBS PI | MBS EI | ΔG°bind (kcal/mol) COX2 | LE LELP |
|---|---|---|---|---|---|---|---|---|
| (4) | 2–O–E–caffeoyl–l–threonic acid O=C(C=Cc1ccc(O)c(O)c1)O[C@H](C(=O)O)[C@@H](O)CO | 298 | 21 | −0.49 | −0.13 | 0.34 | −7.6 ± 0.1 | 0.36 –1.36 |
| (1) | Danshensu c1(ccc(c(c1)O)O)C[C@@H](O)C(=O)O | 198 | 14 | −0.25 | −0.27 | 0.13 | −6.8 ± 0.1 | 0.49 –0.51 |
| (6) | Chlorogenic acid O=C(C=Cc1ccc(O)c(O)c1)O[C@@H]2C[C@](O)(C(=O)O)C[C@@H](O)[C@H]2O | 354 | 25 | −0.45 | 0.25 | 0.62 | −7.9 ± 0.2 | 0.32 –1.41 |
| (37) | Globoidnan A c1(cc2cc(c(cc2c(c1)c1ccc(c(c1)O)O)O)O)C(=O)O[C@@H](C(=O)O)Cc1ccc(c(c1)O)O | 492 | 36 | 3.12 | 0.11 | 0.26 | −8.7 ± 0.3 | 0.24 13.00 |
| (18) | Globoidnan B O([C@H](Cc1cc(c(cc1)O)O)C(=O)O)C(=O)c1c(c(c2c(c1)cc(c(c2)O)O)c1cc(c(cc1)O)O)C(=O)O | 537 | 39 | 2.28 | 0.09 | 0.28 | −6.9 ± 0.2 | 0.18 12.67 |
| (31) | Lithospermic acid A c12[C@@H]([C@@H](Oc1c(ccc2/C=C\C(=O)O[C@@H](Cc1cc(c(cc1)O)O)C(=O)O)O)c1cc(c(cc1)O)O)C(=O)O | 539 | 39 | 1.57 | 0.06 | 0.28 | −7.8± 0.1 | 0.20 7.85 |
| (2) | Menisdaurin O1[C@@H]([C@H]([C@@H]([C@H]([C@@H]1O[C@H]1/C(=C\C#N)/C=C[C@H](C1)O)O)O)O)CO | 313 | 22 | −1.79 | 0.28 | 0.91 | −6.2 ± 0.2 | 0.28 –6.39 |
| (29) | Monardic acid A O1[C@@H]([C@H](c2c1c(ccc2/C=C/C(=O)O[C@@H](Cc1cc(c(cc1)O)O)C(=O)O)O)C(=O)O)c1cc(c(cc1)O)O | 539 | 39 | 1.57 | 0.06 | 0.28 | −7.6 ± 0.5 | 0.20 7.85 |
| (27) | Rosmarinic acid [C@@H](C(=O)O)(OC(=O)/C=C/c1ccc(c(c1)O)O)Cc1ccc(c(c1)O)O | 360 | 26 | 1.53 | 0.15 | 0.24 | −8.3 ± 0.1 | 0.32 4.78 |
| (23) | Quercetin 3–O–(6’’–O–malonyl)–β–glucoside O1[C@@H]([C@H]([C@@H]([C@H]([C@@H]1Oc1c(oc2c(c1=O)c(cc(c2)O)O)c1cc(c(cc1)O)O)O)O)O)COC(=O)CC(=O)O | 550 | 39 | −0.66 | −0.03 | 0.35 | −6.3 ± 0.3 | 0.16 –4.13 |
| (26) | Shimobashiric acid C [C@@H]1([C@@H]([C@@H]([C@H]1c1cc(c(cc1)O)O)C(=O)O[C@@H](C(=O)O)Cc1cc(c(cc1)O)O)c1cc(c(cc1)O)O)C(=O)O[C@@H](C(=O)O)Cc1cc(c(cc1)O)O | 721 | 52 | 2.98 | −0.52 | −1.21 | −3.6 ± 0.7 | 0.07 42.57 |
| (33) | Salvianolic acid H O([C@H](Cc1cc(c(cc1)O)O)C(=O)O)C(=O)/C=C/c1c(c(c(cc1)O)O)/C=C/c1cc(c(cc1)O)O | 495 | 36 | 3.01 | 0.08 | 0.21 | −8.8 ± 0.4 | 0.24 12.54 |
| (22) | Yunnaneic acid E [C@@H]1([C@H](C(=C[C@H]([C@@H]1c1ccc(c(c1)O)O)C(=O)O)/C=C/C(=O)O[C@@H](C(=O)O)Cc1ccc(c(c1)O)O)C(=O)O)C(=O)O | 573 | 41 | 1.03 | −0.01 | 0.06 | −7.7 ± 0.3 | 0.19 5.42 |
| (36) | Yunnaneic acid B O1[C@]2([C@@](O[C@@]31[C@H]1[C@@H]([C@H]([C@@H](C3=O)[C@H](C1)CCC(=O)O[C@@H](C(=O)O)Cc1cc(c(cc1)O)O)C(=O)O)c1cc(c(cc1)O)O)([C@H]1[C@@H]([C@H]([C@@H]2[C@H](C1)/C=C/C(=O)O[C@@H](C(=O)O)Cc1cc(c(cc1)O)O)C(=O)O)c1cc(c(cc1)O)O)O)O | 1001 | 79 | 1.95 | −3.72 | −3.78 | −10.2 ± 0.1 | 0.13 15 |
| Indomethacin C(=O)(n1c(C)c(c2cc(ccc12)OC)CC(=O)O)c1ccc(Cl)cc1 | 358 | 25 | 3.99 | −0.11 | 0.30 | −9.4 ± 1.3 | 0.38 10.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Ponczek, M.B.; Pecio, Ł.; Nowak, P.; Kolodziejczyk-Czepas, J. Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors–In Vitro and In Silico Studies. Molecules 2021, 26, 631. https://doi.org/10.3390/molecules26030631
Krzyżanowska-Kowalczyk J, Kowalczyk M, Ponczek MB, Pecio Ł, Nowak P, Kolodziejczyk-Czepas J. Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors–In Vitro and In Silico Studies. Molecules. 2021; 26(3):631. https://doi.org/10.3390/molecules26030631
Chicago/Turabian StyleKrzyżanowska-Kowalczyk, Justyna, Mariusz Kowalczyk, Michał B. Ponczek, Łukasz Pecio, Paweł Nowak, and Joanna Kolodziejczyk-Czepas. 2021. "Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors–In Vitro and In Silico Studies" Molecules 26, no. 3: 631. https://doi.org/10.3390/molecules26030631
APA StyleKrzyżanowska-Kowalczyk, J., Kowalczyk, M., Ponczek, M. B., Pecio, Ł., Nowak, P., & Kolodziejczyk-Czepas, J. (2021). Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors–In Vitro and In Silico Studies. Molecules, 26(3), 631. https://doi.org/10.3390/molecules26030631









