Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds
Abstract
:1. Introduction
2. The Main Chemical Categories behind Wine Aroma
3. Grape and Wine Aroma Composition
4. Impact of Non-Saccharomyces Species on Wine Aroma
4.1. Torulaspora delbrueckii
4.2. Hanseniaspora spp.
4.3. Lachancea thermotolerans
4.4. Metschnikowia pulcherrima
4.5. Candida zemplinina
4.6. Pichia spp.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early Fermentation Volatile Metabolite Profile of Non-Saccharomyces Yeasts in Red and White Grape Must: A Targeted Approach. LWT-Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Mateo, J.J.; Maicas, S. Application of Non-Saccharomyces Yeasts to Wine-Making Process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and Non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufariello, M.; Maiorano, G.; Rampino, P.; Spano, G.; Grieco, F.; Perrotta, C.; Capozzi, V.; Grieco, F. Selection of an Autochthonous Yeast Starter Culture for Industrial Production of Primitivo “Gioia Del Colle” PDO/DOC in Apulia (Southern Italy). LWT 2019, 99, 188–196. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tasting the Terroir of Wine Yeast Innovation. FEMS Yeast Res. 2020, 20. [Google Scholar] [CrossRef] [Green Version]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora uvarum in Simultaneous and Sequential Co-Fermentation with Saccharomyces cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New Insights on the Use of Wine Yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Role and Use of Non-Saccharomyces Yeasts in Wine Production. S. Afr. J. Enol. Vitic. 2006, 27, 15–38. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Benito, S. The Impact of Torulaspora delbrueckii Yeast in Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter Cultures for Sparkling Wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of Autochthonous Saccharomyces cerevisiae Strains on Volatile Profile of Negroamaro Wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile Profile of Reduced Alcohol Wines Fermented with Selected Non-Saccharomyces Yeasts under Different Aeration Conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Hoff, J.; Jolly, N. Modulation of Wine Flavor Using Hanseniaspora uvarum in Combination with Different Saccharomyces cerevisiae, Lactic Acid Bacteria Strains and Malolactic Fermentation Strategies. Fermentation 2019, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Ebeler, S.E.; Thorngate, J.H. Wine Chemistry and Flavor: Looking into the Crystal Glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [Green Version]
- Savoi, S.; Wong, D.C.J.; Degu, A.; Herrera, J.C.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Multi-Omics and Integrated Network Analyses Reveal New Insights into the Systems Relationships between Metabolites, Structural Genes, and Transcriptional Regulators in Developing Grape Berries (Vitis vinifera L.) Exposed to Water Deficit. Front. Plant Sci. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, M.; Battista, F.; Panighel, A.; Flamini, R.; Tomasi, D. Effect of Pre-Bloom Leaf Removal on Grape Aroma Composition and Wine Sensory Profile of Semillon Cultivar. J. Sci. Food Agric. 2018, 98, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; De Rosso, M.; Dalla Vedova, A.; Flamini, R. High-Resolution Mass Spectrometry Identification of Secondary Metabolites in Four Red Grape Varieties Potentially Useful as Traceability Markers of Wines. Beverages 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The Physiology of Drought Stress in Grapevine: Towards an Integrative Definition of Drought Tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of Viticulture Practices on Grape Aroma Precursors and Their Relation with Wine Aroma. J. Sci. Food Agric. 2015, 95, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Fragasso, M.; Antonacci, D.; Pati, S.; Tufariello, M.; Baiano, A.; Forleo, L.R.; Caputo, A.R.; Notte, E.L. Influence of Training System on Volatile and Sensory Profiles of Primitivo Grapes and Wines. Am. J. Enol. Vitic. 2012. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate Ester Formation in Wine by Mixed Cultures in Laboratory Fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Shen, J.-Y.; Duan, C.-Q.; Yan, G.-L. Use of Indigenous Hanseniaspora vineae and Metschnikowia pulcherrima Co-Fermentation With Saccharomyces cerevisiae to Improve the Aroma Diversity of Vidal Blanc Icewine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef]
- Du Toit, M.; Pretorius, I.S. Microbial Spoilage and Preservation of Wine: Using Weapons from Nature’s Own Arsenal -A Review. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage Yeasts in the Wine Industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Godoy, L.; Acuña-Fontecilla, A.; Catrileo, D. Formation of Aromatic and Flavor Compounds in Wine: A Perspective of Positive and Negative Contributions of Non-Saccharomyces Yeasts. Winemak. Stab. Aging Chem. Biochem. 2020. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine Aroma Response to Different Participation of Selected Hanseniaspora uvarum in Mixed Fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mendes Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The Role of Non-Saccharomyces Species in Releasing Glycosidic Bound Fraction of Grape Aroma Components--a Preliminary Study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of Yeast Species and Strains in Wine Flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- King, A.; Richard Dickinson, J. Biotransformation of Monoterpene Alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast Chichester Engl. 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L. Effect of Sequential Fermentations and Grape Cultivars on Volatile Compounds and Sensory Profiles of Danish Wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina Can Reduce Acetic Acid Produced by Saccharomyces cerevisiae in Sweet Wine Fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.-C.; Bely, M. Increase of Fruity Aroma during Mixed T. delbrueckii/S. cerevisiae Wine Fermentation Is Linked to Specific Esters Enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae Mixed Cultures on Fermentation and Aroma of Amarone Wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in Varietal Thiol (3-SH and 4-MSP) Release in Wine Sequential Fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on Cv. Emir Wine Fermentation. Yeast Chichester Engl. 2016, 33, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia pulcherrima in the Volatile Profile of Verdejo White Wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile Flavour Profile of Reduced Alcohol Wines Fermented with the Non-Conventional Yeast Species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Russo, P.; Tufariello, M.; Renna, R.; Tristezza, M.; Taurino, M.; Palombi, L.; Capozzi, V.; Rizzello, C.G.; Grieco, F. New Insights into the Oenological Significance of Candida zemplinina: Impact of Selected Autochthonous Strains on the Volatile Profile of Apulian Wines. Microorganisms 2020, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging Trends in the Application of Malolactic Fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-Fermentation with Pichia kluyveri Increases Varietal Thiol Concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Perpetuini, G.; Tittarelli, F.; Battistelli, N.; Suzzi, G.; Tofalo, R. Contribution of Pichia manshurica Strains to Aroma Profile of Organic Wines. Eur. Food Res. Technol. 2020, 246, 1405–1417. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Wine Yeasts. Available online: https://www.lallemandwine.com/en/new-zealand/products/catalogue/wine-yeasts/ (accessed on 23 November 2020).
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Mol. Basel Switz. 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [Green Version]
- Saez, J.S.; Lopes, C.A.; Kirs, V.E.; Sangorrín, M. Production of Volatile Phenols by Pichia manshurica and Pichia membranifaciens Isolated from Spoiled Wines and Cellar Environment in Patagonia. Food Microbiol. 2011, 28, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M.; Veurink, J.H.; Euverink, G.-J.W.; Dijkhuizen, L. Growth of the Salt-Tolerant Yeast Zygosaccharomyces rouxii in Microtiter Plates: Effects of NaCl, PH and Temperature on Growth and Fusel Alcohol Production from Branched-Chain Amino Acids. FEMS Yeast Res. 2003, 3, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, J.J.; Jiménez, M. Monoterpenes in Grape Juice and Wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Manzanares, P.; Rojas, V.; Genovés, S.; Vallés, S. A Preliminary Search for Anthocyanin-β-d-Glucosidase Activity in Non-Saccharomyces Wine Yeasts. Int. J. Food Sci. Technol. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Strauss, M.L.; Jolly, N.P.; Lambrechts, M.G.; van Rensburg, P. Screening for the Production of Extracellular Hydrolytic Enzymes by Non-Saccharomyces Wine Yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine Analysis and Influence of Viticultural and Enological Procedures on Their Levels in Grapes, Musts, and Wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional Characterisation of Three Members of the Vitis vinifera L. Carotenoid Cleavage Dioxygenase Gene Family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef] [Green Version]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-Analysis of the Core Aroma Components of Grape and Wine Aroma. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Lund, S.T.; Bohlmann, J. The Molecular Basis for Wine Grape Quality--a Volatile Subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrau, F.M.; Boido, E.; Dellacassa, E. Terpenoids in Grapes and Wines: Origin and Micrometabolism during the Vinification Process. Nat. Prod. Commun. 2008, 3, 1934578X0800300419. [Google Scholar] [CrossRef] [Green Version]
- Swangkeaw, J.; Vichitphan, S.; Butzke, C.E.; Vichitphan, K. The Characterisation of a Novel Pichia anomala β-Glucosidase with Potentially Aroma-Enhancing Capabilities in Wine. Ann. Microbiol. 2009, 59, 335. [Google Scholar] [CrossRef]
- Marais, J.; Versini, G.; van Wyk, C.J.; Rapp, A. Effect of Region on Free and Bound Monoterpene and C13-N Orisoprenoid Concentrations in Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 1992, 13, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Rapp, A. Possibilities of characterizing wine varieties by means of volatile flavor compounds. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1703–1722. [Google Scholar]
- Strauss, C.R.; Wilson, B.; Gooley, P.R.; Williams, P.J. Role of Monoterpenes in Grape and Wine Flavor. In Biogeneration of Aromas; American Chemical Society: Washington, DC, USA, 1986; Volume 317, pp. 222–242. ISBN 978-0-8412-0987-9. [Google Scholar]
- Rapp, A.; Mandery, H.; Niebergall, H. Neue Monoterpendiole in Traubenmost Und Wein Sowie in Kulturen von Botrytis Cinerea. VITIS J. Grapevine Res. 1986, 25, 79. [Google Scholar] [CrossRef]
- Winterhalter, P.; Baderschneider, B.; Bonnländer, B. Analysis, Structure, and Reactivity of Labile Terpenoid Aroma Precursors in Riesling Wine. In Chemistry of Wine Flavor; American Chemical Society: Washington, DC, USA, 1998; Volume 714, pp. 1–12. ISBN 978-0-8412-3592-2. [Google Scholar]
- Bitteur, S.M.; Baumes, R.L.; Bayonove, C.L.; Versini, G.; Martin, C.A.; Dalla Serra, A. 2-Exo-Hydroxy-1,8-Cineole: A New Component from Grape Var. Sauvignon. J. Agric. Food Chem. 1990, 38, 1210–1213. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene Compounds as Possible Precursors of 1,8-Cineole in Red Grapes and Wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B. Hydroxylated Linalool Derivatives as Precursors of Volatile Monoterpenes of Muscat Grapes. J. Agric. Food Chem. 1980, 28, 766–771. [Google Scholar] [CrossRef]
- Gramatica, P.; Manitto, P.; Ranzi, B.M.; Delbianco, A.; Francavilla, M. Stereospecific Reduction of Geraniol to (R)-(+)-Citronellol By Saccharomyces cerevisiae. Experientia 1982, 38, 775–776. [Google Scholar] [CrossRef]
- Williams, P.J.; Sefton, M.A.; Wilson, B. Nonvolatile Conjugates of Secondary Metabolites as Precursors of Varietal Grape Flavor Components. In Flavor Chemistry; American Chemical Society: Washington, DC, USA, 1989; Volume 388, pp. 35–48. ISBN 978-0-8412-1570-2. [Google Scholar]
- Flamini, R.; Dalla Vedova, A.; Panighel, A.; Biscaro, S.; Borgo, M.; Calò, A. Characterization of Torbato (Vitis vinifera L.) aroma and study of leaf roll effects on the grape aroma compounds [varieties; Sardinia]. Riv. Vitic. E Enol. Italy 2006, 59, 13–26. [Google Scholar]
- Williams, P.J.; Sefton, M.A.; Francis, I.L. Glycosidic Precursors of Varietal Grape and Wine Flavor. ACS Symp. Ser. USA 1992, 7, 74–86. [Google Scholar]
- Winterhalter, P.; Sefton, M.A.; Williams, P.J. Two-Dimensional GC-DCCC Analysis of the Glycoconjugates of Monoterpenes, Norisoprenoids, and Shikimate-Derived Metabolites from Riesling Wine. J. Agric. Food Chem. 1990, 38, 1041–1048. [Google Scholar] [CrossRef]
- Winterhalter, P. Oxygenated C13-Norisoprenoids. In Flavor Precursors; American Chemical Society: Washington, DC, USA, 1992; Volume 490, pp. 98–115. ISBN 978-0-8412-2222-9. [Google Scholar]
- Knapp, H.; Straubinger, M.; Stingl, C.; Winterhalter, P. Analysis of Norisoprenoid Aroma Precursors. In Carotenoid-Derived Aroma Compounds; American Chemical Society: Washington, DC, USA, 2001; Volume 802, pp. 20–35. ISBN 978-0-8412-3729-2. [Google Scholar]
- Versini, G.; Carlin, S.; Dalla Serra, A.; Nicolini, G.; Rapp, A. Formation of 1,1,6-Trimethyl-1,2-Dihydronaphthalene and Other Norisoprenoids in Wine: Considerations on the Kinetics. In Carotenoid-Derived Aroma Compounds; American Chemical Society: Washington, DC, USA, 2001; Volume 802, pp. 285–299. ISBN 978-0-8412-3729-2. [Google Scholar]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaghenaufi, D.; Perello, M.-C.; Marchand-Marion, S.; Tempere, S.; de Revel, G. Quantitative Solid Phase Microextraction – Gas Chromatography Mass Spectrometry Analysis of Five Megastigmatrienone Isomers in Aged Wine. Anal. Chim. Acta 2014, 813, 63–69. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Delgado, J.A.; Ferrer, M.A.; Viñas, M.A.G. The Aroma of La Mancha Chelva Wines: Chemical and Sensory Characterization. Food Res. Int. 2019, 119, 135–142. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile Composition and Aroma Profile of Uruguayan Tannat Wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Lonardi, A.; Landi, L.; Mattivi, F. Aromatic Complexity in Verdicchio Wines: A Case Study. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B.; Massy-Westropp, R.A. Glycosides of 2-Phenylethanol and Benzyl Alcohol in Vitis Vinifera Grapes. Phytochemistry 1983, 22, 2039–2041. [Google Scholar] [CrossRef]
- López, R.; Ezpeleta, E.; Sánchez, I.; Cacho, J.; Ferreira, V. Analysis of the Aroma Intensities of Volatile Compounds Released from Mild Acid Hydrolysates of Odourless Precursors Extracted from Tempranillo and Grenache Grapes Using Gas Chromatography-Olfactometry. Food Chem. 2004, 88, 95–103. [Google Scholar] [CrossRef]
- Flamini, R.; Dalla Vedova, A.; Cancian, D.; Panighel, A.; De Rosso, M. GC/MS-Positive Ion Chemical Ionization and MS/MS Study of Volatile Benzene Compounds in Five Different Woods Used in Barrel Making. J. Mass Spectrom. JMS 2007, 42, 641–646. [Google Scholar] [CrossRef]
- De Rosso, M.; Cancian, D.; Panighel, A.; Dalla Vedova, A.; Flamini, R. Chemical Compounds Released from Five Different Woods Used to Make Barrels for Aging Wines and Spirits: Volatile Compounds and Polyphenols. Wood Sci. Technol. 2009, 43, 375–385. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-Active Compounds of American, French, Hungarian and Russian Oak Woods, Studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of Oak-Derived Compounds on the Olfactory Perception of Barrel-Aged Wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar]
- Chira, K.; Teissedre, P.-L. Chemical and Sensory Evaluation of Wine Matured in Oak Barrel: Effect of Oak Species Involved and Toasting Process. Eur. Food Res. Technol. 2015, 240, 533–547. [Google Scholar] [CrossRef]
- Snakkers, G.; Boulesteix, J.-M.; Estréguil, S.; Gaschet, J.; Lablanquie, O.; Faure, A.; Cantagrel, R. Effect of Oak Wood Heating on Cognac Spirit Matured in New Barrel: A Pilot Study. OENO One 2003, 37, 243–255. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in Volatile Composition of Madeira Wines during Their Oxidative Ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, K.; Samuta, T. Grape Maturity and Light Exposure Affect Berry Methoxypyrazine Concentration. Am. J. Enol. Vitic. 1999, 50, 194–198. [Google Scholar]
- Lacey, M.J.; Allen, M.S.; Harris, R.L.N.; Brown, W.V. Methoxypyrazines in Sauvignon Blanc Grapes and Wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- Hashizume, K.; Umeda, N. Methoxypyrazine Content of Japanese Red Wines. Biosci. Biotechnol. Biochem. 1996, 60, 802–805. [Google Scholar] [CrossRef]
- Hashizume, K.; Samuta, T. Green Odorants of Grape Cluster Stem and Their Ability To Cause a Wine Stemmy Flavor. J. Agric. Food Chem. 1997, 45, 1333–1337. [Google Scholar] [CrossRef]
- De Boubée, D.R.; Cumsille, A.M.; Pons, M.; Dubourdieu, D. Location of 2-Methoxy-3-Isobutylpyrazine in Cabernet Sauvignon Grape Bunches and Its Extractability during Vinification. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of Methoxypyrazines to Sauvignon Blanc Wine Aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Siebert, T.E.; Wood, C.; Elsey, G.M.; Pollnitz, A.P. Determination of Rotundone, the Pepper Aroma Impact Compound, in Grapes and Wine. J. Agric. Food Chem. 2008, 56, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of Organic Sulfur Compounds in Wine Aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Varietal Aroma. In Handbook of Enology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 205–230. ISBN 978-0-470-01039-6. [Google Scholar]
- Rapp, A.; Güntert, M.; Almy, J. Identification and Significance of Several Sulfur-Containing Compounds in Wine. Am. J. Enol. Vitic. 1985, 36, 219–221. [Google Scholar]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Swiegers, J.H.; Pretorius, I.S. Modulation of Volatile Sulfur Compounds by Wine Yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Versini, G.; Dellacassa, E.; Carlin, S.; Fedrizzi, B.; Magno, F. Analysis of Aroma Compounds in Wine. In Hyphenated Techniques in Grape and Wine Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 173–225. ISBN 978-0-470-75432-0. [Google Scholar]
- Beloqui, A.A.; de Pinho, P.G.; Bertrand, A. Bis(2-Hydroxyethyl) Disulfide, a New Sulfur Compound Found in Wine. Its Influence in Wine Aroma. Am. J. Enol. Vitic. 1995, 46, 84–87. [Google Scholar]
- Bouchilloux, P.; Darriet, P.; Dubourdieu, D. Identification d’un Thiol Fortement Odorant, Le 2-Méthyl-3-Furanthiol, Dans Les Vins. VITIS J. Grapevine Res. 1998, 37, 177. [Google Scholar] [CrossRef]
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical Implications of Malolactic Fermentation: A Review. Am. J. Enol. Vitic. 1985, 36, 290–301. [Google Scholar]
- Flamini, R.; Luca, G.D.; Stefano, R.D. Changes in Carbonyl Compounds in Chardonnay and Cabernet Sauvignon Wines as a Consequence of Malolactic Fermentation. VITIS-J. Grapevine Res. 2002, 41, 107. [Google Scholar] [CrossRef]
- Sauvageot, F.; Vivier, P. Effects of Malolactic Fermentation on Sensory Properties of Four Burgundy Wines. Am. J. Enol. Vitic. 1997, 48, 187–192. [Google Scholar]
- De Revel, G.; Bertrand, A. Dicarbonyl compounds and their reduction products in wine. Identification of wine aldehydes. In Trends in Flavour Research; Maarse, H., van der Heij, D.G., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; pp. 353–361. [Google Scholar]
- Allen, M.S. What Level of Methoxypyrazines Is Desired in Red Wines? The Flavour Perspective of the Classic Red Wines of Bordeaux. Aust Grapegrow. Winemak. 1995, 381, 7–9. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D. Identification of Substances Responsible for the ‘Sawdust’ Aroma in Oak Wood. J. Sci. Food Agric. 1998, 76, 179–188. [Google Scholar] [CrossRef]
- Revel, G.D.; Bertrand, A. A Method for the Detection of Carbonyl Compounds in Wine: Glyoxal and Methylglyoxal. J. Sci. Food Agric. 1993, 61, 267–272. [Google Scholar] [CrossRef]
- Guillou, I.; Bertrand, A.; de Revel, G.; Barbe, J.C. Occurrence of Hydroxypropanedial in Certain Musts and Wines. J. Agric. Food Chem. 1997, 45, 3382–3386. [Google Scholar] [CrossRef]
- Flamini, R.; Dalla Vedova, A. Glyoxal/Glycolaldehyde: A Redox System Involved in Malolactic Fermentation of Wine. J. Agric. Food Chem. 2003, 51, 2300–2303. [Google Scholar] [CrossRef]
- Kenneth Fugelsang, P. Brettanomyces: Dr Jekyll ou Mr Hyde des vins? Biofutur 1998, 1998, 22–23. [Google Scholar] [CrossRef]
- Edlin, D.A.N.; Narbad, A.; Dickinson, J.R.; Lloyd, D. The Biotransformation of Simple Phenolic Compounds by Brettanomyces anomalus. FEMS Microbiol. Lett. 1995, 125, 311–315. [Google Scholar] [CrossRef]
- Edlin, D.A.N.; Narbad, A.; Gasson, M.J.; Dickinson, J.R.; Lloyd, D. Purification and Characterization of Hydroxycinnamate Decarboxylase from Brettanomyces anomalus. Enzyme Microb. Technol. 1998, 22, 232–239. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.; Pons, M. The Origin of Ethylphenols in Wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Chatonnet, P.; Viala, C.; Dubourdieu, D. Influence of Polyphenolic Components of Red Wines on the Microbial Synthesis of Volatile Phenols. Am. J. Enol. Vitic. 1997, 48, 443–448. [Google Scholar]
- Dias, L.; Dias, S.; Sancho, T.; Stender, H.; Querol, A.; Malfeito-Ferreira, M.; Loureiro, V. Identification of Yeasts Isolated from Wine-Related Environments and Capable of Producing 4-Ethylphenol. Food Microbiol. 2003, 20, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Couto, J.A.; Campos, F.M.; Figueiredo, A.R.; Hogg, T.A. Ability of Lactic Acid Bacteria to Produce Volatile Phenols. Am. J. Enol. Vitic. 2006, 57, 166–171. [Google Scholar]
- Barata, A.; Nobre, A.; Correia, P.; Malfeito-Ferreira, M.; Loureiro, V. Growth and 4-Ethylphenol Production by the Yeast Pichia guilliermondii in Grape Juices. Am. J. Enol. Vitic. 2006, 57, 133–138. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N. The Influence of Brettanomyces/Dekkera Sp. Yeasts and Lactic Acid Bacteria on the Ethylphenol Content of Red Wines. Am. J. Enol. Vitic. 1995, 46, 463–468. [Google Scholar]
- Rodrigues, N.; Gonçalves, G.; Pereira-da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and Use of a New Medium to Detect Yeasts of the Genera Dekkera/Brettanomyces. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of Volatile Phenols by Saccharomyces cerevisiae in Wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Flüchtige phenolische Verbindungen in Wein. Deutsche. Lebensm. Rundsch. 1996, 92, 42–48. [Google Scholar]
- Piggott, J.R. Flavour of Distilled Beverages: Origin and Development; Society of Chemical Industry (Great Britain), Food Group, Sensory Panel, Ed.; E. Horwood Ltd.: Chichester, UK, 1983; ISBN 978-0-89573-131-9. [Google Scholar]
- Van Wyk, C.J.; Rogers, L.M. A “Phenolic” Off-Odour in White Table Wines: Causes and Methods to Diminish Its Occurrence. S. Afr. J. Enol. Vitic. 2000, 21, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Versini, G.; Serra, A.D.; Falcetti, M.; Sferlazzo, G. Rôle du clone, du millésime et de l’époque de la récolte sur le potentiel aromatique du raisin de Chardonnay. Rev. Oenologues 1992, 18, 19–23. [Google Scholar]
- Versini, G. Sull’aroma del vino Traminer Aromatico o Gewürztraminer. Vignevini 1995, 12, 57–65. [Google Scholar]
- Hjelmeland, A.K.; Zweigenbaum, J.; Ebeler, S.E. Profiling Monoterpenol Glycoconjugation in Vitis vinifera L. Cv. Muscat of Alexandria Using a Novel Putative Compound Database Approach, High Resolution Mass Spectrometry and Collision Induced Dissociation Fragmentation Analysis. Anal. Chim. Acta 2015, 887, 138–147. [Google Scholar] [CrossRef]
- Flamini, R.; Rosso, M.D.; Panighel, A.; Vedova, A.D.; Marchi, F.D.; Bavaresco, L. Profiling of Grape Monoterpene Glycosides (Aroma Precursors) by Ultra-High Performance-Liquid Chromatography-High Resolution Mass Spectrometry (UHPLC/QTOF). J. Mass Spectrom. 2014, 49, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Godshaw, J.; Hjelmeland, A.K.; Zweigenbaum, J.; Ebeler, S.E. Changes in Glycosylation Patterns of Monoterpenes during Grape Berry Maturation in Six Cultivars of Vitis vinifera. Food Chem. 2019, 297, 124921. [Google Scholar] [CrossRef]
- Caffrey, A.J.; Lerno, L.A.; Zweigenbaum, J.; Ebeler, S.E. Direct Analysis of Glycosidic Aroma Precursors Containing Multiple Aglycone Classes in Vitis vinifera Berries. J. Agric. Food Chem. 2020, 68, 3817–3833. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene Content of Wine from the Aromatic Grape Variety ‘Irsai Oliver’ (Vitis vinifera L.) Depends on Maceration Time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the Aroma of White Wines by Controlled Torulaspora delbrueckii Cultures in Association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Trinh, T.T.T.; Woon, W.Y.; Yu, B.; Curran, P.; Liu, S.-Q. Effect of L-Isoleucine and L-Phenylalanine Addition on Aroma Compound Formation During Longan Juice Fermentation by a Co-Culture of Saccharomyces cerevisiae and Williopsis saturnus. S. Afr. J. Enol. Vitic. 2010, 31, 116–124. [Google Scholar] [CrossRef]
- Mestre, M.V.; Maturano, Y.P.; Gallardo, C.; Combina, M.; Mercado, L.; Toro, M.E.; Carrau, F.; Vazquez, F.; Dellacassa, E. Impact on Sensory and Aromatic Profile of Low Ethanol Malbec Wines Fermented by Sequential Culture of Hanseniaspora uvarum and Saccharomyces cerevisiae Native Yeasts. Fermentation 2019, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of Non-Saccharomyces Yeasts to Be Used in Grape Musts with High Alcoholic Potential: A Strategy to Obtain Wines with Reduced Ethanol Content. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzi, V.; Berbegal, C.; Tufariello, M.; Grieco, F.; Spano, G.; Grieco, F. Impact of Co-Inoculation of Saccharomyces cerevisiae, Hanseniaspora uvarum and Oenococcus oeni Autochthonous Strains in Controlled Multi Starter Grape Must Fermentations. LWT 2019, 109, 241–249. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The Complexity of Wine: Clarifying the Role of Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Bauer, F.F.; Setati, M.E. The Diversity and Dynamics of Indigenous Yeast Communities in Grape Must from Vineyards Employing Different Agronomic Practices and Their Influence on Wine Fermentation. S. Afr. J. Enol. Vitic. 2015, 36, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of Non-Saccharomyces Yeasts to Wine Volatile and Sensory Diversity: A Study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris Strains Isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The Grape Must Non-Saccharomyces Microbial Community: Impact on Volatile Thiol Release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a Future for Non-Saccharomyces Yeasts: Selection of Putative Spoilage Wine Strains to Be Used in Association with Saccharomyces cerevisiae for Grape Juice Fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Cachón, D.C.; Rantsiou, K.; Blanco, P.; Petrozziello, M.; Pollon, M.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Effect of Mixed Species Alcoholic Fermentation on Growth and Malolactic Activity of Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 7687–7702. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae Mixed Fermentations to Reduce Ethanol Content in Wine. Appl. Microbiol. Biotechnol. 2016, 100, 5515–5526. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative Evaluation of Some Oenological Properties in Wine Strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Russo, P.; Englezos, V.; Capozzi, V.; Pollon, M.; Río Segade, S.; Rantsiou, K.; Spano, G.; Cocolin, L. Effect of Mixed Fermentations with Starmerella bacillaris and Saccharomyces cerevisiae on Management of Malolactic Fermentation. Food Res. Int. 2020, 134, 109246. [Google Scholar] [CrossRef]
- Englezos, V.; Giacosa, S.; Rantsiou, K.; Rolle, L.; Cocolin, L. Starmerella bacillaris in Winemaking: Opportunities and Risks. Curr. Opin. Food Sci. 2017, 17, 30–35. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-Yeast Interactions Revealed by Aromatic Profile Analysis of Sauvignon Blanc Wine Fermented by Single or Co-Culture of Non-Saccharomyces and Saccharomyces Yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Sáez, J.S.; Lopes, C.A.; Kirs, V.C.; Sangorrín, M.P. Enhanced Volatile Phenols in Wine Fermented with Saccharomyces cerevisiae and Spoiled with Pichia guilliermondii and Dekkera bruxellensis. Lett. Appl. Microbiol. 2010, 51, 170–176. [Google Scholar] [CrossRef]


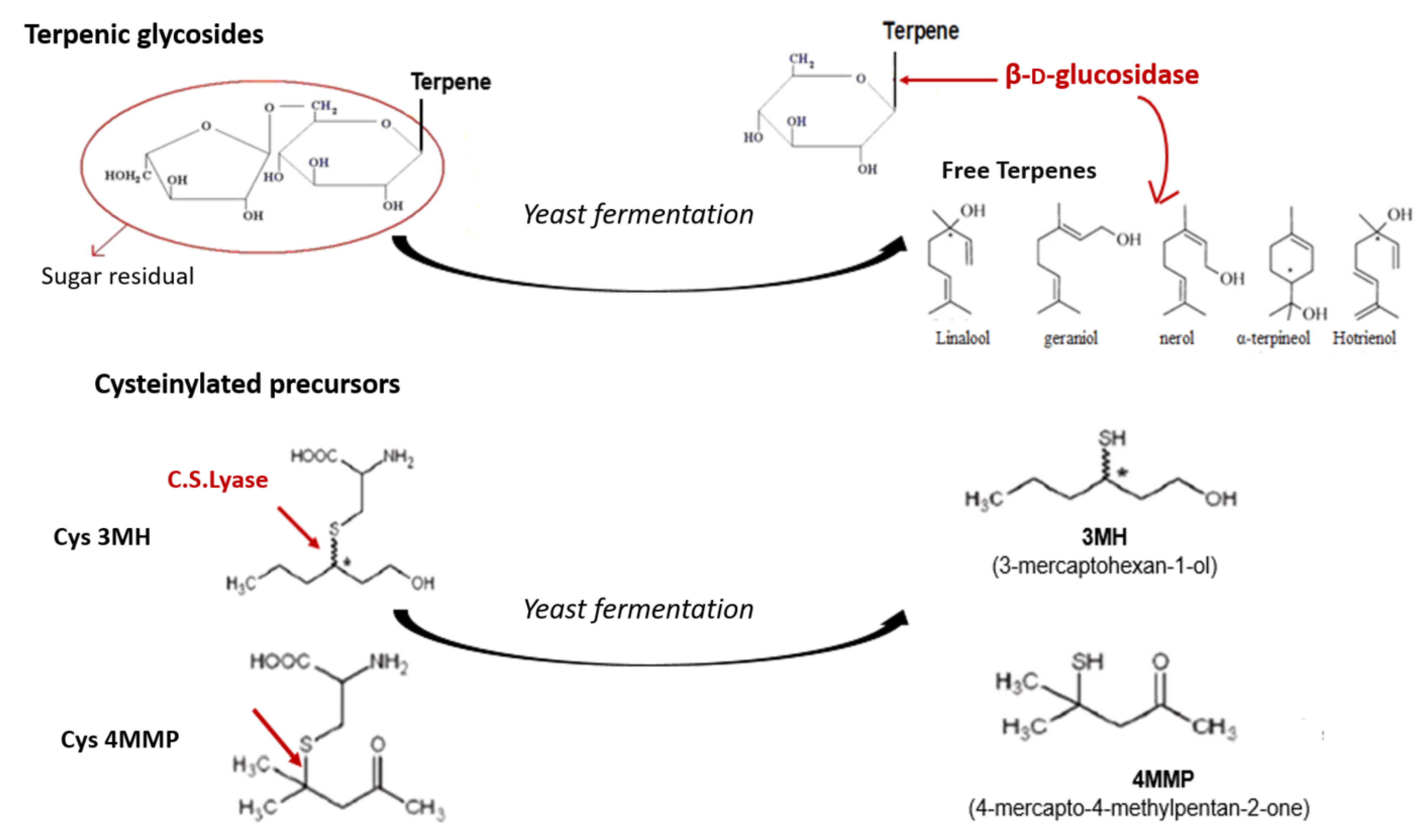




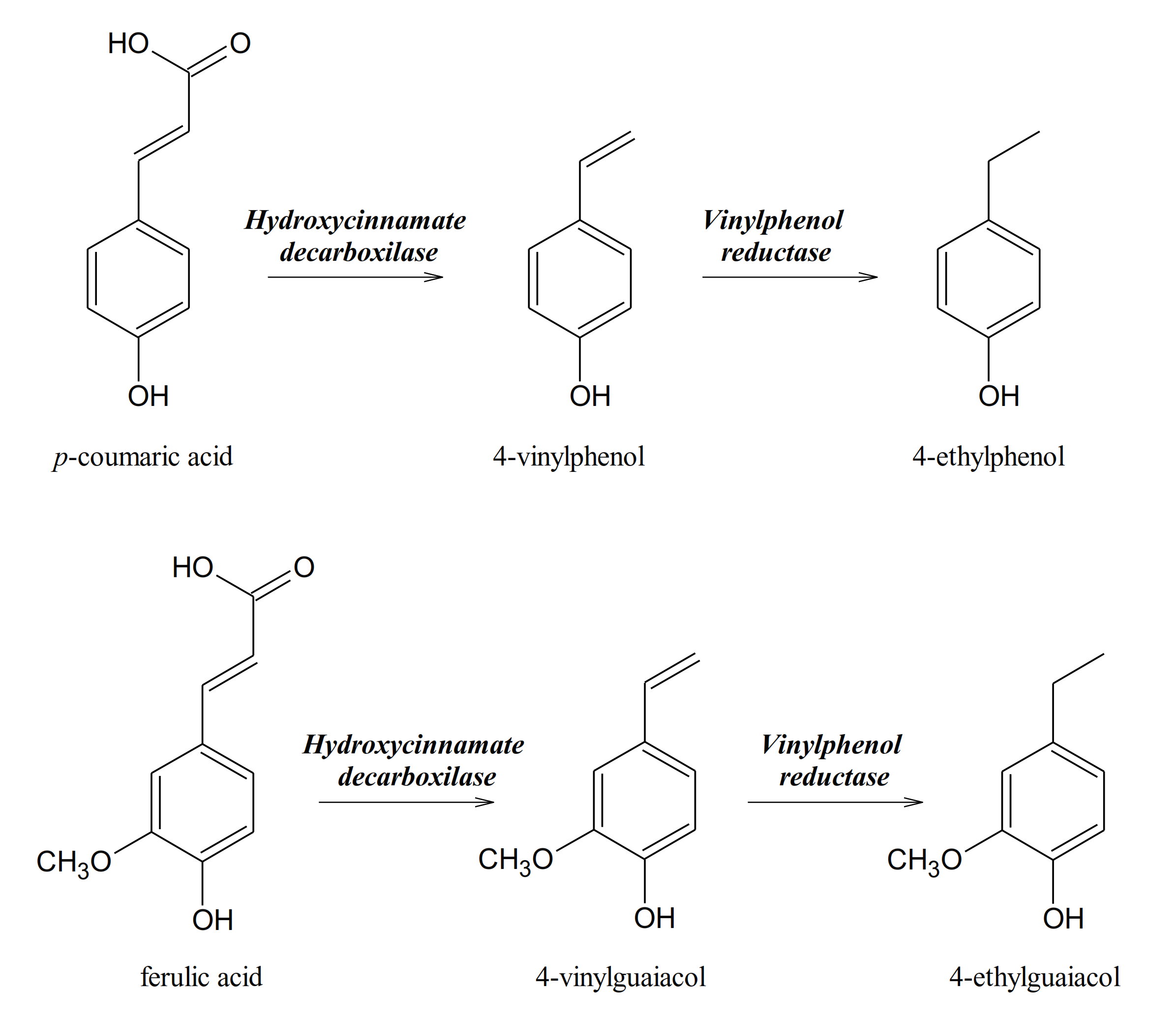
| Species | Metabolites | References |
|---|---|---|
| Hanseniaspora spp. | Acetate esters | [28] |
| C6 alcohols | [29] | |
| Acetic acid | [30,31] | |
| Sulphur compounds | [32] | |
| Hanseniaspora uvarum | Phenylethanol | [7] |
| Acetate esters and ethyl esters | [7,33] | |
| Terpens and norisoprenoids | [33,34] | |
| Acetic acid | [35] | |
| Torulaspora delbrueckii | Linalool | [36] |
| Higher alcohols, esters | [35,37,38] | |
| Acetate esters | [39] | |
| Lactones | [40] | |
| 3-Methyltio-1-propanol | [1] | |
| 4-methyl-4-sulfanylpentan-2-one | [41] | |
| Lachancea thermotolerans | Esters, terpenes | [12,42] |
| 3-Methylthio-1-propanol | [1] | |
| Metschnikowia pulcherrima | Terpenes | [32] |
| 4-Methyl-4-sulfanylpentan-2-one | [43] | |
| Phenylethanol | [43] | |
| β-Damascenone | [29] | |
| Ethyl octanoate, ethyl acetate, 2-phenylethyl acetate | [37,44] | |
| Candida zemplinina | Higher alcohols | [38,45,46] |
| Ethyl esters | [38,45,46,47] | |
| Terpenes | [48,49,50] | |
| Pichia spp. | Acetate esters | [51] |
| Terpenes | [52] | |
| 3-Mercaptohexyl acetate | [48] | |
| Volatile phenols | [49,53] | |
| 3-Methylthio-1-propanol | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. https://doi.org/10.3390/molecules26030644
Tufariello M, Fragasso M, Pico J, Panighel A, Castellarin SD, Flamini R, Grieco F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules. 2021; 26(3):644. https://doi.org/10.3390/molecules26030644
Chicago/Turabian StyleTufariello, Maria, Mariagiovanna Fragasso, Joana Pico, Annarita Panighel, Simone Diego Castellarin, Riccardo Flamini, and Francesco Grieco. 2021. "Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds" Molecules 26, no. 3: 644. https://doi.org/10.3390/molecules26030644







