Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers
Abstract
:1. Introduction
2. Results
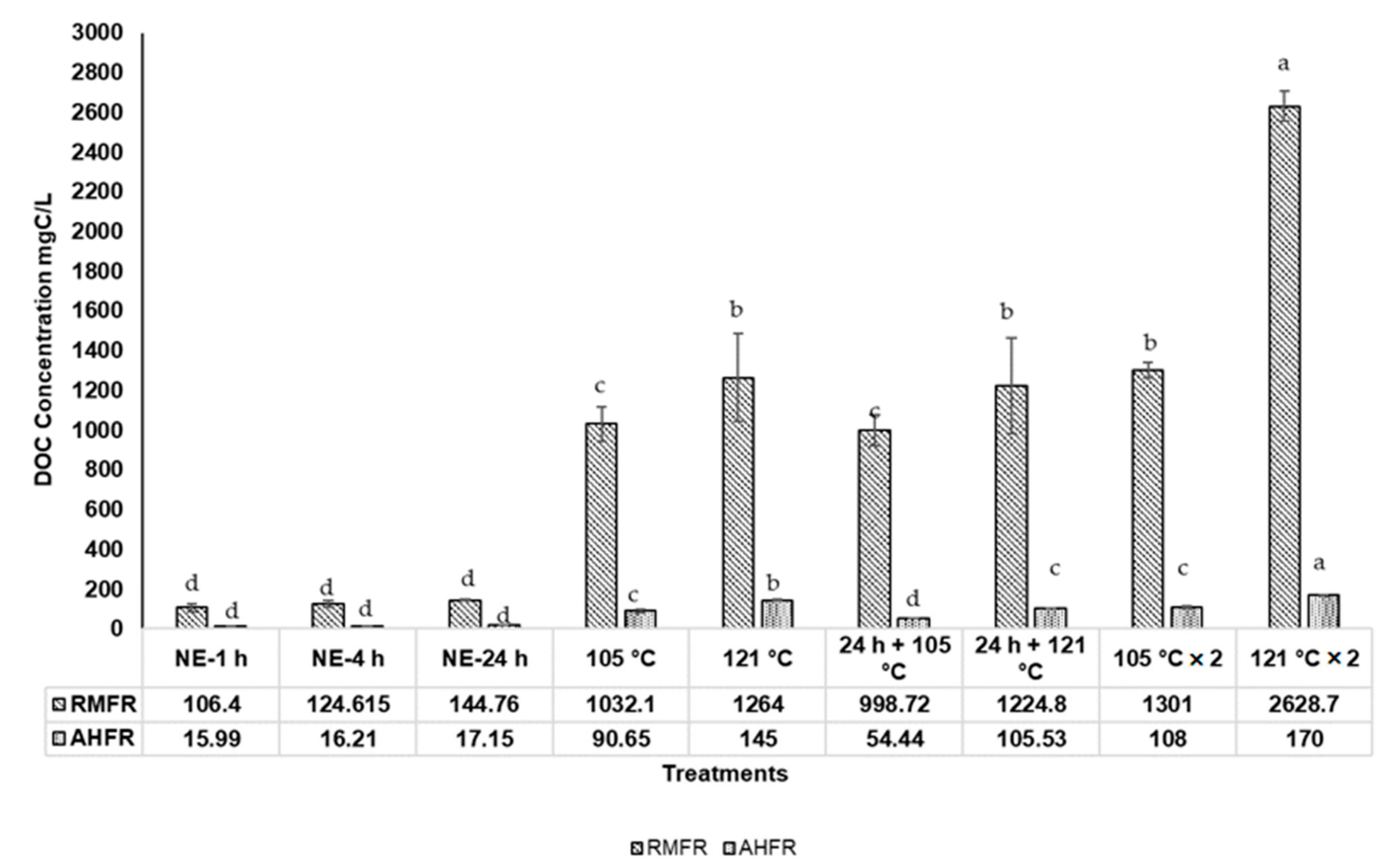
2.1. Total Dissolved Nitrogen (TDN), Total Dissolved Phosphorus (TDP), and Dissolved Organic Carbon (DOC) Concentrations in Soil Extracts with Natural Extractions
2.2. Total Dissolved Nitrogen (TDN), Total Dissolved Phosphorus (TDP), and Dissolved Organic Carbon (DOC) Concentrations in Soil Extracts with Autoclave Extractions
2.3. Comparison of Carbon, Nitrogen, and Phosphorus Recovery
2.4. Effect of Ammonia and Nitrate on Microalgae Growth
2.5. Effect of Modified Soil Extract on the Targeted Microalgae Growth
3. Discussion
3.1. Total Dissolved Nitrogen (TDN), Total Dissolved Phosphorus (TDP), and Dissolved Organic Carbon (DOC) Concentrations in Soil Extracts with Natural Extractions
3.2. Total Dissolved Nitrogen (TDN), Total Dissolved Phosphorus (TDP), and Dissolved Organic Carbon (DOC) Concentrations in Soil Extracts with Autoclaved Extractions Method
3.3. Effect of Ammonia and Nitrate on the Growth of Microalgae
3.4. Effect of Modified Soil Extract on the Targeted Microalgae Growth
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Soil Extraction
4.3. Sample Analyses
4.4. Microalgae
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 1, 246–249. [Google Scholar] [CrossRef]
- Delgado, A.; Gómez, J.A. The soil. Physical, chemical and biological properties. In Principles of Agronomy for Sustainable Agriculture; Springer: Cham, Switzerland, 2016; pp. 15–26. [Google Scholar]
- Ontl, T.A.; Schulte, L.A. Soil carbon storage. Nat. Educ. Knowl. 2012, 3, 35. [Google Scholar]
- Teo, C.L.; Idris, A.; Akhtar, J. Effect of Soil Extract in Different Culture Medium for Marine Microalgae’s Biomass and Lipid Production in Biodiesel Production. J. Life Sci. Technol. 2016, 4, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.L. Nature of the factor in soil-extract responsible for bacterial growth-stimulation. Nature 1951, 168, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.N.; Fujioka, R.S. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 1998, 38, 171–174. [Google Scholar] [CrossRef]
- Liebeke, M.; Brözel, V.S.; Hecker, M.; Lalk, M. Chemical characterization of soil extract as growth media for the ecophysiological study of bacteria. Appl. Microbiol. Biotechnol. 2009, 83, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Laboratory techniques for the cultivation of microalgae. In Handbook of Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Abingdon, UK, 1986; pp. 117–146. [Google Scholar]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lou, C.; Luo, L.; Wang, X.C. Insight into nitrogen and phosphorus coupling effects on mixotrophic Chlorella vulgaris growth under stably controlled nutrient conditions. Sci. Total Environ. 2020, 752, 141747. [Google Scholar]
- Kapdan, I.; Aslan, S. Application of the Stover–Kincannon kinetic model to nitrogen removal by Chlorella vulgaris in a continuously operated immobilized photobioreactor system. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 998–1005. [Google Scholar]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2020, 752, 142168. [Google Scholar]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production (No. 80); Food & Agriculture Organization: Rome, Italy, 2005. [Google Scholar]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing nitrogen in combination with rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 2020, 10, 1513. [Google Scholar] [CrossRef]
- Nouri, H.J.; Mohammadi Roushandeh, A.; Hallajisani, A.; Golzary; Daliry, S. The effects of glucose, nitrate, and pH on cultivation of Chlorella sp. Microalgae. Glob. J. Environ. Sci. Manag. 2021, 7, 103–116. [Google Scholar]
- Ghosh, D.; Brahmachari, K.; Skalicky, M.; Hossain, A.; Sarkar, S.; Dinda, N.K.; Das, A.; Pramanick, B.; Moulick, D.; Brestic, M.; et al. Nutrients Supplementation through Organic Manures Influence the Growth of Weeds and Maize Productivity. Molecules 2020, 25, 4924. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide 2020, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Neto, V.; Ainuddin, N.A.; Wong, M.Y.; Ting, H.L. Contributions of forest biomass and organic matter to above-and belowground carbon contents at Ayer Hitam Forest Reserve, Malaysia. J. Trop. For. Sci. 2012, 24, 217–230. [Google Scholar]
- Yeh, K.L.; Chang, J.S.; Chen, W.M. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Ross, M.E.; Davis, K.; McColl, R.; Stanley, M.S.; Day, J.G.; Semião, A.J. Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res. 2018, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Gao, B.; Liu, J.; Zhang, C.; Van de Waal, D.B. Biological stoichiometry of oleaginous microalgal lipid synthesis: The role of N: P supply ratios and growth rate on microalgal elemental and biochemical composition. Algal Res. 2018, 32, 353–361. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; López-Chicharro, D.; Morales, V.; Espada, J.J.; Puyol, D.; Martínez, F.; Astals, S.; Vicente, G.; Bautista, L.F.; Rodríguez, R. Biodiesel and biogas production from Isochrysis galbana using dry and wet lipid extraction: A biorefinery approach. Renew. Energy 2020, 146, 188–195. [Google Scholar] [CrossRef]
- Arumugam, K.; Ahmad, M.F.; Yaacob, N.S.; Ikram, W.M.; Maniyam, M.N.; Abdullah, H.; Kuwahara, V.S. Enhancement of targeted microalgae species growth using aquaculture sludge extracts. Heliyon 2020, 6, e04556. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, A.M.; Kothawala, D.N.; Dittmar, T.; Tranvik, L.J. Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat. Geosci. 2015, 8, 454–457. [Google Scholar] [CrossRef]
- Wolf, D.C.; Skipper, H.D. Soil sterilization. Methods of Soil Analysis: Part 2. Microbiol. Biochem. Prop. 1994, 5, 41–51. [Google Scholar]
- Truhlar, D.G. Interpretation of the activation energy. J. Chem. Educ. 1978, 55, 309. [Google Scholar] [CrossRef]
- Steinweg, M.J.; Jagadamma, S.; Frerichs, J.; Mayes, M.A. Activation energy of extracellular enzymes in soils from different biomes. PLoS ONE 2013, 8, e59963. [Google Scholar] [CrossRef]
- Murayama, S.; Bakar, Z.A. Decomposition of Tropical Peat Soils. 1. Decomposition kinetics of organic matter of peat soils. J. Agric. Res. Q. 1996, 30, 145–151. [Google Scholar]
- Mouginot, C.; Kawamura, R.; Matulich, K.L.; Berlemont, R.; Allison, S.D.; Amend, A.S.; Martiny, A.C. Elemental stoichiometry of Fungi and Bacteria strains from grassland leaf litter. Soil Biol. Biochem. 2014, 76, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.H.; Magdoff, F.R. Autoclaving soil samples affects algal-available phosphorus. J. Environ. Qual. 2005, 34, 1958–1963. [Google Scholar] [CrossRef] [Green Version]
- Inselsbacher, E. Recovery of individual soil nitrogen forms after sieving and extraction. Soil Biol. Biochem. 2014, 71, 76–86. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Elkin, K.R.; Liu, C.W.; Bryant, R.B.; Kleinman, P.J.; Moore, P.A. Characterizing the phosphorus forms extracted from soil by the Mehlich III soil test. Geochem. Trans. 2018, 19, 7. [Google Scholar] [CrossRef]
- Guigue, J.; Mathieu, O.; Lévêque, J.; Mounier, S.; Laffont, R.; Maron, P.A.; Navarro, N.; Chateau, C.; Amiotte Suchet, P.; Lucas, Y. A comparison of extraction procedures for water-extractable organic matter in soils. Eur. J. Soil Sci. 2014, 65, 520–530. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Pape, M.L.; Angelidaki, I. Characterization of nutrient removal and microalgal biomass production on an industrial waste-stream by application of the deceleration-stat technique. Water Res. 2015, 75, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Mehrnia, M.R.; Shayegan, J.; Sarrafzadeh, M.H.; Amini, E. Evaluation of nutrient removal and biomass production through mixotrophic, heterotrophic, and photoautotrophic cultivation of chlorella in nitrate and ammonium wastewater. Int. J. Environ. Res. 2018, 12, 167–178. [Google Scholar] [CrossRef]
- Ramaraj, R.; Tsai, D.D.W.; Chen, P.H. Carbon dioxide fixation of freshwater microalgae growth on natural water medium. Ecol. Eng. 2015, 75, 86–92. [Google Scholar] [CrossRef]
- Sun, J.; Simsek, H. Bioavailability of wastewater derived dissolved organic nitrogen to green microalgae Selenastrum capricornutum, Chlamydomonas reinhardtii, and Chlorella vulgaris with/without presence of bacteria. J. Environ. Sci. 2017, 57, 346–355. [Google Scholar] [CrossRef]
- Haug, R.T. Lessons in Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Xu, N.; Zhang, X.; Fan, X.; Han, L.; Zeng, C. Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J. Appl. Phycol. 2001, 13, 463–469. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- El-Kassas, H.Y. Growth and fatty acid profile of the marine microalga Picochlorum sp. grown under nutrient stress conditions. Egypt J. Aquat. Res. 2013, 39, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Campos, H.; Boeing, W.J.; Dungan, B.N.; Schaub, T. Cultivating the marine microalga Nannochloropsis salina under various nitrogen sources: Effect on biovolume yields, lipid content and composition, and invasive organisms. Biomass Bioenergy 2014, 66, 301–307. [Google Scholar] [CrossRef]
- Giordano, M.; Raven, J.A. Nitrogen and sulfur assimilation in plants and algae. Aquat. Bot. 2014, 118, 45–61. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Isolation and biochemical characterisation of two thermophilic green algal species-Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res. 2018, 30, 28–37. [Google Scholar] [CrossRef]
- Kamako, S.I.; Hoshina, R.; Ueno, S.; Imamura, N. Establishment of axenic endosymbiotic strains of Japanese Paramecium bursaria and the utilization of carbohydrate and nitrogen compounds by the isolated algae. Eur. J. Protistol. 2005, 41, 193–202. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Taziki, M.; Ahmadzadeh, H.; Murry, M.A.; Lyon, S.R. Nitrate and nitrite removal from wastewater using algae. Curr. Biotechnol. 2015, 4, 426–440. [Google Scholar] [CrossRef]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Analysis of the elemental composition and uptake mechanism of Chlorella sorokiniana for nutrient removal in agricultural wastewater under optimized response surface methodology (RSM) conditions. J. Clean. Prod. 2019, 210, 673–686. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef] [Green Version]
- Hii, Y.S.; Soo, C.L.; Chuah, T.S.; Mohd-Azmi, A.; Abol-Munafi, A.B. UPTAKE BY Nannochloropsis sp. J. Sustain. Sci. Manag. 2011, 6, 60–68. [Google Scholar]
- Goto, M.; Nagao, N.; Yusoff, F.M.; Kamarudin, M.S.; Katayama, T.; Kurosawa, N.; Toda, T. High ammonia tolerance on growth rate of marine microalga Chlorella vulgaris. J. Environ. Biol. 2018, 39, 843–848. [Google Scholar] [CrossRef]
- Podevin, M.; De Francisci, D.; Holdt, S.L.; Angelidaki, I. Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. J. Appl. Phycol. 2015, 27, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Khalili, A.; Najafpour, G.D.; Amini, G.; Samkhaniyani, F. Influence of nutrients and LED light intensities on biomass production of microalgae Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2015, 20, 284–290. [Google Scholar] [CrossRef]
- Chen, S.Y.; Pan, L.Y.; Hong, M.J.; Lee, A.C. The effects of temperature on the growth of and ammonia uptake by marine microalgae. Bot. Stud. 2012, 53, 125–133. [Google Scholar]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paskuliakova, A.; McGowan, T.; Tonry, S.; Touzet, N. Microalgal bioremediation of nitrogenous compounds in landfill leachate–The importance of micronutrient balance in the treatment of leachates of variable composition. Algal Res. 2018, 32, 162–171. [Google Scholar] [CrossRef]
- Idris, A.; Atta, M.; Bukhari, A. Effect of soil extracts concentration on specific growth rate and lipid content of Chlorella vulgaris in bolds basal medium. Malays. J. Fundam. Appl. Sci. 2014, 10, 96–100. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Sidella, S.; Coppa, M.; Broccanello, C.; Gennari, M.; Baglieri, A. Biostimulant activity of humic-like substances from agro-industrial waste on Chlorella vulgaris and Scenedesmus quadricauda. Eur. J. Phycol. 2018, 53, 433–442. [Google Scholar] [CrossRef]
- Allaguvatova, R.; Myasina, Y.; Zakharenko, V.; Gaysina, L. A simple method for the cultivation of algae Chlorella vulgaris Bejerinck. In IOP Conference Series, Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 390, p. 12020. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef]
- Marjakangas, J.M.; Chen, C.Y.; Lakaniemi, A.M.; Puhakka, J.A.; Whang, L.M.; Chang, J.S. Simultaneous nutrient removal and lipid production with Chlorella vulgaris on sterilized and non-sterilized anaerobically pretreated piggery wastewater. Biochem. Eng. J. 2015, 103, 177–184. [Google Scholar] [CrossRef]
- Warcup, J.H. Chemical and biological aspects of soil sterilization. Soils Fertil. 1957, 20, 1–5. [Google Scholar]
- Powlson, D.S.; Jenkinson, D.S. Effects of biocidal treatments on metabolism in soil. 2. gamma-irradiation, autoclaving, air-drying and fumigation. Soil Biol. Biochem. 1976, 8, 179–188. [Google Scholar] [CrossRef]
- Berns, A.E.; Philipp, H.; Narres, H.D. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur. J. Soil Sci. 2008, 59, 540–550. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Pruvost, J.G.; Vooren, G.; Cogne, G.; Legrand, J. Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour. Technol 2009, 100, 5988–5995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, A.; Hernández-Mireles, I.; García-Martínez, C.; Álvarez, M.M. Microplates as a microreactor platform for microalgae research. Biotechnol. Prog. 2013, 29, 638–644. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Holdt, S.L.; De Francisci, D.; Valverde-Pérez, B.; Plósz, B.G.; Angelidaki, I. Microplate-based method for high-throughput screening of microalgae growth potential. Bioresour. Technol. 2014, 169, 566–572. [Google Scholar] [CrossRef]
- Kong, W.; Song, H.; Cao, Y.; Yang, H.; Hua, S.; Xia, C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotechnol. 2011, 10, 11620–11630. [Google Scholar]
- Hammoud, N.; Mayhoub, H.; Allaan, T. The influence of illumination and soil extract concentration on growth rate and protein content of Cosmarium subtumidum microalgae. J. Entomol. Zool. Stud. 2015, 3, 27–31. [Google Scholar]
- Page-Dumroese, D.; Harvey, A.; Jurgensen, M. A Guide to Soil Samplng and Analysis on the National Forests of the Inland Northwest United States; General Technical Report (GTR); U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Albany, FL, USA, 1995.
- Khatoon, H.; Banerjee, S.; Syakir Syahiran, M. Re-use of aquaculture wastewater in cultivating microalgae as live feed for aquaculture organisms. Desalination Water Treat. 2016, 57, 29295–29302. [Google Scholar] [CrossRef]
- Blaise, C.; Férard, J.F. Overview of contemporary toxicity testing. In Small-Scale Freshwater Toxicity Investigations; Springer: Dordrecht, The Netherlands, 2005; pp. 1–68. [Google Scholar]
- St-Laurent, D.; Blaise, C.; MacQuarrie, P.; Scroggins, R.; Trottier, B. Comparative assessment of herbicide phytotoxicity to Selenastrum capricornutum using microplate and flask bioassay procedures. Environ. Toxicol. Water Qual. 1992, 7, 35–48. [Google Scholar] [CrossRef]
- Rojíčková, R.; Dvořáková, D.; Maršálek, B. The use of miniaturized algal bioassays in comparison to the standard flask assay. Environ. Toxicol. Water Qual. Int. J. 1998, 13, 235–241. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Patel, A. Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, G.T.; Takriff, M.S.; Salihon, S.; Syukri, M.; Rahaman, A.B.D. Feasiblity of the Optical Density (OD) in the Determination of the Microalgal Biomass using Palm Oil Mill Effluent (POME) as Medium. In Proceedings of the 50th The IIER International Conference, Zurich, Switzerland, 26 December 2015. [Google Scholar]
- Bohutskyi, P.; Liu, K.; Nasr, L.K. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef] [PubMed]









| Extraction Methods | RMFR/AHFR | ||
|---|---|---|---|
| N Ratio | P Ratio | C Ratio | |
| NE-1 h | 4.7 ± 0.02 c | 9.2 ± 0.01 a | 6.7 ± 0.04 d |
| NE-4 h | 7.7 ± 0.04 abc | 6.0 ± 0.03 a | 7.7 ± 0.04 d |
| NE-24 h | 9.2 ± 0.06 abc | 6.2 ± 0.02 a | 8.4 ± 0.09 d |
| 105 °C | 6.5 ± 0.01 ab | 18.2 ± 0.08 a | 11.4 ± 0.07 c |
| 121 °C | 7.8 ± 0.08 abc | 11.9 ± 0.02 a | 8.7 ± 0.04 d |
| NE + 105 °C | 10.5 ± 0.02 abc | 18.2 ± 0.02 a | 18.3 ± 0.08 a |
| NE + 121 °C | 13.6 ± 0.07 a | 19.7 ± 0.08 a | 11.6 ± 0.02 d |
| 105 °C × 2 | 7.0 ± 0.05 abc | 17.5 ± 0.09 a | 12.0 ± 0.05 d |
| 121 °C × 2 | 12 ± 0.04 ab | 9.2 ± 0.03 a | 15.46 ± 0.07 b |
| Raja Musa Forest Reserve (RMFR) | Ayer Hitam Forest Reserve (AHFR) | |||||
|---|---|---|---|---|---|---|
| C/N | C/P | N/P | C/N | C/P | N/P | |
| NE-1 h | 10.7 ± 0.08 ab | 62.2 ± 0.05 b | 5.8 ± 0.04 a | 7.3 ± 0.03 b | 86.4 ± 0.01 a | 11.9 ± 0.03 a |
| NE-4 h | 7.7 ± 0.08 b | 138.5 ± 0.05 ab | 18.1 ± 0.09 a | 7.7 ± 0.016 b | 108.1 ± 0.02 a | 14.0 ± 0.03 a |
| NE-24 h | 9.1 ± 0.01 b | 156.5 ± 0.06 ab | 17.2 ± 0.09 a | 9.5 ± 0.09 ab | 114.3 ± 0.02 a | 12.0 ± 0.06 a |
| 105 °C | 22.8 ± 0.04 a | 149.6 ± 0.04 ab | 6.6 ± 0.05 a | 13.0 ± 0.07 ab | 238.6 ± 0.07 a | 18.4 ± 0.04 a |
| 121 °C | 12.5 ± 0.06 ab | 166.3 ± 0.02 ab | 13.4 ± 0.01 a | 11.2 ± 0.01 ab | 226.6 ± 0.07 a | 20.3 ± 0.09 a |
| NE + 105 °C | 17.7 ± 0.01 ab | 161.1 ± 0.01 ab | 9.1 ± 0.01 a | 10.1 ± 0.02 ab | 160.1 ± 0.04 a | 15.9 ± 0.01 a |
| NE + 121 °C | 12.8 ± 0.02 ab | 144.1 ± 0.01 ab | 11.2 ± 0.02 a | 15.1 ± 0.04 a | 229.4 ± 0.08 a | 15.2 ± 0.07 a |
| 105 °C × 2 | 19.1 ± 0.07ab | 132.8 ± 0.00 ab | 6.9 ± 0.07 a | 12.0 ± 0.06 ab | 192.9 ± 0.09 a | 16.1 ± 0.02 a |
| 121 °C × 2 | 19.9 ± 0.04 ab | 246.8 ± 0.05 a | 12.4 ± 0.09 a | 15.5 ± 0.01 a | 236.1 ± 0.02 a | 15.3 ± 0.01 a |
| Types of SE | Microalgae | Control | Modified SE | ||||
|---|---|---|---|---|---|---|---|
| Media + 105 °C | Media + 105 °C Twice | Media + 121 °C | Media + 121 °C Twice | Media + 24 h | |||
| RM SE | N.oceanica | 0.49 ± 0.03 a | 0.32 ± 0.05 b | 0.32 ± 0.11 b | 0.29 ± 0.03 b | 0.23 ± 0.08 b | 0.31 ± 0.10 b |
| C. vulgaris | 0.37 ± 0.03 c | 0.36 ± 0.21 c | 0.59 ± 0.19 b | 0.60 ± 0.11 b | 0.81 ± 0.03 a | 0.78 ± 0.02 a | |
| AH SE | N.oceanica | 0.00 ± 0.03 c | 0.38 ± 0.00 b | 0.30 ± 0.00 b | 0.34 ± 0.03 b | 0.33 ± 0.03 b | 0.31 ± 0.00 b |
| C.vulgaris | 0.37 ± 0.04 b | 0.31 ± 0.04 b | 0.28 ± 0.15 b | 0.26 ± 0.11 b | 0.42 ± 0.01 a | 0.29 ± 0.06 b | |
| Types of SE | Microalgae | Control | Modified SE | ||||
|---|---|---|---|---|---|---|---|
| Media + 105 °C | Media + 105 °C Twice | Media + 121 °C | Media + 121 °C Twice | Media + 24 h | |||
| RM SE | N. oceanica | 0.17 ± 0.00 a | 0.03 ± 0.09 a | 0.03 ± 0.08 a | 0.06 ± 0.11 a | 0.04 ± 0.05 a | 0.10 ± 0.13 a |
| C. vulgaris | 0.12 ± 0.02 a | 0.13 ± 0.07 a | 0.01 ± 0.23 a | 0.19 ± 0.05 a | 0.19 ± 0.23 a | 0.21 ± 0.07 a | |
| AH SE | N. oceanica | 0.02 ± 0.05 a | 0.14 ± 0.05 a | 0.12 ± 0.06 a | 0.13 ± 0.05 a | 0.12 ± 0.06 a | 0.12 ± 0.09 a |
| C. vulgaris | 0.12 ± 0.02 a | 0.13 ± 0.14 a | 0.01 ± 0.15 a | 0.18 ± 0.13 a | 0.19 ± 0.36 a | 0.21 ± 0.09 a | |
| Extraction Methods | Procedure |
|---|---|
| Natural Extraction 1 h (NE-1 h) | Soil extracted at room temperature for 1 h |
| Natural Extraction 4 h (NE-4 h) | Soil extracted at room temperature for 4 h |
| Natural Extraction 24 h (NE-24 h) | Soil extracted at room temperature for 24 h |
| Autoclave 105 °C (105 °C) | Soil extracted at 105 °C autoclave for 1 h |
| Autoclave 121 °C (121 °C) | Soil extracted at 121 °C autoclave for 1 h |
| Autoclave 105 °C after Natural Extraction 24 h (NE + 105 °C) | Soil extracted at 105 °C autoclave for 1 h before soil extracted at room temperature for 24 h |
| Autoclave 121 °C after Natural Extraction 24 h (NE + 121 °C) | Soil extracted at 121 °C autoclave for 1 h before soil extracted at room temperature for 24 h |
| Autoclave 105 °C twice (×2) (105 °C × 2) | Soil extracted at 105 °C autoclave for 1 h, and after cooled (~30 min), soil extracted at 105 °C autoclave for 1 h |
| Autoclave 121 °C twice (×2) (121 °C × 2) | Soil extracted at 121 °C autoclave for 1 h, and after cooled (~30 min), soil extracted at 121 °C autoclave for 1 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacob, N.S.; Ahmad, M.F.; Kawasaki, N.; Maniyam, M.N.; Abdullah, H.; Hashim, E.F.; Sjahrir, F.; Wan Mohd Zamri, W.M.I.; Komatsu, K.; Kuwahara, V.S. Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers. Molecules 2021, 26, 653. https://doi.org/10.3390/molecules26030653
Yaacob NS, Ahmad MF, Kawasaki N, Maniyam MN, Abdullah H, Hashim EF, Sjahrir F, Wan Mohd Zamri WMI, Komatsu K, Kuwahara VS. Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers. Molecules. 2021; 26(3):653. https://doi.org/10.3390/molecules26030653
Chicago/Turabian StyleYaacob, Nor Suhaila, Mohd Fadzli Ahmad, Nobuyuki Kawasaki, Maegala Nallapan Maniyam, Hasdianty Abdullah, Emi Fazlina Hashim, Fridelina Sjahrir, Wan Muhammad Ikram Wan Mohd Zamri, Kazuhiro Komatsu, and Victor S. Kuwahara. 2021. "Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers" Molecules 26, no. 3: 653. https://doi.org/10.3390/molecules26030653
APA StyleYaacob, N. S., Ahmad, M. F., Kawasaki, N., Maniyam, M. N., Abdullah, H., Hashim, E. F., Sjahrir, F., Wan Mohd Zamri, W. M. I., Komatsu, K., & Kuwahara, V. S. (2021). Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers. Molecules, 26(3), 653. https://doi.org/10.3390/molecules26030653








