New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Prediction of Anti-Inflammatory Activity of Designed Compounds
2.2. In Vivo Inhibition of the Carrageenin-Induced Edema
2.3. In Vitro Study of COX Inhibition
2.4. In Vitro Studies of LOX Inhibitory Activity
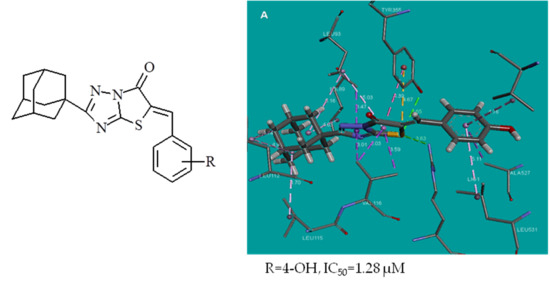
2.5. Docking Studies
3. Materials and Methods
3.1. Chemistry
3.2. In Vivo Experiments. Inhibition of the Carrageenin-Induced Edema
3.3. COX Inhibitor Screening Assay in Vitro
3.4. Soybean Lipoxygenase Inhibition Study in Vitro
3.5. Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borne, R.F. Non-steroidal anti-inflammatory drugs. In Foye’s Principles of Medicinal Chemistry, 5th ed.; Williams, D.A., Lemke, T.L., Eds.; Lippincott Williams & Wilkins: New York, NY, USA, 2002; pp. 751–793. [Google Scholar]
- Morris, C.J. Carrageenan-Induced Paw Edema in the Rat and Mouse. In Inflammation Protocols. Methods in Molecular Biology; Winyard, P.G., Willoughby, D.A., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 225. [Google Scholar] [CrossRef]
- Gilligan, J.P.; Lovato, S.J.; Erion, M.D.; Jeng, A.Y. Modulation of carrageenan-induced hind paw edema by substance P. Inflammation 1994, 18, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Hedqvist, P.; Gautam, N.; Lindbom, L. Interactions between Leukotrienes and Other Inflammatory Mediators/Modulators in the Microvasculature. Am. J. Respir. Crit. Care Med. 2000, 161, S117–S119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shaffer, A.; Portanova, J.; Seibert, K.; Isakson, P.C. Inhibition of Cyclooxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production. J. Pharmacol. Exp. Ther. 1997, 283, 1069–1075. [Google Scholar] [PubMed]
- Vellani, V.; Moschetti, G.; Franchi, S.; Giacomoni, C.; Sacerdote, P.; Amodeo, C. Effects of NSAIDs on the Release of Calcitonin Gene-Related Peptide and Prostaglandin E2 from Rat Trigeminal Ganglia. Mediat. Inflamm. 2017, 9547056. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.J.; Bronson, J.J.; Barrett, J.F.; DenBleyker, K.L.; Discotto, D.L.; Fung-Tomc, J.C.; Ueda, Y. Antimicrobial evaluation of nocathiacins, a thiazole peptide class of antibiotics. Antimicrob. Agents Chemother. 2004, 48, 3697–3701. [Google Scholar] [CrossRef] [Green Version]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New Series of Thiazole Derivatives: Synthesis, Structural Elucidation, Antimicrobial Activity, Molecular Modeling and MOE Docking. Molecules 2019, 24, 1741–1764. [Google Scholar] [CrossRef] [Green Version]
- Bikobo, D.S.N.; Vodnar, D.C.; Stana, A.; Tiperciuc, B.; Nastasa, C.; Douchet, M.; Oniga, O. Synthesis of 2-phenylamino-thiazole derivatives as antimicrobial agents. J. Saudi Chem. Soc. 2017, 21, 861–868. [Google Scholar] [CrossRef]
- Geronikaki, A.; Vicini, P.; Theophilidis, G.; Lagunin, A.; Poroikov, V.; Dabarakis, N.; Modarresi, H.; Dearden, J.C. Evaluation the local anaesthetic activity of derivatives of 3-amino-1,2-[d]benzoisothiazoles on sciatic nerve of rat. Eur. J. Med. Chem. 2009, 44, 473–481. [Google Scholar] [CrossRef]
- Kamble, R.D.; Meshram, R.J.; Hese, S.V.; More, R.A.; Kamble, S.S.; Gacche, R.N.; Dawane, B.S. Synthesis and in silico investigation of thiazoles bearing pyrazolesderivatives as anti-inflammatory agents. Comput. Biol. Chem. 2016, 61, 86–96. [Google Scholar] [CrossRef]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Faheem Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine- and Thiazole-Based Hydrazides with Promising Anti-inflammatory and Antimicrobial Activities along with Their In Silico Studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.A.; Geronikaki, A.; Eleftheriou, P.T.; Hadjipavlou-Litina, D.I.; Filimonov, D.A.; Poroikov, V.V. Computer-aided discovery of potential anti-inflammatory thiazolidinones with dual 5-LOX/COX inhibition. J. Med. Chem. 2008, 51, 1601–1609. [Google Scholar]
- Mohareb, R.M.; Al-Omran, F.; Abdelaziz, M.A.; Ibrahim, R.A. Anti-inflammatory and Anti-ulcer Activities of New Fused Thiazole Derivatives Derived from 2-(2-Oxo-2H-chromen-3-yl)thiazol-4(5H)-one. Acta Chim. Slov. 2017, 64, 349–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Sunday, N.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Pitta, E.; Crespan, E.; Geronikaki, A.; Maga, G.; Samuele, A. Novel thiazolidinone derivatives with an uncommon mechanism of inhibition towards HIV-1 Reverse Transcriptase. Lett. Drug Des. Discov. 2010, 7, 228–234. [Google Scholar] [CrossRef]
- Pitta, E.; Geronikaki, A.; Surmava, S.; Eleftheriou, P.; Mehta, V.; Van der Eycken, E. Synthesis and HIV-1 RT inhibitory action of novel (4/6-substituted benzo[d]thiazol-2-yl)thiazolidin-4-ones. Divergence from the non-competitive inhibition mechanism. J. Enzyme Inhib. Med. Chem. 2013, 28, 113–122. [Google Scholar] [CrossRef]
- Brzezińska, E.; Stolarska, J.; Sobańska, A. A structure-activity relationship study of thiazole derivatives with H1-antihistamine activity. Acta Pol. Pharm. 2011, 68, 677–686. [Google Scholar]
- Sowjanya, C.H.; Swamy, S.S.; Gomathi, S.; Babu, K.A. Synthesis, Chemistry and Anti-Hypertensive Activity of Some New Thiazole-Thiadiazole Derivatives. Int. J. Adv. Res. Med. Pharm. Sci. 2016, 1, 1–9. [Google Scholar]
- Kelleher, J.P.; Centorrino, F.; Albert, M.J.; Baldessarini, R.J. Advances in atypical antipsychotics for the treatment of schizophrenia: New formulations and new agents. CNS Drugs 2002, 14, 249–261. [Google Scholar] [CrossRef]
- Siddiqui, N.; Arshad, M.F.; Ahsan, W.; Alam, M.S. Thiazoles: A Valuable Insight into the Recent Advances and Biological Activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar]
- Kumar, S.G.V.; Mishra, D.N. Analgesic, antiinflammatory, and ulcerogenic studies of meloxicam solid dispersion prepared with polyethylene glycol 6000. Methods Find Exp. Clin. Pharmacol. 2006, 28, 419. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, I.; Liaras, K.; Geronikaki, A.; Hadjipavlou-Litina, D.; Gavalas, A.; Sokovic, M.; Glamočlija, J.; Ciric, A. Synthesis and biological evaluation of some 5-arylidene-2-(1,3-thiazol-2-ylimino)-1,3-thiazolidin-4-ones as dual antiinflammatory/antimicrobial agent. Bioorg. Med. Chem. 2013, 21, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Bari, S.B.; Sandip, D.; Firake, S.D. Exploring Anti-inflammatory Potential of Thiazolidinone Derivatives of Benzenesulfonamide via Synthesis, Molecular Docking and Biological Evaluation. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 44–53. [Google Scholar] [CrossRef]
- Atta-Allah, S.R.; Nassar, I.F.; El-Sayed, W.A. Design, synthesis and anti-inflammatory vel 5-(Indol-3-yl)thiazolidinone derivatives as COX-2 inhibitors. J. Pharmacol. Ther. Res. 2020, 5, 3–16. [Google Scholar] [CrossRef]
- Ali, Y.; Alam, M.S.; Hamid, H.; Husain, A.; Dhulap, A.; Hussain, F.; Bano, S.; Kharbanda, C. Molecular modeling and synthesis of some new 2-imino-4-thiazolidinone derivatives with promising TNF-ainhibitory activity. New J. Chem. 2016, 40, 711–725. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, R.; Sonar, P.K.; Saraf, S.K. Novel 4-Thiazolidinone Derivatives as Anti-Infective Agents: Synthesis, Characterization, and Antimicrobial Evaluation. Biochem. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, P.S.; Karale, S.N.; Khandebharad, A.U.; Agrawal, B.R.; Sarda, S.R. Design, Synthesis, and Biological Evaluation of Newer Arylidene Incorporated 4-ThiazolidinonesDerivatives as Potential Antimicrobial Agents. Polycycl. Aromat. Compd. 2020, 40, 437–445. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Leja, M.L.; Kaminskyy, D.V.; Binduga, U.E.; Pinyazhko, O.R.; Lesyk, R.B.; Gminski, J. Study of novel anticancer 4-thiazolidinone derivatives. Chem. Biol. Interact. 2017, 262, 46–56. [Google Scholar] [CrossRef]
- Ansari, M.F.; Idrees, D.; Hassan Md, I.; Ahmad, K.; Avecilla, F.; Azam, A. Design, synthesis and biological evaluation of novel pyridine-thiazolidinone derivatives as anticancer agents: Targeting human carbonic anhydrase IX. Eur. J. Med. Chem. 2018, 144, 544–556. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Fadaly, W.A.A.; Kamel, G.M.; Elshaier, Y.A.M.M.; El-Magd, M.A. Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γagonists and anti-inflammatory COX-2 selective inhibitors. Bioorg. Chem. 2019, 82, 86–99. [Google Scholar] [CrossRef]
- Petrou, A.; Eleftheriou, P.; Geronikaki, A.; Akrivou, M.G.; Vizirianakis, I. Novel Thiazolidin-4-ones as Potential Non-nucleoside Inhibitors of HIV-1 Reverse Transcriptase. Molecules 2019, 24, 3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, A.S.; Lather, V.; Deepti Pandita, D.; Dalal, R. Synthesis, Docking and Anti-Inflammatory Activity of Triazole Amine Derivatives as Potential Phosphodiesterase-4 Inhibitors. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Yong, Y.; Shin, S.Y.; Jung, H.; Park, K.H.; Lee, Y.H.; Lim, Y.; Jung, K.-Y. Synthesis and biological evaluation of phenyl-1H-1,2,3-triazolederivatives as anti-inflammatory agents. Bioorg. Chem. 2015, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; El-Sayed, W.A. Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers. Molecules 2020, 25, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Qadri, T.; Hussain, Z.; Saeed, A.; Channar, P.A.; Shehzadi, S.A.; Hassan, M.; Larik, F.A.; Tarique Mahmood, T.; Malik, A. Synthesis, antibacterial activity and molecular docking study of vanillin derived 1,4-disubstituted 1,2,3-triazoles as inhibitors of bacterial DNA synthesis. Heliyon 2019, 5, 02812. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-I.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 1, 183–189. [Google Scholar] [CrossRef]
- Asif, M. Antiviral and antiparasitic activities of various substituted triazole derivatives: A mini review. Chem. Int. 2015, 1, 71–80. [Google Scholar]
- Ye, G.-I.; Lan, T.; Huang, Z.-X.; Cheng, X.-N.; Cai, C.-Y.; Ding, S.-M.; Xie, M.-L.; Wang, B. Design and synthesis of novel xanthone-triazole derivatives as potential antidiabetic agents: α-Glucosidase inhibition and glucose uptake promotion. Eur. J. Med. Chem. 2019, 177, 362–373. [Google Scholar] [CrossRef]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Microwave-assisted synthesis, antioxidant and antimicrobial evaluation of 2-indolinone-based bis-1,2,3-triazole derivatives. Mol. Divers. 2018, 22, 57–70. [Google Scholar] [CrossRef]
- Tratrat, C.; Haroun, M.; Paparisva, A.; Geronikaki, A.; Kamoutsis, C.; Ciric, A.; Glamočlija, J.; Sokovic, M.; Fotakis, C.; Zoumpoulakis, P.; et al. Design, synthesis and biological evaluation of new substituted 5-benzylideno-2-adamantylthiazol[3,2-b][1,2,4]triazol-6(5H)ones. Pharmacophore modelsfor antifungal activity. Arabian J. Chem. 2018, 11, 573–590. [Google Scholar] [CrossRef] [Green Version]
- Sarıgöl, D.; Baran, A.U.; Tel, B.; Tozkoparan, B. Novel thiazolo[3,2-b]-1,2,4-triazoles derived from naproxen with analgesic/anti-inflammatory properties: Synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Slivka, M.; Korol, N.; Fizer, M.; Baumer, V.; Lendel, V. [1,3]Thiazolo[3,2-b][1,2,4]triazol-ium salts: Synthesis, properties and structural studies. Heterocycl. Commun. 2018, 24, 197–203. [Google Scholar] [CrossRef]
- Pardali, V.; Giannakopoulou, E.; Konstantinidi, A.; Kolocouris, A.; Zoidis, G. 1,2-Αnnulated Adamantane Heterocyclic Derivatives as Anti-Influenza Α Virus Agents. Croat. Chem. Acta 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Shen, Z.; Lou, K.; Wang, W. New small-molecule drug design strategies for fighting resistant influenza A. Acta Pharm. Sin. B 2015, 5, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzarini, J.; Orzeszko-Krzesinszka, B.; Maurin, J.K.; Orzeszko, A. Synthesis amnd anti-HIV studies of 2 and 3-adamantyl substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2009, 44, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S.; Asiri, H.H.; Siham Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Doverpress 2014, 8, 505–518. [Google Scholar]
- Kalita, U.; Kaping, S.; Nongkynrih, R.; Singha, L.I.; Vishwakarma, J.N. Novel tetrahydropyrimidine–adamantane hybrids as anti-inflammatory agents: Synthesis, structure and biological evaluation. Med. Chem. Res. 2015, 24, 2742–2755. [Google Scholar] [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Web Site for PASS. Available online: http://www.ibmc.msk.ru/PASS (accessed on 10 December 2020).
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse set of drug-likestructures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and Their Receptors: Contributions to Physiological Control and the Mechanisms of Disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [Green Version]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.; Schuligoi, R.; Lanz, I.; Donnerer, J. Histamine-induced edema in the rat paw—Effect of capsaicin denervation and a CGRP receptor antagonist. Eur. J. Pharmacol. 1995, 279, 227–231. [Google Scholar] [CrossRef]
- Barghash, R.F.; Geronikaki, A.; Abdou, W.M. Synthesis of a Series of Substituted Thiazole Derivatives:New COX-2 Enzyme Inhibitors for Colon Cancer andInflammation Treatment. ChemistrySelect 2018, 3, 13329–13337. [Google Scholar] [CrossRef]
- Assali, M.; Abualhasan, M.; Sawaftah, H.; Hawash, M. Synthesis, Biological Activity, and Molecular Modeling Studies of Pyrazole and Triazole Derivatives as Selective COX-2 Inhibitors. Hindawi J. Chem. 2020, 2020, 6393428. [Google Scholar] [CrossRef] [Green Version]
- Kouatly, O.; Eleftheriou, P.; Petrou, A.; Hadjipavlou-Litina, D.; Geronikaki, A. Docking assisted design of novel 4-adamantanyl-2-thiazolylimino-5-arylidene-4-thiazolidinones as potent NSAIDs. SAR QSAR Environ. Res. 2018, 29, 83–101. [Google Scholar] [CrossRef]
- Eleftheriou, P.; Petrou, A.; Geronikaki, A.; Liaras, L.; Dirnal, S.M. Prediction of enzyme inhibition and modeof inhibitory action based on calculation ofdistances between hydrogen bond donor/acceptorgroups of the molecule and docking analysis: Anapplication on the discovery of novel effectivePTP1B inhibitors. SAR QSAR Environ. Res. 2015, 26, 557–576. [Google Scholar] [CrossRef]
- Selinsky, B.S.; Gupta, K.; Sharkey, C.T.; Loll, P.J. Structural analysis of NSAID binding by prostaglandin H2 synthase: Time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry 2001, 40, 5172–5180. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [Green Version]




| Com. | R | CPE, a % | Pa b | Com | R | CPE a % | Pa b |
|---|---|---|---|---|---|---|---|
| 1 | H | 40 | 0.407 | 14 | 4-NO2 | 42 | 0.409 |
| 2 | 3-OH | 36 | 0.551 | 15 | 3-F | 51 | 0.581 |
| 3 | 4-OH | 37 | 0.554 | 16 | 4-F | 56 | 0.566 |
| 4 | 2,4-OH | 49 | 0.480 | 17 | 2-Br | 67 | 0.274 |
| 5 | 2-OH, 3-OMe | 58 | 0.485 | 18 | 3-Br | 61 | 0.360 |
| 6 | 4-OH-3-OMe | 46 | 0.503 | 19 | 4-Br | 53 | 0.393 |
| 7 | 4-OH, 3,5-OMe | 36 | 0.567 | 20 | 2-Cl | 47 | 0.585 |
| 8 | 4-OMe | 62 | 0.539 | 21 | 3-Cl | 45 | 0.636 |
| 9 | 3,4-OMe | 44 | 0.538 | 22 | 4-Cl | 61 | 0.552 |
| 10 | 3,4,5-OMe | 49 | 0.503 | 23 | 2,3-Cl | 37 | 0366 |
| 11 | 4-N(CH3)2 | 55 | 0.407 | 24 | 2,4-Cl | 37 | |
| 12 | 2-NO2 | 40 | 0.409 | 25 | 2,6-Cl | 32 | 0.585 |
| 13 | 3-NO2 | 15 | - | indomethacin | 47 | ||
| A/A | R | COX-1 * (%) | COX-1 IC50 (μM) | COX-2 * (%) | COX-2 IC50 (μM) | LOX (%) |
|---|---|---|---|---|---|---|
| 2 | 3-OH | 18 | 64 | >50 | 5.82 | |
| 3 | 4-OH | 93 | 1.28 | 25 | 28.58 | |
| 6 | 4-OH 3 OCH3 | 26.2 | 66 | >50 | 6.67 | |
| 7 | 4-OH 3,5 di-OCH3 | 22 | 25 | 16.13 | ||
| 8 | 4-OCH3 | 60.5 | 41.88 | 0 | 5.72 | |
| 15 | 3-F | 36 | 23 | 27.15 | ||
| 16 | 4-F | 19 | 0 | 16.13 | ||
| 17 | 2-Br | 16 | 0 | 17.15 | ||
| 18 | 3-Br | 0 | 37 | 31.43 | ||
| 19 | 4-Br | 16 | 36 | 8.07 | ||
| 23 | 2,3 di-Cl | 46 | 20 | 11.43 | ||
| Naproxen | - | 60 | 40.10 | 65 | 50 | |
| NDGA | - | - | - | - | 94 |
| Compound | R | Est. Binding Energy (kcal/mol) | Binding Affinity Dcore | I-H | Residues Involved at Hydrogen Bond Formation |
|---|---|---|---|---|---|
| Flurbiprofen | −8.8 | −38.21 | 3 | ARG120, TYR355 | |
| 3 | 4-OH | −8.2 | −31.67 | 2 | ARG120, TYR355 |
| 8 | 4-OMe | −7.0 | −23.49 | 1 | TYR355 |
| 18 | 3-Br | −4.4 | −14.88 | 1 | SER530 |
| Compound | R | Est. Binding Energy (kcal/mol) | Binding Affinity Dcore | I-H | Residues Involved at Hydrogen Bond Formation |
|---|---|---|---|---|---|
| diclofenac | −9.0 | −32.95 | 3 | Tyr385, SER530 | |
| 2 | 3-OH | −8.8 | −29.99 | 2 | TYR355, ARG120 |
| 6 | 4-OH,3-OMe | −8.6 | −30.82 | 2 | TYR355, ARG120 |
| 16 | 4-F | −0.9 | −5.78 | 0 | - |
| Naproxen | −8.0 | −28.67 | 1 | ARG120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tratrat, C.; Haroun, M.; Paparisva, A.; Kamoutsis, C.; Petrou, A.; Gavalas, A.; Eleftheriou, P.; Geronikaki, A.; Venugopala, K.N.; Kochkar, H.; et al. New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents. Molecules 2021, 26, 659. https://doi.org/10.3390/molecules26030659
Tratrat C, Haroun M, Paparisva A, Kamoutsis C, Petrou A, Gavalas A, Eleftheriou P, Geronikaki A, Venugopala KN, Kochkar H, et al. New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents. Molecules. 2021; 26(3):659. https://doi.org/10.3390/molecules26030659
Chicago/Turabian StyleTratrat, Christophe, Michelyne Haroun, Aliki Paparisva, Charalmpos Kamoutsis, Anthi Petrou, Antonis Gavalas, Phaedra Eleftheriou, Athina Geronikaki, Katharigatta N. Venugopala, Hafedh Kochkar, and et al. 2021. "New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents" Molecules 26, no. 3: 659. https://doi.org/10.3390/molecules26030659
APA StyleTratrat, C., Haroun, M., Paparisva, A., Kamoutsis, C., Petrou, A., Gavalas, A., Eleftheriou, P., Geronikaki, A., Venugopala, K. N., Kochkar, H., & Nair, A. B. (2021). New Substituted 5-Benzylideno-2-Adamantylthiazol[3,2-b][1,2,4]Triazol-6(5H)ones as Possible Anti-Inflammatory Agents. Molecules, 26(3), 659. https://doi.org/10.3390/molecules26030659