Ionotropic Gelation of Chitosan Flat Structures and Potential Applications
Abstract
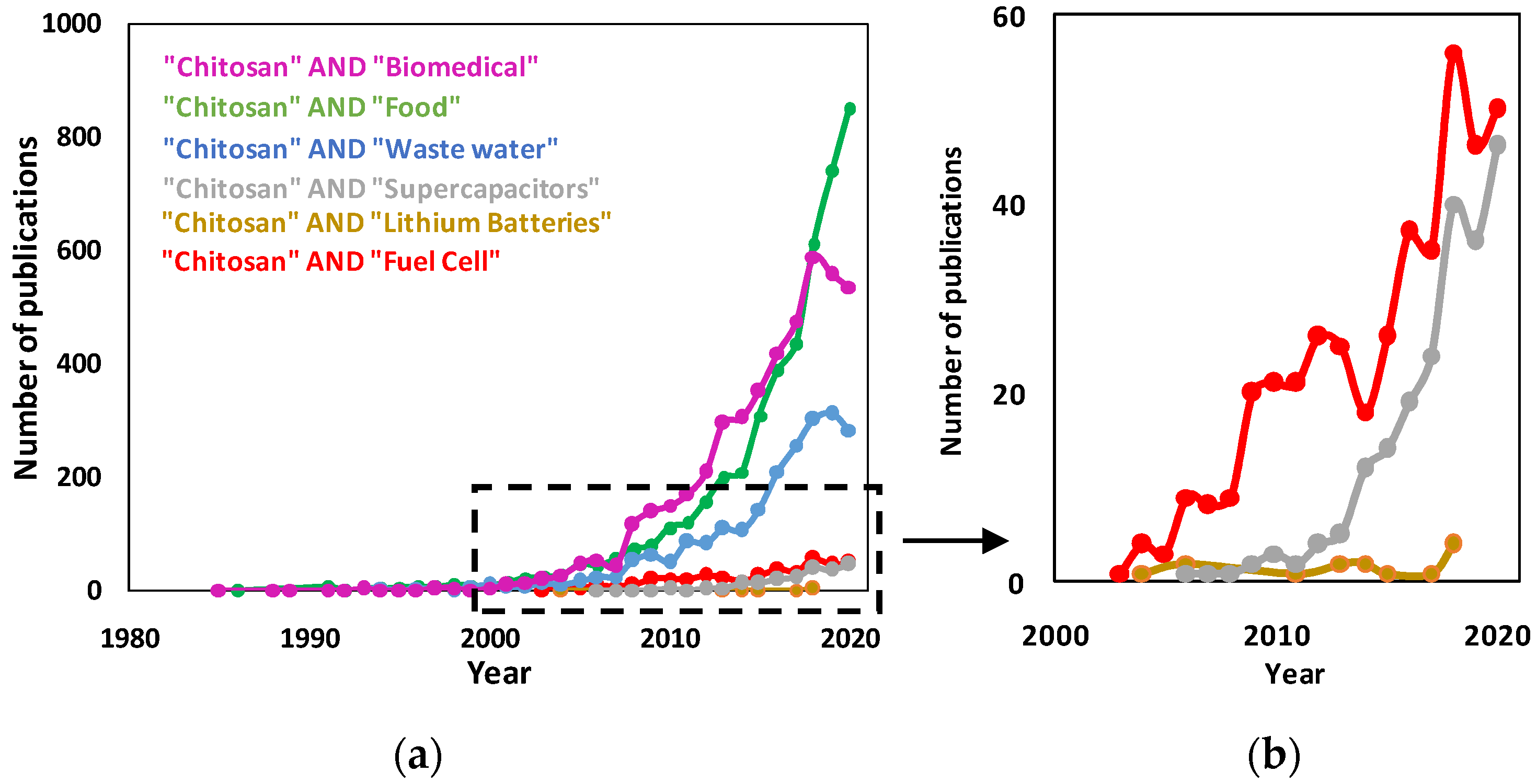
1. Introduction
2. An Overview of Chitosan Properties and Methods of Synthesis of Flat Structures
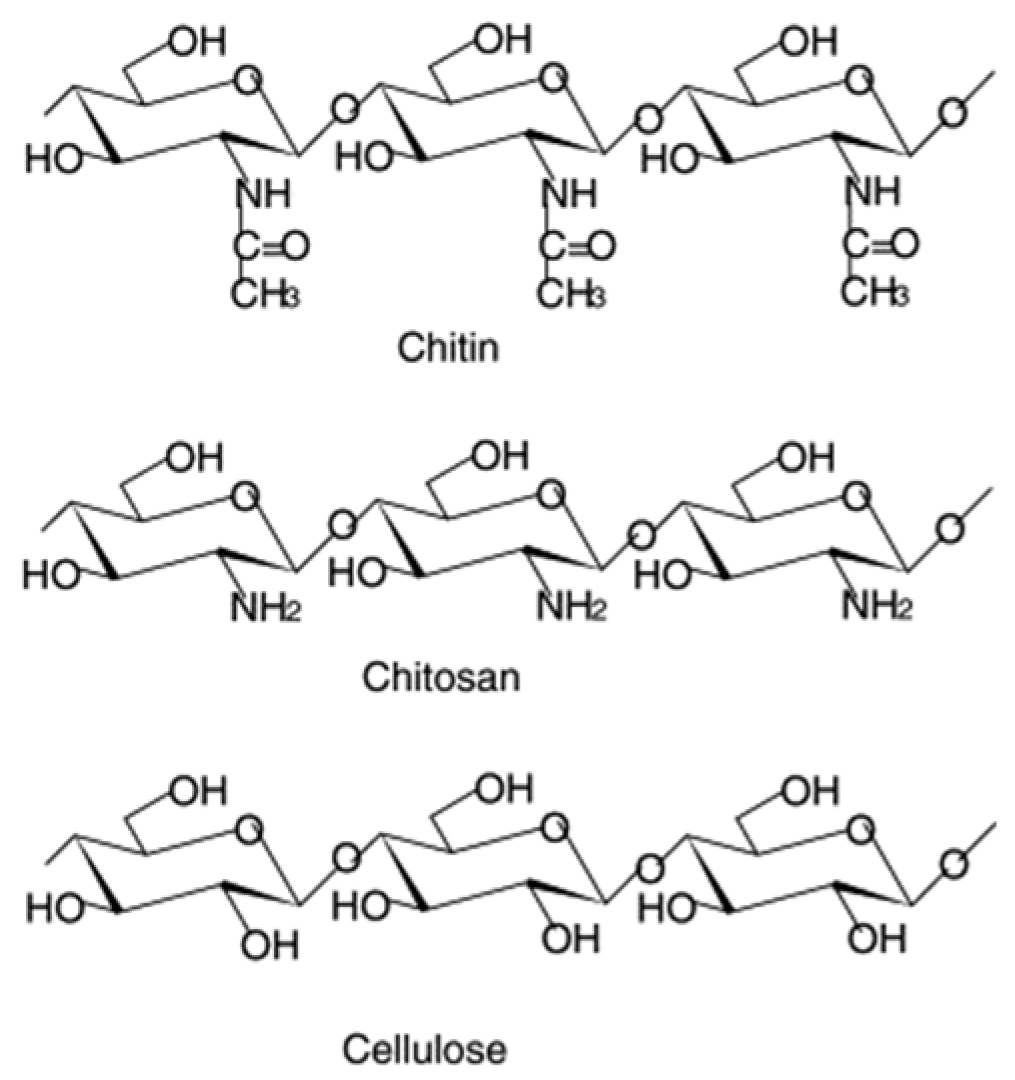
2.1. Chitosan
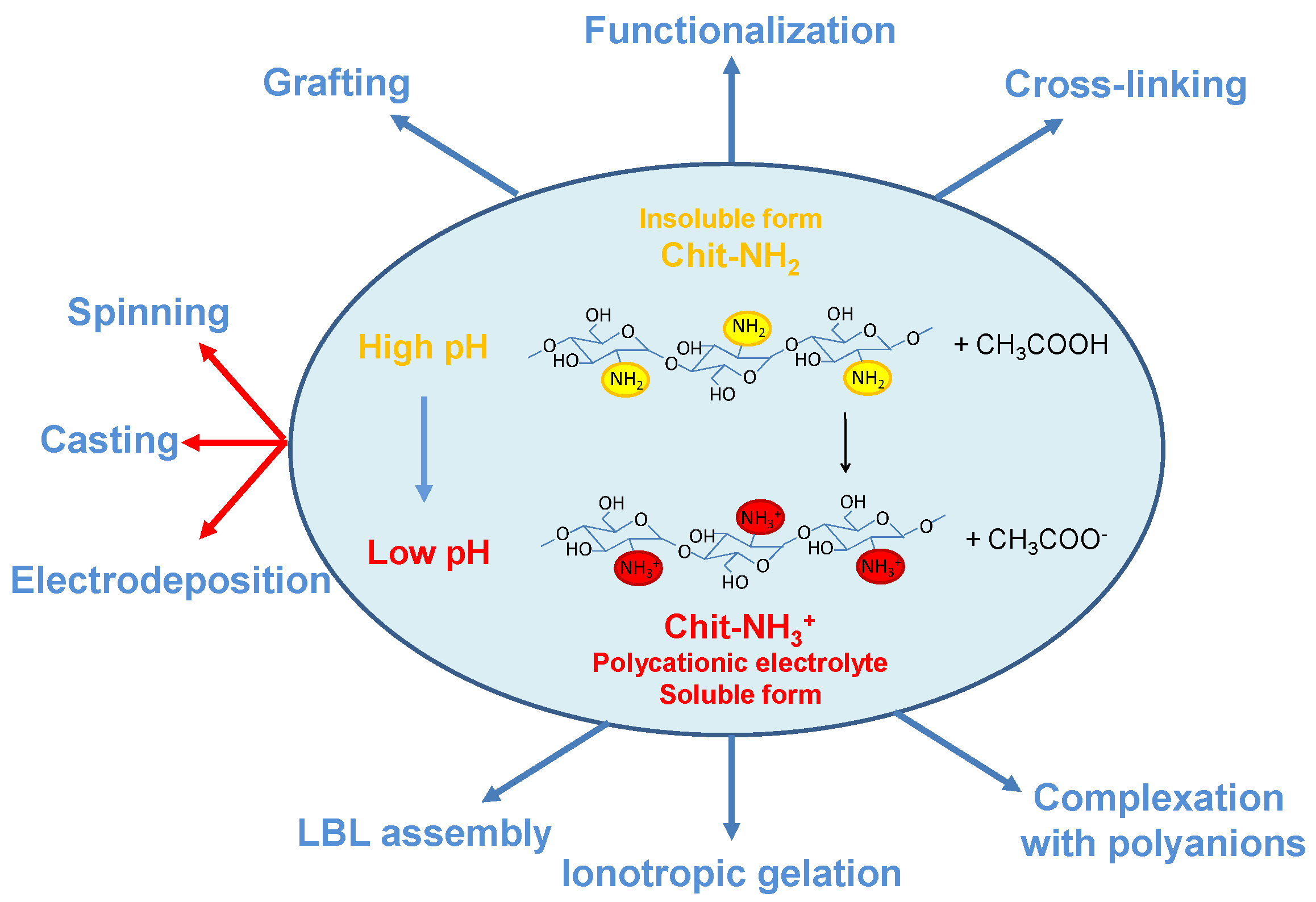
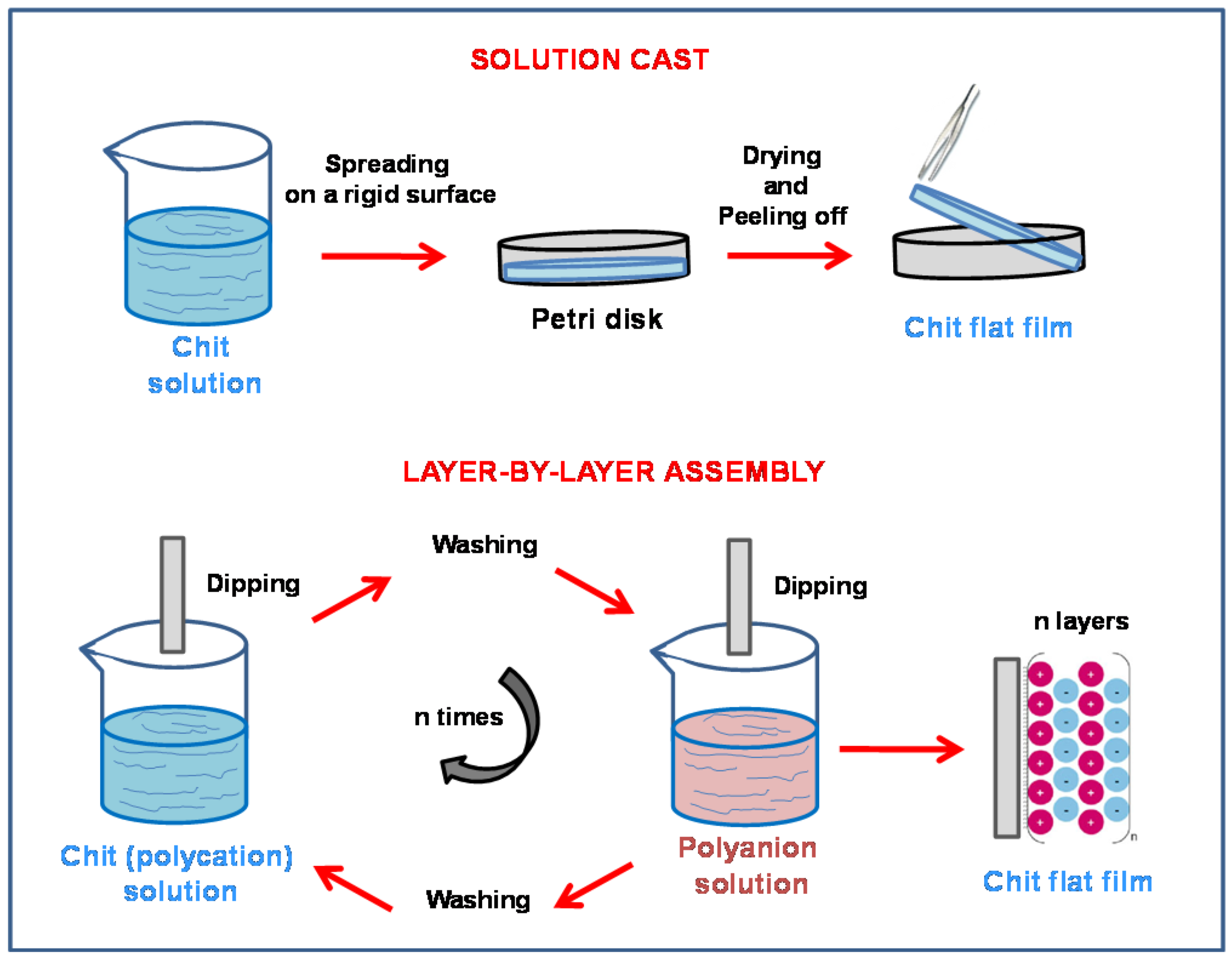
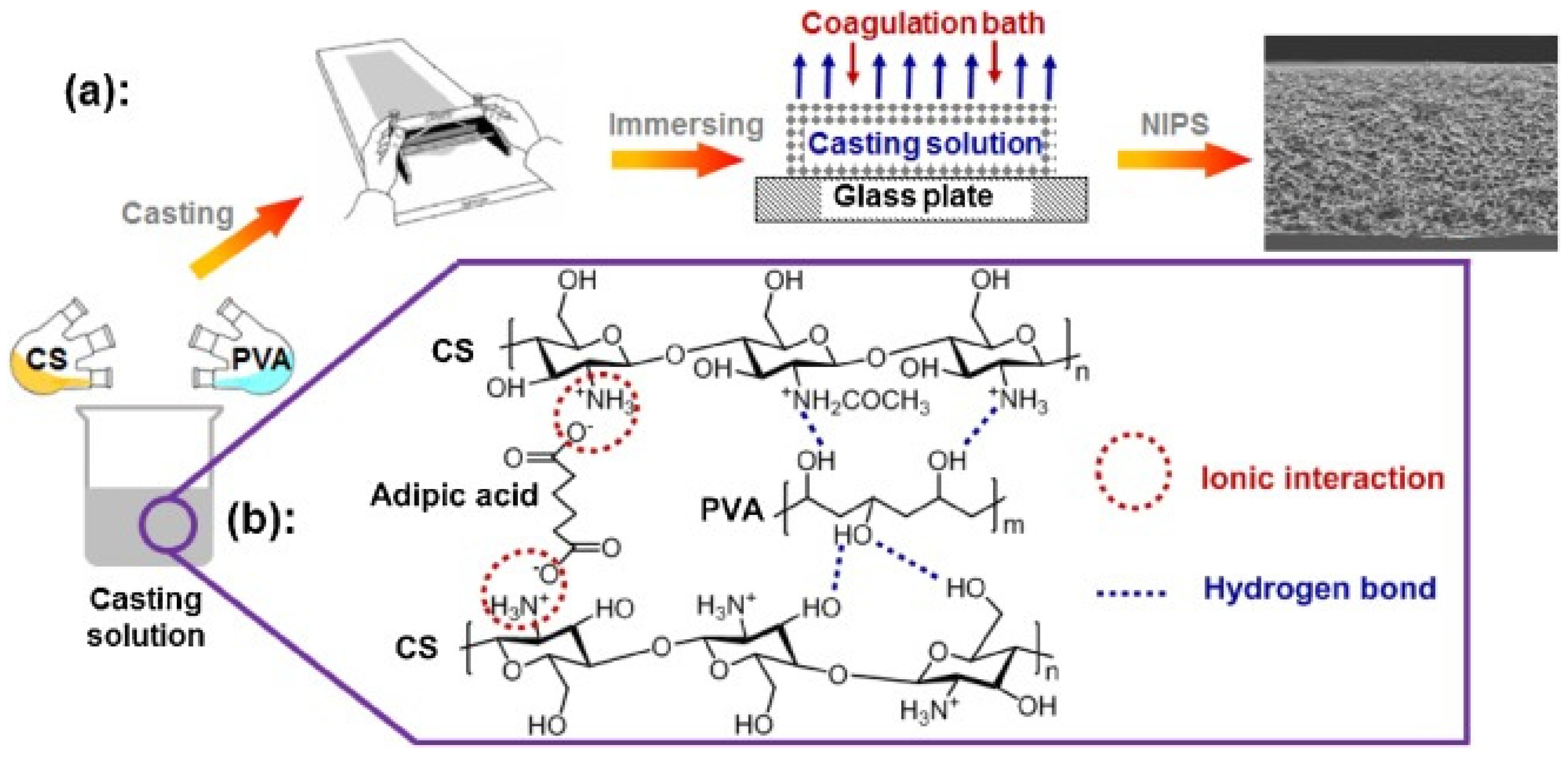
2.2. Flat Chitosan Structures: Methods of Synthesis
O2 + 2 H2O + 4e− → 4 OH−
3. Applications of Chitosan Flat Structures
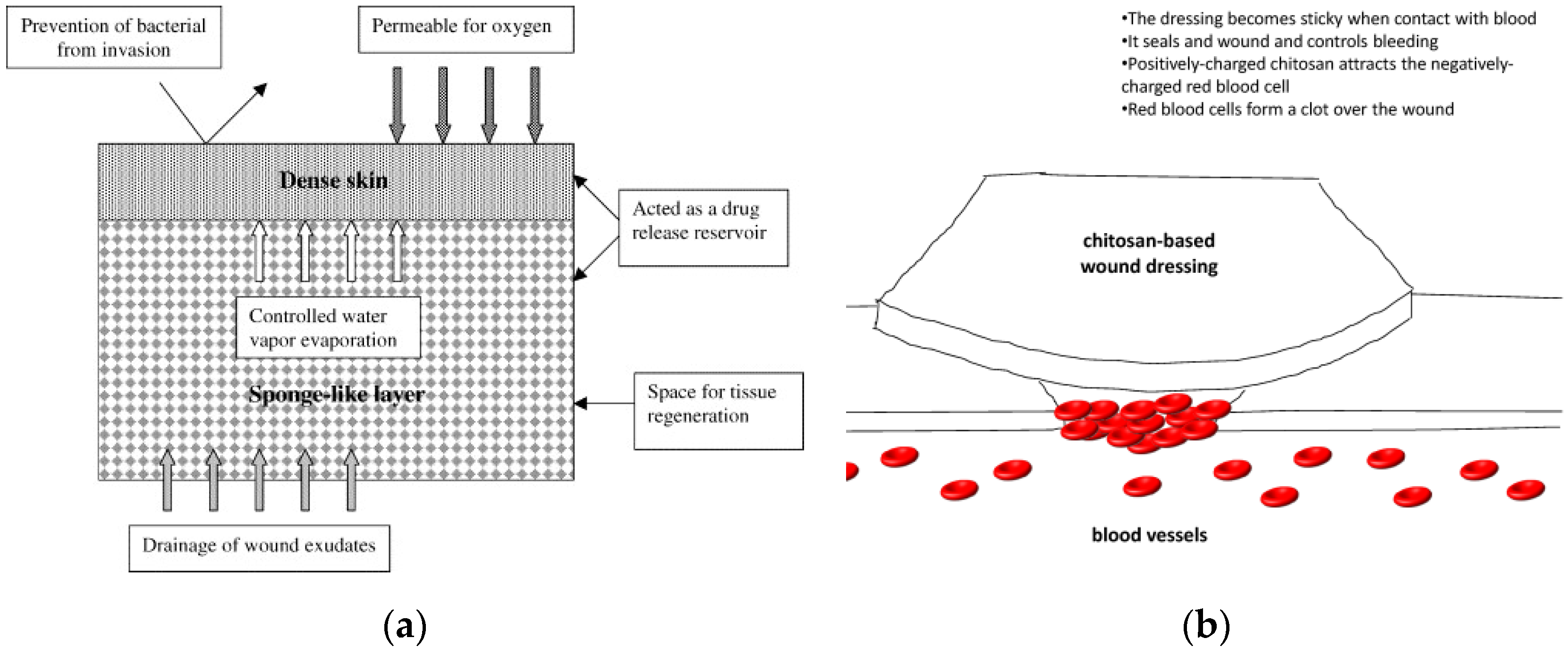
3.1. Biomedicine
3.2. Energy Storage and Conversion Devices
3.3. Food Packaging
3.4. Wastewater Treatment and Air Filtration
4. Mechanisms of Ionotropic Gelation
4.1. Fundamentals of Ionotropic Gelation
4.2. Ionotropic Gelation for the Synthesis of Nano/Microparticles
4.3. Advantages and Disadvantages of Ionotropic Gelation
4.4. Ionotropic Gelation of Chitosan
5. Ionotropic Gelation of Chitosan Flat Structures
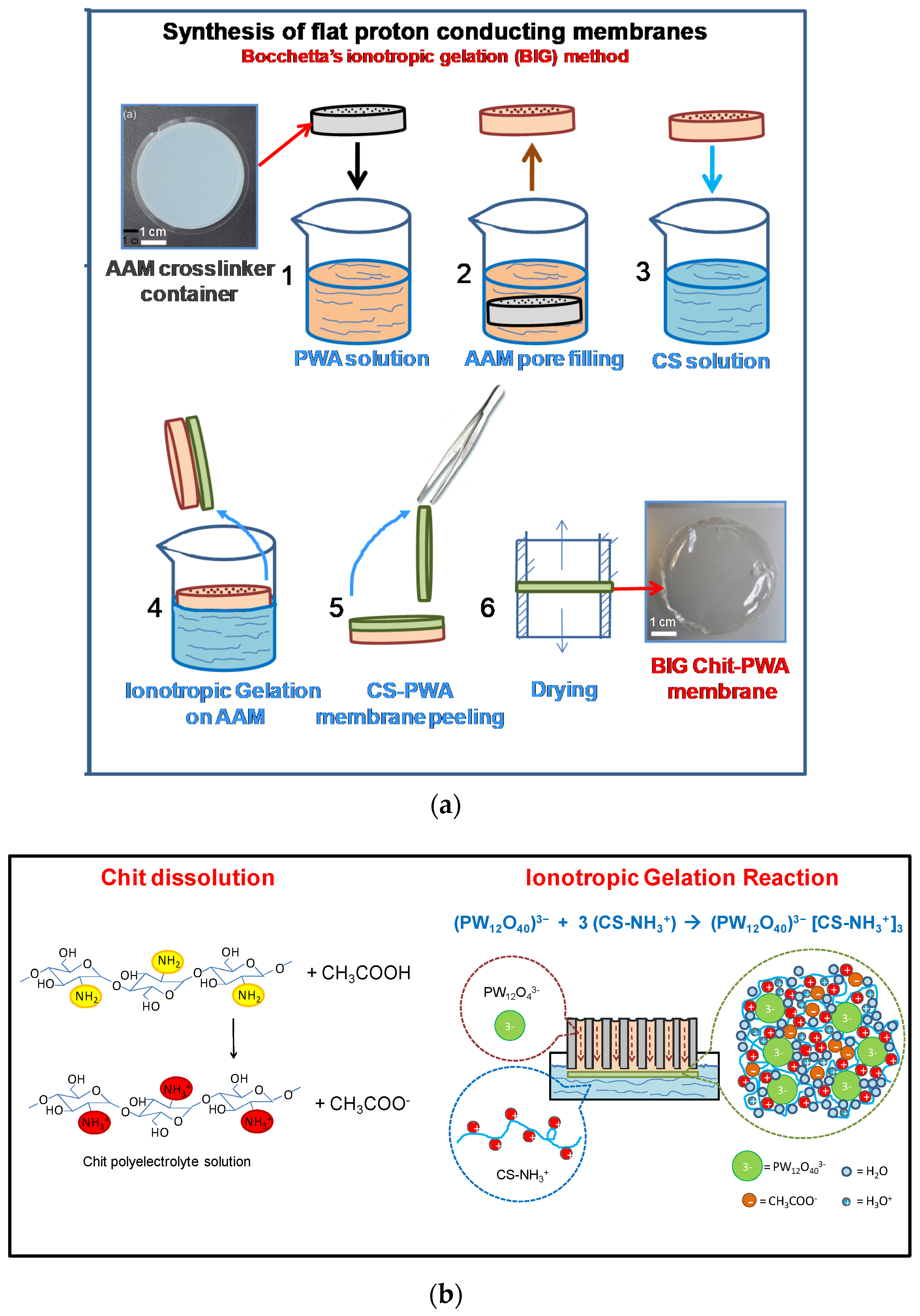
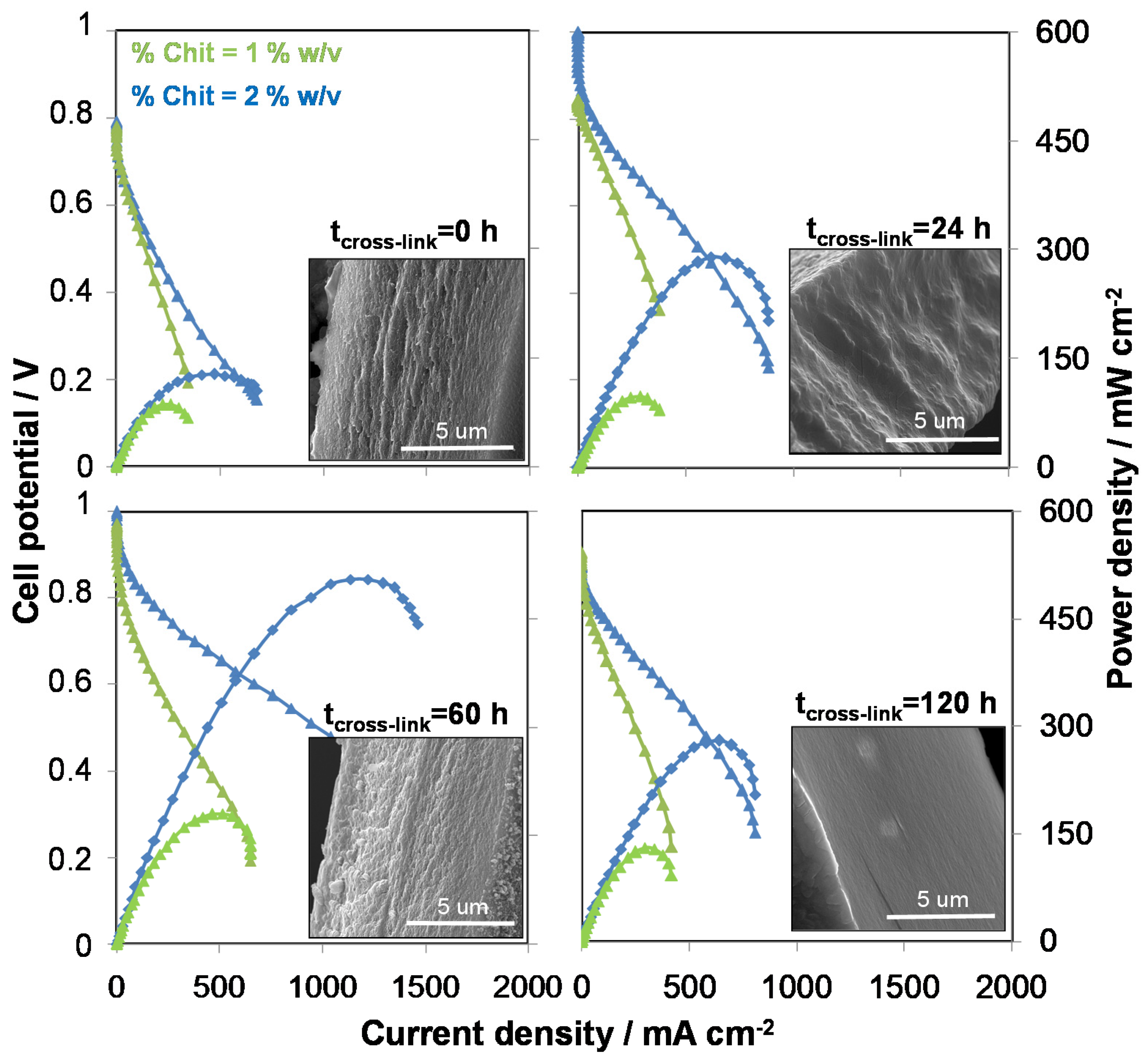
5.1. Ionotropic Gelation of Chit Flat Structures through Porous Alumina Support
5.2. Ionotropic Gelation through Slow Diffusion of the Cross-Linker
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Arguelles-Monal, W.; Sarabia-Sainz, A.; Lucero-Acuña, A.; del Castillo-Castro, T.; San Román, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature stimuli-responsive nanoparticles from chitosan-graft-poly (N-vinylcaprolactam) as a drugdeliverysystem. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; LamazaresArcia, E.; Fleitas-Salazar, N.; Gancino Guevara, M.; Mansilla, R.; Gomez-Gaete, C.; Altamirano, C.; Fernández, K.; Ruiz, A.; Toledo Alonso, J.R. Polymeric nanoencapsulation of alpha interferon increases drug bioavailability and induces a sustained antiviral response in vivo. Mater. Sci. Eng. C 2020, 116, 111260. [Google Scholar] [CrossRef]
- Canepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Jamil, S.N. Synthesis and Optimization of Chitosan Nanoparticles Loaded with L-Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E.; Winnicka, K. Stability of Chitosan-A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Advanced Pharmaceutical Technology Solution. Available online: https://www.micro-encapsulation.com/expertise/ionotropic-gelation (accessed on 26 January 2021).
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar]
- Bocchetta, P. Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures. Molecules 2020, 25, 1632. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Vårum, K.M.; Smidsrød, O. Structure-Property Relationship in Chitosans. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 625–642. [Google Scholar]
- JafarizadehMalmiri, H.; Ali GhazJahanian, M.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-Based Networks from Homogeneous Chitosan- Tripolyphosphate Hydrogels: Synthesis and Characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Peters, M.G. Chitin Handbook; Università di Ancona: Breman, Germany; European Chitin Society: Venice, Italy, 1997; pp. 437–438. [Google Scholar]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular conformation of chitosan in dilute solution: A new global hydrodynamic approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Teruel-Juanes, R.; Arenga, F.; Salaberria, A.M.; Baschetti, M.G.; Labidi, J.; Badia, J.D.; Ribes-Greus, A. Crosslinked chitosan/poly(vinyl alcohol)-based polyelectrolytes for proton exchange membrane. React. Funct. Polym. 2019, 142, 213–222. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinki, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Barra, A.; Alves, Z.; Ferreira, N.M.; Martins, M.A.; Oliveira, H.; Ferreira, L.P.; Cruz, M.M.; Carvalho, M.; Neumayer, S.M.; Rodriguez, B.J.; et al. Biocompatible chitosan-based composites with properties suitable for hyperthermia therapy. J. Mater. Chem. B 2020, 8, 1256–1265. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Machado, J.A.; Costa, R.A.; Wanderley, A. Electrophoretic deposition and characterization of chitosan-molybdenum composite coatings. Carbohydr. Polym. 2021, 255, 117382. [Google Scholar]
- Biswas, S.; Rashid, T.U.; Debnath, T.; Haque, P.; Rahman, M.M. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. J. Compos. Sci. 2020, 4, 16. [Google Scholar]
- Zhuang, S.; Zhu, K.; Wang, J. Fibrous chitosan/cellulose composite as an efficient adsorbent for Co(II) removal. J. Clean. Prod. 2021, 285, 124911. [Google Scholar]
- Bocchetta, P.; Amati, M.; Bozzini, B.; Catalano, M.; Gianoncelli, A.; Gregoratti, L.; Taurino, A.; Kiskinova, M. Quasi-in-Situ Single-Grain Photoelectron Microspectroscopy of Co/PPy Nanocomposites under Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 19621–19629. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, P.; Chen, J.; Cui, B.; Zhang, C.; Wu, F. Conducting Polymer-Based Composite Materials for Therapeutic Implantations: From Advanced Drug Delivery System to Minimally Invasive Electronics. Int. J. Polym. Sci. 2020, 2020, 5659682. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. Design of Polymer Blends Based on Chitosan: POZ with Improved Dielectric Constant for Application in Polymer Electrolytes and Flexible Electronics. Adv. Polym. Technol. 2020, 2020, 8586136. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with Chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F. Applications and properties of chitosan. J. Bioact. Comp. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Hudson, S.M.; Smith, C.; Kaplan, D.L. (Eds.) Biopolymers from Renewable Resources; Springer: Berlin, Germany, 1998; p. 96. [Google Scholar]
- Monteiro, O.A.C.; Airoldi, C. Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, C.; Lu, T.; Xing, W. Polyelectrolyte complexes of chitosan and phosphotungstic acid asproton-conducting membranes for direct methanol fuel cells. J. Power Sources 2007, 167, 94–99. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Liu, C.; Liao, J.; Zhang, H. Chitosan/heteropolyacid composite membranes for direct methanol fuel cell. J. Power Sources 2009, 188, 24–29. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology: A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-da-Silva, F.; Dessì, M.; Gloria, A.; Ambrosio, L.; Gonçalves, R.M.; Barbosa, M.A. Layer-by-Layer Self-Assembly of Chitosan and Poly(γ-glutamic acid) into Polyelectrolyte Complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Farhat, T.; Hammond, P. Designing a New Generation of Proton-Exchange Membranes Using Layer-by-Layer Deposition of Polyelectrolytes. Adv. Funct. Mater. 2005, 15, 945–954. [Google Scholar] [CrossRef]
- Yuan, W.; Weng, G.M.; Lipton, J.; Li, C.M.; Van Tassel, P.R.; Taylor, A.D. Weak polyelectrolyte-based multilayers via layer-by-layer assembly: Approaches, properties, and applications. Adv. Colloid Interface Sci. 2020, 282, 102200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lin, H.; Cui, Z.; Li, X.; Na, H.; Xing, W. Highly conductive, methanol resistant fuel cell membranes fabricated by layer-by-layer self-assembly of inorganic heteropolyacid. J. Power Sources 2009, 194, 168–174. [Google Scholar]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Lai, J.Y.; Lin, F.C.; Wang, C.C.; Wang, D.M. Effect of nonsolvent additives on the porosity and morphology of asymmetric TPX membranes. J Membrane Sci. 1996, 118, 49–61. [Google Scholar] [CrossRef]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Chao, A.C.; Lai, J.Y.; Su, C.C. Asymmetric chitosan membranes prepared by dry/wet phase separation: A new type of wound dressing for controlled antibacterial release. J. Membr. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Szklarska, M.; Łosiewicz, B.; Dercz, G.; Maszybrocka, J.; Rams-Baron, M.; Stach, S. Electrophoretic deposition of chitosan coatings on the Ti15Mo biomedical alloy from a citric acid solution. RSC Adv. 2020, 10, 13386–13393. [Google Scholar] [CrossRef]
- Bernards, M.T. 2—Environmentally Responsive Polyelectrolytes and Zwitterionic Polymers. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 45–64. [Google Scholar]
- Kudo, S.; Konno, M.; Saito, S. Swelling equilibria of cationic polyelectrolyte gels in aqueous solutions of various electrolytes. Polymer 1993, 34, 2370–2373. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan:An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef]
- Kumar, S.; Kesharwani, S.S.; Kuppast, B.; Bakkari, M.A.; Tummala, H. Pathogenmimicking vaccine deliverysystem designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses. J. Control. Release 2017, 261, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesharwani, S.S.; Kuppast, B.; Rajput, M.; Ali Bakkari, M.; Tummala, H. Discovery of inulinacetate as a novel immune-active polymer and vaccine adjuvant: Synthesis, material characterization, andbiological evaluation as a toll-like receptor-4 agonist. J. Mater. Chem. B 2016, 4, 7950–7960. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Philippou, K.; Krasia-Christoforou, T.; Pashalidis, I. Uranium adsorption bypolyvinylpyrrolidone/chitosan blended nanofibers. Carbohydr. Polym. 2019, 219, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing chitosan based eco-friendly multifunctionalsoil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Owczarek, A.; Nadolna, K.; Sionkowska, A. The film-forming properties of chitosan withtannic acid addition. Mater. Lett. 2019, 245, 22–24. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Kurasova, M.N.; Volkova, O.V.; Meledina, T.V.; Lipkan, N.A.; Tskhovrebov, A.G.; Kurliuk, A.V.; Shakola, T.V.; Dysin, A.P.; et al. Novel non-toxic high e_cientantibacterialazido chitosan derivatives with potential application in food coatings. Food Chem. 2019, 301, 125247. [Google Scholar]
- Lin, Y.-H.; Kang, P.-L.; Xin, W.; Yen, C.-S.; Hwang, L.-C.; Chen, C.-J.; Liu, J.-T.; Chang, S.J. Preparation andevaluation of chitosan biocompatible electronic skin. Comput. Ind. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Stefanescu, C.; Daly, W.H.; Negulescu, I.I. Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose. Carbohydr. Polym. 2012, 87, 435–443. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use asbiomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosanand its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Song, J.G.; Han, H.K. Development of pH-responsive organic-inorganic hybrid nanocomposites asaneective oral delivery system of protein drugs. J. Control. Release 2019, 311–312, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Almodovar, J.; Kipper, M.J. Coating electrospun chitosan nanofibers withpolyelectrolyte multilayers usingthe polysaccharides heparin and N,N,N-trimethyl chitosan. Macromol. Biosci. 2011, 11, 72–76. [Google Scholar] [CrossRef]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.K.M.C.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.L.P.M.; et al. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Nasseri, B.A.; Vacanti, J.P. Tissue engineering: A 21st century solution to surgical reconstruction. Ann. Thorac. Surg. 2001, 72, 577. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Martins, N.I.; Sousa, M.P.; Custódio, C.A.; Pinto, V.C.; Sousa, P.J.; Minas, G.; Cleymand, F.; Mano, J.F. Multilayered membranes with tuned well arrays to be used as regenerative patches. Acta Biomater. 2017, 57, 313–323. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar, R.A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Pathomthongtaweechai, N.; Soodvilai, S.; Pichyangkura, R.; Muanprasat, C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules 2020, 25, 5589. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Coralli, A.; Sarruf, B.J.M.; Emılio, P.; de Miranda, V.; Osmieri, L.; Specchia, S.; Minh, N.Q. Fuel Cells, Chapter 2, in Hydrogen Production and Practical Applications in Energy Generation. Sci. Eng. Hydrog. Based Energy Technol. 2019, 39–122. [Google Scholar] [CrossRef]
- Bozzini, B.; Amati, M.; Bocchetta, P.; Zilio, S.D.; Knop-Gericke, A.; Vesselli, E.; Kiskinova, M. An in situ near-ambient pressure X-ray Photoelectron Spectroscopy study of Mn polarised anodically in a cell with solid oxide electrolyte. Electrochim. Acta 2015, 174, 532–541. [Google Scholar]
- Li, X. Principles of Fuel Cells; Taylor and Francis Group: New York, NY, USA; London, UK, 1962. [Google Scholar]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Cooper, H.W. A future in fuel cells. Chem. Eng. Prog. 2007, 103, 34–43. [Google Scholar]
- Sopian, K.; Wan Daud, R.W. Challenges and future developments in proton exchange membrane fuel cells. Renew. Energy 2006, 35, 719–727. [Google Scholar] [CrossRef]
- Zaidi, S.M.J. Development of Proton Conducting Composite Membranes for Fuel Cell Applications. Ph.D. Thesis, Laval University, Quebec, QC, Canada, 2000. [Google Scholar]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Bocchetta, P.; Conciauro, F.; Santamaria, M.; Di Quarto, F. Cs0.86(NH4)1.14SO4Te(OH)6 in porous anodic alumina for micro fuel cell applications. Electrochim. Acta 2011, 56, 3845–3851. [Google Scholar]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Wu, H.; Hou, W.; Wang, J.; Xiao, L.; Jiang, Z. Preparation and properties of hybrid direct methanol fuel cell membranes by embedding organophosphorylated titania submicrospheres into a chitosan polymer matrix. J. Power Sources 2010, 195, 4104–4113. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, Q.; He, B.; Chen, H.; Li, Q.; Ma, C.; Jin, S.; Liu, Z. H3PO4 imbibed polyacrylamide-graft-chitosan frameworks for high-temperature proton exchange membranes. J. Power Sources 2014, 249, 277–284. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Zhang, S.; Wang, H.; Cheng, B.; Zhuang, X.; Li, Z. Proton-conducting amino acid-modified chitosan nanofibers for nanocomposite proton exchange membranes. Eur. Polym. J. 2019, 119, 327–334. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar]
- Odeh, A.O.; Osifo, P.; Noemagus, H. Chitosan: A Low Cost Material for the Production of Membrane for Use in PEMFC-A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 152–163. [Google Scholar] [CrossRef]
- Xiaoa, Y.; Xianga, Y.; Xiua, R.; Lu, S. Development of cesium phosphotungstate salt and chitosan composite membrane for direct methanol fuel cells. Carbohydr. Polym. 2013, 98, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Jayakumar, R.; Selvamurugan, N.; Nair, S.V.; Tokura, S.; Tamura, H. Preparative methods of phosphorylated chitin and chitosan—An overview. Int. J. Biol. Macromol. 2008, 43, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Uragami, T.; Aketa, T.; Gobodani, S.; Sugihara, M. Studies of syntheses and permeabilities of special polymer membranes. 61. New method for enzyme immobilization by a polyion complex membrane. Polym. Bull. 1986, 15, 101–106. [Google Scholar]
- Singh, K.; Suri, R.; Tiwary, A.K.; Rana, V. Chitosan films: Crosslinking with EDTA modifies physicochemical and mechanical properties. J. Mater. Sci. Mater. Med. 2012, 23, 687–695. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Fabrication and comprehensive investigation of physicochemical and electrochemical properties of chitosan-silica supported silicotungstic acid nanocomposite membranes for fuel cell applications. Energy 2018, 142, 313–330. [Google Scholar]
- Cui, Z.; De Moraes, M.A.; Cocenza, D.S.; da Cruz Vasconcellos, F.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar]
- Mat, N.; Liong, A. Chitosan-poly (vinyl alcohol) and calcium oxide composite membrane for direct methanol fuel cell applications. Eng. Lett. 2009, 116, 1017–1029. [Google Scholar]
- Campos, M.G.N.; Ferreira Grosso, C.R.; Cárdenas, G.; Inocentinni Mei, L.H. Effects of Neutralization Process on Preparation and Characterization of Chitosan Membranes for Wound Dressing. Macromol. Symp. 2005, 229, 253–257. [Google Scholar] [CrossRef]
- Kaiser, V.; Stropnik, C.; Musil, V.; Brumen, B. Morphology of solidified polysulfone structures obtained by wet phase separation. Eur. Polym. J. 2007, 43, 2515–2524. [Google Scholar] [CrossRef]
- Bocchetta, P.; Conciauro, F.; Santamaria, M.; Di Quarto, F. Fuel Cell Performances of Bio-Membranes Made of Chitosan-Polyelectrolyte Thin Films and Nanowires into Anodic Alumina Membranes. In Proceedings of the 220th ECS Meeting, Boston, MA, USA, 9–14 October 2011. [Google Scholar]
- Santamaria, M.; Pecoraro, C.M.; Di Quarto, F.; Bocchetta, P. Chitosanphosphotungstic acid complex as membranes for low temperature H2-O2 fuelcell. J. Power Sources 2015, 276, 189–194. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Ma, J.; Sahai, Y. High performance and eco-friendly chitosan hydrogel membrane electrolytes for direct borohydride fuel cells. J. Power Sources 2012, 210, 358–365. [Google Scholar] [CrossRef]
- Feketeföldi, B.; Cermenek, B.; Spirk, C.; Schenk, A.; Grimmer, C.; Bodner, M.; Koller, M.; Ribitsch, V.; Hacker, V. Chitosan-Based Anion Exchange Membranes for Direct Ethanol Fuel Cells. J. Membr. Sci. Technol. 2016, 6. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternizedpoly(vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosanmembranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Structure and ionic conductivity of a series of di-o-butyryl chitosan membranes. J. Appl. Polym. Sci. 2004, 94, 2309–2323. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity and related properties of cross-linked chitosan membranes. J. Appl. Polym. Sci. 2003, 89, 306–317. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Synthesis, characterization and ionic conductive properties of phosphorylated chitosan membranes. Macromol. Chem. Phys. 2003, 204, 850–858. [Google Scholar] [CrossRef]
- Klotzbach, T.; Watt, M.; Ansari, Y.; Minteer, S.D. Effects of hydrophobic modificationof chitosan and Nafion on transport properties, ion-exchange capacities, andenzyme immobilization. J. Membr. Sci. 2006, 282, 276–283. [Google Scholar]
- Pecoraro, C.M.; Santamaria, M.; Bocchetta, P.; Di Quarto, F. Influence of synthesis conditions on the performance of chitosan-heteropolyacid complexes as membranes for low temperature H2-O2 fuel cell. Int. J. Hydrog. Energy 2015, 40, 14616–14626. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Nam, D.H.; Cho, M.; Kim, J.; Chanthad, C.; Lee, Y. Epoxidized Natural Rubber/Chitosan Network Binder for Silicon Anode in Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 16449–16457. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-ion Battery Si-based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Ozeki, T.; Han, Z.J.; Shimomura, K.; Yui, H.; Katayama, Y.; Miura, T. Comparative Study of SodiumPolyacrylate and Poly(vinylidene fluoride) as Binders for HighCapacity Si-graphite Composite Negative Electrodes in Li-ionBatteries. J. Phys. Chem. C 2012, 116, 1380–1389. [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.H.; Cho, M.; Kim, J.; Lee, Y. Cross-Linked Chitosan as an Efficient Binder for Si Anode of Li-ionBatteries. ACS Appl. Mater. Interfaces 2016, 8, 2658–2665. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. Multifunctional Chitosan–rGO Network Binderfor Enhancing the Cycle Stability of Li–S Batteries. Adv. Funct. Mater. 2020, 30, 1907680. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhao, X.; Yim, C.H.; Du, N.; Abu-Lebdeh, Y. Crosslinked Chitosan Networks as Binders for Silicon/GraphiteComposite Electrodes in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, 1110–1121. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, W.; Jang, J.H.; Goodenough, J.B. Cross-Linked Chitosan as a Polymer Network Binder for anAntimony Anode in Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502130. [Google Scholar]
- Seh, Z.W.; Sun, Y.M.; Zhang, Q.F.; Cui, Y. Designing high energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.Z.; Chung, S.-H.; Zu, C.X.; Su, Y.-S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical ordered mesoporous carbon nanoparticleswith high porosity for lithium-sulfur batteries. Angew. Chem. Int. Ed. 2012, 51, 3591–3595. [Google Scholar] [CrossRef]
- Fang, R.P.; Zhao, S.Y.; Sun, Z.H.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions andProspects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, M.; Wang, X.; Wu, Z.; Zeng, P.; Huang, C.; Wang, Y. MoS2-Coated N-doped Mesoporous Carbon Spherical CompositeCathode and CNT/Chitosan Modified Separator for AdvancedLithium Sulfur Batteries. ACS Sustain. Chem. Eng. 2018, 6, 16828–16837. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Northrop, P.W.; Crothers, A.C.; Jain, S.; Subramanian, V.R. Chitosan hydrogel-based electrode binder and electrolytemembrane for EDLCs: Experimental studies and model validation. J. Appl. Electrochem. 2012, 42, 935–943. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Niu, B.; Jiang, F.; Yang, X.; Li, M. Chitosan-derived hybrid porous carbon with the novel tangerine pith-like surface as supercapacitor electrode. J. Mater. Sci. 2019, 54, 14456–14468. [Google Scholar] [CrossRef]
- Cao, L.; Yang, M.; Wu, D.; Lyu, F.; Sun, Z.; Zhong, X.; Pan, H.; Liu, H.; Lu, Z. Biopolymer-chitosan based supramolecular hydrogels as solid state electrolytes for electrochemical energy storage. Chem. Commun. 2017, 53, 1615–1618. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Kumar Yadav, B. Applications of chitosan in environmental remediation: A review. Chemosphere 2020, 266, 128934. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37. [Google Scholar] [CrossRef]
- Peter, M.J. Applications and Environmental Aspects of Chitin and Chitosan. Macromol. Sci. Pure Appl. Chem. A 2005, 32, 629–640. [Google Scholar]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.M.; Ahmed, W. Preparation and Characterization of Chitosan/Gelatin-Based Active Food Packaging Films Containing Apple Peel Nanoparticles. J. Polym. Environ. 2019, 28, 411–420. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Ploym. 2020, 237, 116142. [Google Scholar]
- Edwards, W.; Leukes, W.D.; Rose, P.D.; Burton, S.G. Immobilization of polyphenol oxidase on chitosan-coated polysulphone capillary membranes for improved phenolic effluent bioremediation. Enzym. Microb. Technol. 1999, 25, 769. [Google Scholar]
- Kumar, G.; Smith, P.J.; Payne, G.F. Enzymatic grafting of a natural product onto chitosan to confer water solubility under basic conditions. Biotechnol. Bioeng. 1999, 63, 154. [Google Scholar] [CrossRef]
- Muzzarelli, C.; Muzzarelli, R.A.A. Reactivity of quinones towards chitosans. Trends Glycosci. Glycotechnol. 2002, 14, 223. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.M.; Yang, Q.; Zeng, G.M.; Shen, X.X.; Zhang, J.; Liu, J.J. Adsorption of Cd(II) and Cu (II) from aqueous solution by carbonate hydroxyapatite derived from egg shell waste. J. Hazard. Mater. 2007, 147, 534–539. [Google Scholar] [CrossRef]
- Han, Y.; Lingyun, Y.; Zhen, Y.; Hu, Y.; Aimin, L.; Rongshi, C. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 229–230, 371–380. [Google Scholar]
- Kamiński, W.; Modrzejewska, Z. Application of chitosan membranes in separation of heavy metal ions. Sep. Sci. Technol. 1997, 32, 2659–2668. [Google Scholar] [CrossRef]
- Taha, S.; Bouvet, P.; Corre, G.; Dorange, G. Study and modelization of some heavy metals removal by ultrafiltration in the presence of soluble chitosan. Adv. Chitin Sci. 1996, 1, 389. [Google Scholar]
- Genç, Ö.; Arpa, Ç.; Bayramoğlu, G.; Arıca, M.Y.; Bektaş, S. Selective recovery of mercury by Procion Brown MX 5BR immobilized poly(hydroxyethylmethacrylate/chitosan) composite membranes. Hydrometallurgy 2002, 67, 53. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, F.; Pei, H.; Li, J.; Cui, Z.; He, B. Antibacterial and environmentally friendly chitosan/polyvinyl alcohol blend membranes for air filtration. Carbohydr. Polym. 2018, 198, 241–248. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A Review. Dig. J. Nanomat. Biostruct. 2010, 5, 241. [Google Scholar]
- Ahmad, M.; Rai, S.M.; Mahmood, A. Hydrogel Microparticles as an Emerging Tool in Pharmaceutical Field: A Review. Adv. Polym. Technol. 2016, 35, 121–128. [Google Scholar]
- Suganeswari, M.; Senthil Kumar, V.; Anto, S.M. Formulation and Evaluation of Metoclopramide Hydrochloride Microbeads by Ionotropic Gelation Method. Int. J. Pharm. Biol. Arch. 2011, 2, 921–925. [Google Scholar]
- Patel, M.A.; AbouGhaly, M.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginatecross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Shiralshetti, S.S. Ionotropically gelled novel hydrogel beads: Preparation, characterization and In vitro evaluation. Indian J. Pharm. Sci. 2011, 73, 504–509. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Boppana Setty, C.M.; Kalyane, N.V. Carboxymethylcellulose-aluminum hydrogel microbeads for prolonged release of simvastatin. Acta Pharm. Sci. 2010, 52, 137–143. [Google Scholar]
- Dhanaraju, M.D.; Sundar, V.D.; NandhaKumar, S.; Bhaskar, K. Development and evaluation of sustained delivery of diclofenac sodium from hydrophilic polymeric beads. J. Young Pharm. Pharm. 2009, 1 (Suppl. 4), 301–304. [Google Scholar] [CrossRef]
- Patil, P.; Chavanke, D.; Wagh, M. A review on ionotropic gelation method: Novel approach for controlled gastroretentivegelispheres. Int. J. Pharm. Pharm. Sci. 2012, 4, 27–32. [Google Scholar]
- Patel, A.; Patel, M.; Yang, X.; Mitra, A.K. Recent advances in protein and peptide drug delivery: A special emphasis on polymeric nanoparticles. Protein Pept. Lett. 2014, 21, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef]
- Jarudilokkul, S.; Tongthammachat, A.; Boonamnuayvittaya, V. Preparation of chitosan nanoparticles for encapsulation and release of protein. Korean. J. Chem. Eng. 2011, 28, 1247–1251. [Google Scholar]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.J.; Zhao, H.Y.; Zheng, J.M.; Xu, H.; Wei, G.; Hao, J.S.; Cui, F.D. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar]
- Wu, J.; Wang, Y.; Yang, H.; Liu, X.; Lu, Z. Preparation and biological activity studies of resveratrol loaded ionically cross-linked chitosan-TPP nanoparticles. Carbohydr. Polym. 2017, 175, 170–177. [Google Scholar] [CrossRef]
- Yan, J.; Guan, Z.Y.; Zhu, W.F.; Zhong, L.Y.; Qiu, Z.Q.; Yue, P.F.; Wu, W.T.; Liu, J.; Huang, X. Preparation of Puerarin chitosan oral nanoparticles by ionic gelation method and its related kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Sousa, A.I.; Carvalho Silva, J.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, A.; Sanz, B.; San Román, J.; Goya, G.F.; Hernández, R.; Mijangos, C. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: From preparation to in vitro studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar]
- Goycoolea, F.M.; Lollo, G.; Remuñán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-Alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remunán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Park, J.S.; Han, T.H.; Lee, K.Y.; Han, S.S.; Hwang, J.J.; Moon, D.H.; Kim, S.Y.; Cho, Y.W. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: Endocytosis, exocytosis and drug release. J. Control. Release 2006, 115, 37–45. [Google Scholar] [CrossRef]
- Vila, A.; Sánchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Jato, J.L.V.; Alonso, M.J. Low moleculer weight chitosan nanoparticles as new carriers for nasal vaccire delivery in mice. Eur. J. Pharm. Biopharm. 2003, 57, 123–131. [Google Scholar] [CrossRef]
- Pérez Quiñones, J.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the Degree of Acetylation and the Electrostatic Properties of Chitin and Chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, M.W.; Smidsrød, O. Hydrogen ion titration of chitosans with varying degrees of N-acetylation by monitoring induced 1H-NMR chemical shifts. Carbohydr. Polym. 1995, 26, 303–305. [Google Scholar] [CrossRef]
- Strand, S.P.; Tømmeraas, K.; Vårum, K.M.; Østgaard, K. Electrophoretic light scattering studies of chitosans with different degrees of N-acetylation. Biomacromolecules 2001, 2, 1310–1314. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Determining the colloidal behavior of ionically cross-linked polyelectrolytes with isothermal titration calorimetry. J. Phys. Chem. B 2013, 117, 9548–9557. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Vårum, K.M.; Marsich, E. Butyrate-Loaded Chitosan/Hyaluronan Nanoparticles: A Suitable Tool for Sustained Inhibition of ROS Release by Activated Neutrophils. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic acid (HA) presentation as a tool to modulate and control the receptor-mediated uptake of HA-coated nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Müllner, M.; Ping, Y.; Cui, J.; Kempe, K.; Cortez-Jugo, C.; Caruso, F. Shape-Dependent Activation of Cytokine Secretion by Polymer Capsules in Human Monocyte-Derived Macrophages. Biomacromolecules 2016, 17, 1205–1212. [Google Scholar] [CrossRef]
- Palomba, R.; Palange, A.L.; Rizzuti, I.F.; Ferreira, M.; Cervadoro, A.; Barbato, M.G.; Canale, C.; Decuzzi, P. Modulating Phagocytic Cell Sequestration by Tailoring Nanoconstruct Softness. ACS Nano 2018, 12, 1433–1444. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Donati, I.; Paoletti, S.; Marsich, E. Chitosan Acetylation Degree Influences the Physical Properties of Polysaccharide Nanoparticles: Implication for the Innate Immune Cells Response. ACS Appl. Mater. Interfaces 2019, 11, 9794–9803. [Google Scholar] [CrossRef]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, Y. Ionically crosslinked polyelectrolyte nanoparticle formation mechanisms: The significance of mixing. Soft Matter 2019, 15, 9871–9880. [Google Scholar] [CrossRef]
- Hirano, S.; Kondo, S.; Ohe, Y. Chitosan gel: A novelpolysaccharide gel. Polymer 1975, 16, 622. [Google Scholar] [CrossRef]
- Vachoud, L.; Zydowicz, N.; Domard, A. Formation and characterisation of a physical chitin gel. Carbohydr. Res. 1997, 302, 169–177. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric Study of the Gelation of Chitosan in Aqueous Solution without Cross-Linking Agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic Hydrogel Based on Chitosan Cross-Linked with 6-Phosphogluconic Trisodium Salt as a Drug Delivery System. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef]
- Khong, T.T.; Aarstad, O.A.; Skjåk-Bræk, G.; Draget, K.I.; Vårum, K.M. Gelling concept combining chitosan and alginate-proof of principle. Biomacromolecules 2013, 14, 2765–2771. [Google Scholar] [CrossRef]
- Draget, K.I.; Vårum, K.M.; Moen, E.; Gynnild, H.; Smidsrød, O. Chitosan cross-linked with Mo(VI) polyoxyanions: A new gelling system. Biomaterials 1992, 13, 635–638. [Google Scholar] [CrossRef]
- Vorlop, K.D.; Klein, J. Formation of spherical chitosan biocatalysts by ionotropic gelation. Biotechnol. Lett. 1981, 3, 9–14. [Google Scholar] [CrossRef]
- Bocchetta, P.; Santamaria, M.; di Quarto, F. One-step electrochemical synthesis and physico-chemical characterization of CdSe nanotubes. Electrochim. Acta 2013, 88, 340–346. [Google Scholar]
- Santamaria, M.; Asaro, L.; Bocchetta, P.; Megna, B.; Quarto, F.D. Electrodeposition of ceo2 and co-doped ceo2 nanotubes by cyclic anodization in porous alumina membranes. ECS Electrochem. Lett. 2013, 2, D29–D32. [Google Scholar] [CrossRef]
- Bocchetta, P.; Frattini, D.; Tagliente, M.; Selleri, F. Electrochemical Deposition of Polypyrrole Nanostructures for Energy Applications: A Review. Curr. Nanosci. 2020, 16, 462–477. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Salt-assisted mechanistic analysis of chitosan/tripolyphosphate micro- and nanogel formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the Correlation between the Microscopic Structure and Properties of Phosphate-Cross-Linked Chitosan Gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef] [PubMed]
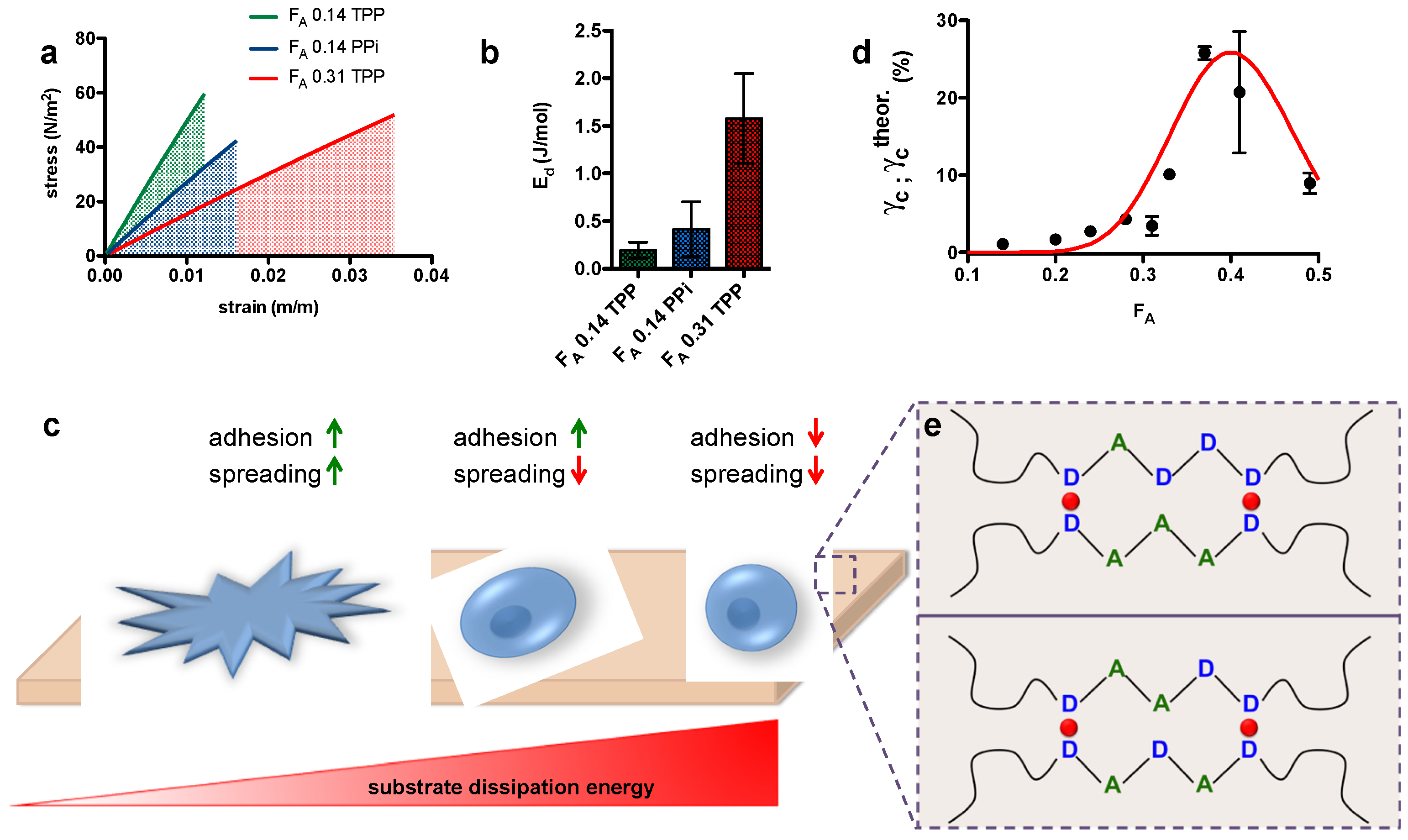
- Sacco, P.; Baj, G.; Asaro, F.; Marsich, E.; Donati, I. Substrate Dissipation Energy Regulates Cell Adhesion and Spreading. Adv. Funct. Mater. 2020, 2001977. [Google Scholar] [CrossRef]
- Cameron, A.R.; Frith, J.E.; Cooper-White, J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 2011, 32, 5979–5993. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6365. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef] [PubMed]



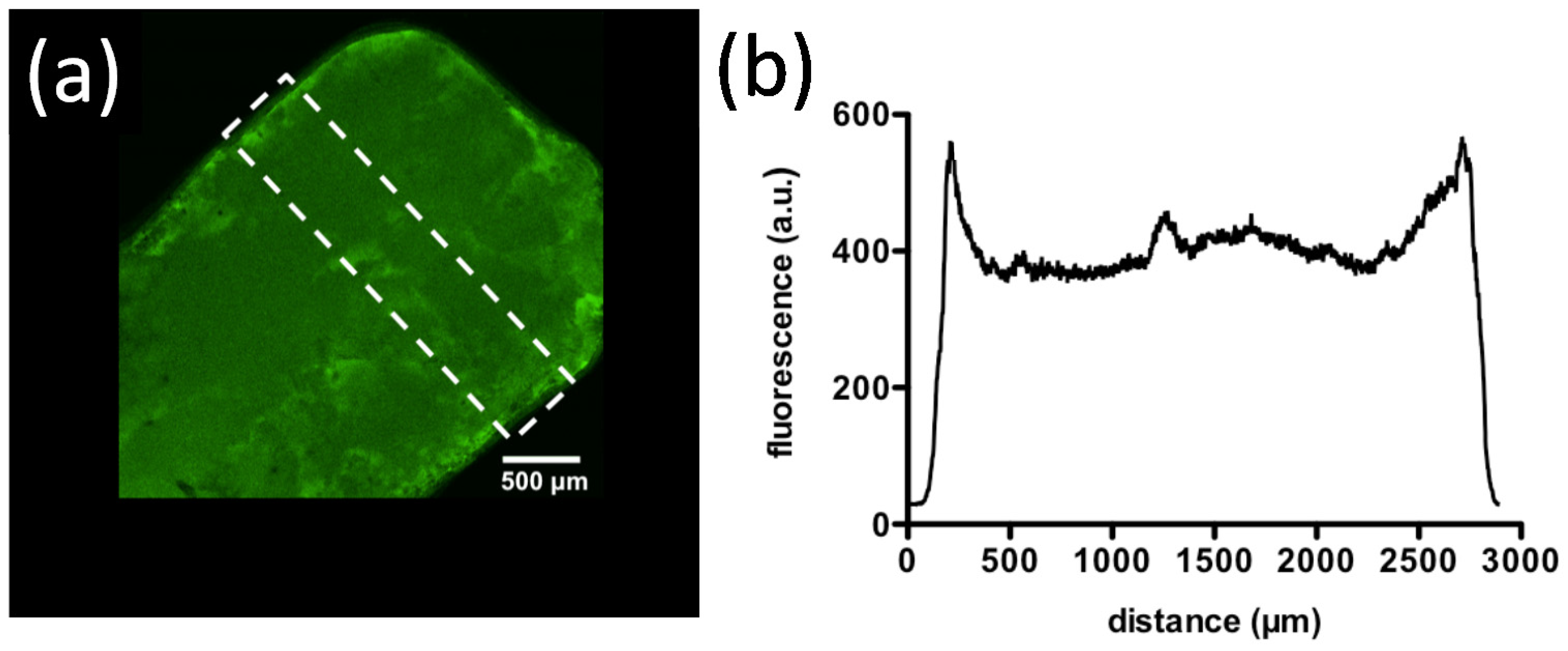
| Natural Polymers | Synthetic Monomers/Polymers | Multivalent Cations/Anions |
|---|---|---|
| Chitosan | Hydroxyethyl methacrylate | Ca2+, Mo7O26−, (PW12O40)3− |
| Alginate | N-(2-Hydroxypropyl) methacrylate | K+ |
| Fibrin | N-Vinyl-2-pyrrolidone | Fe2+, Ba2+, Na+, Mg2+ |
| Collagen | N-isopropylacrylamide | Al3+ |
| Gelatin | Vinyl acetate | Zn2+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660. https://doi.org/10.3390/molecules26030660
Sacco P, Pedroso-Santana S, Kumar Y, Joly N, Martin P, Bocchetta P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules. 2021; 26(3):660. https://doi.org/10.3390/molecules26030660
Chicago/Turabian StyleSacco, Pasquale, Seidy Pedroso-Santana, Yogesh Kumar, Nicolas Joly, Patrick Martin, and Patrizia Bocchetta. 2021. "Ionotropic Gelation of Chitosan Flat Structures and Potential Applications" Molecules 26, no. 3: 660. https://doi.org/10.3390/molecules26030660
APA StyleSacco, P., Pedroso-Santana, S., Kumar, Y., Joly, N., Martin, P., & Bocchetta, P. (2021). Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules, 26(3), 660. https://doi.org/10.3390/molecules26030660











