Acid-Activated Motion Switching of DB24C8 between Two Discrete Platinum(II) Metallacycles
Abstract
1. Introduction
2. Results and Discussion
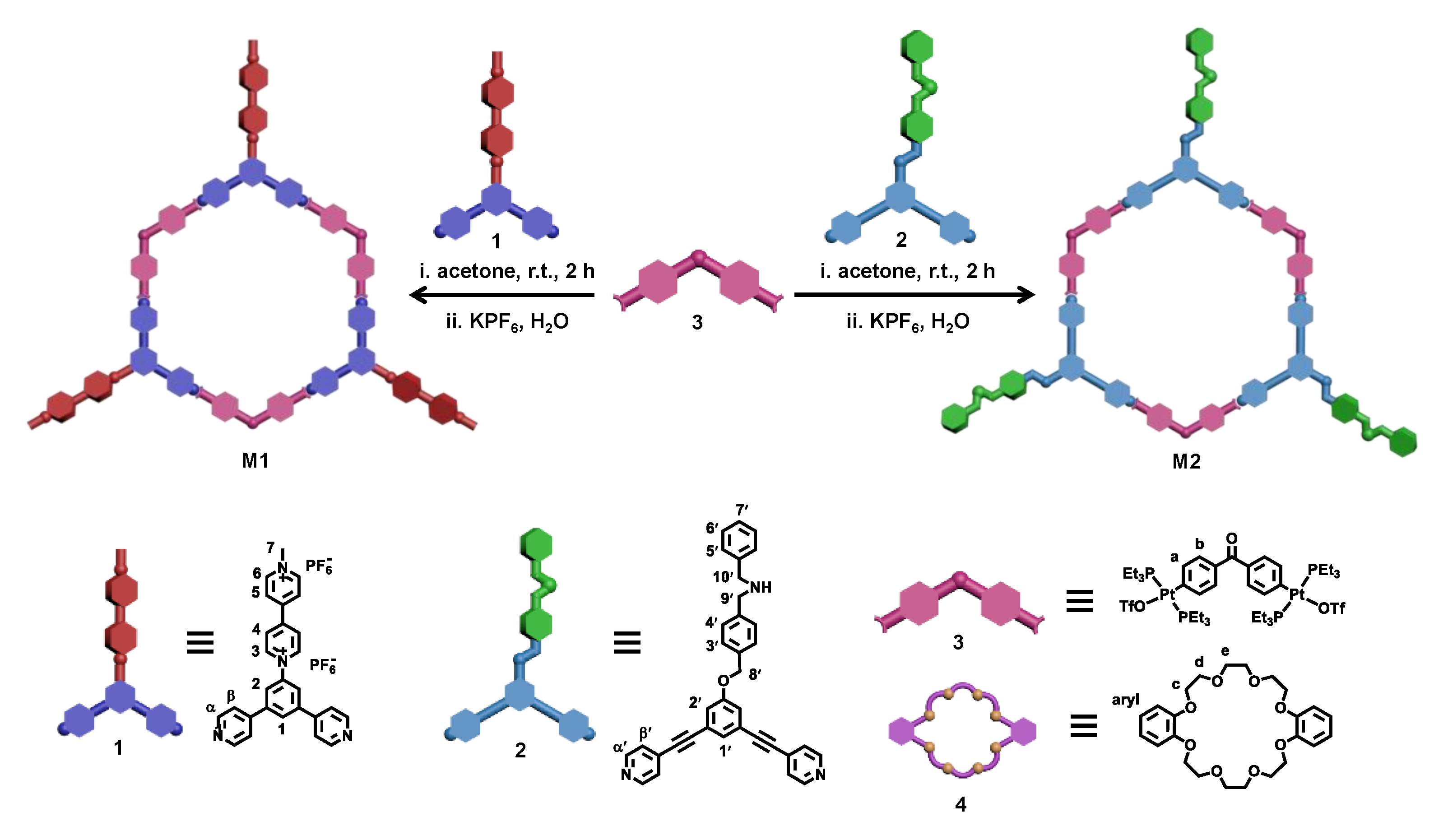
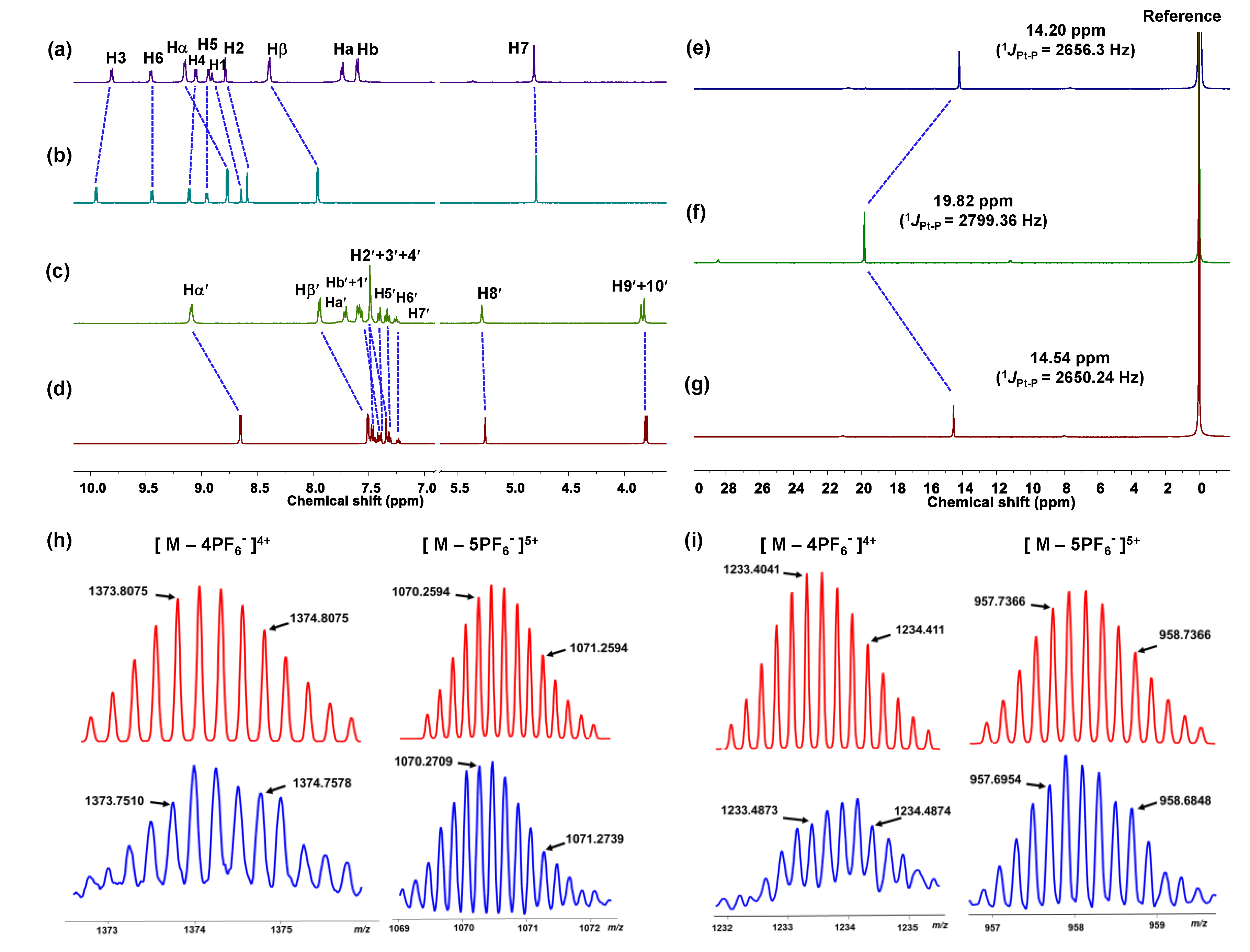
2.1. Synthesis and Characterization
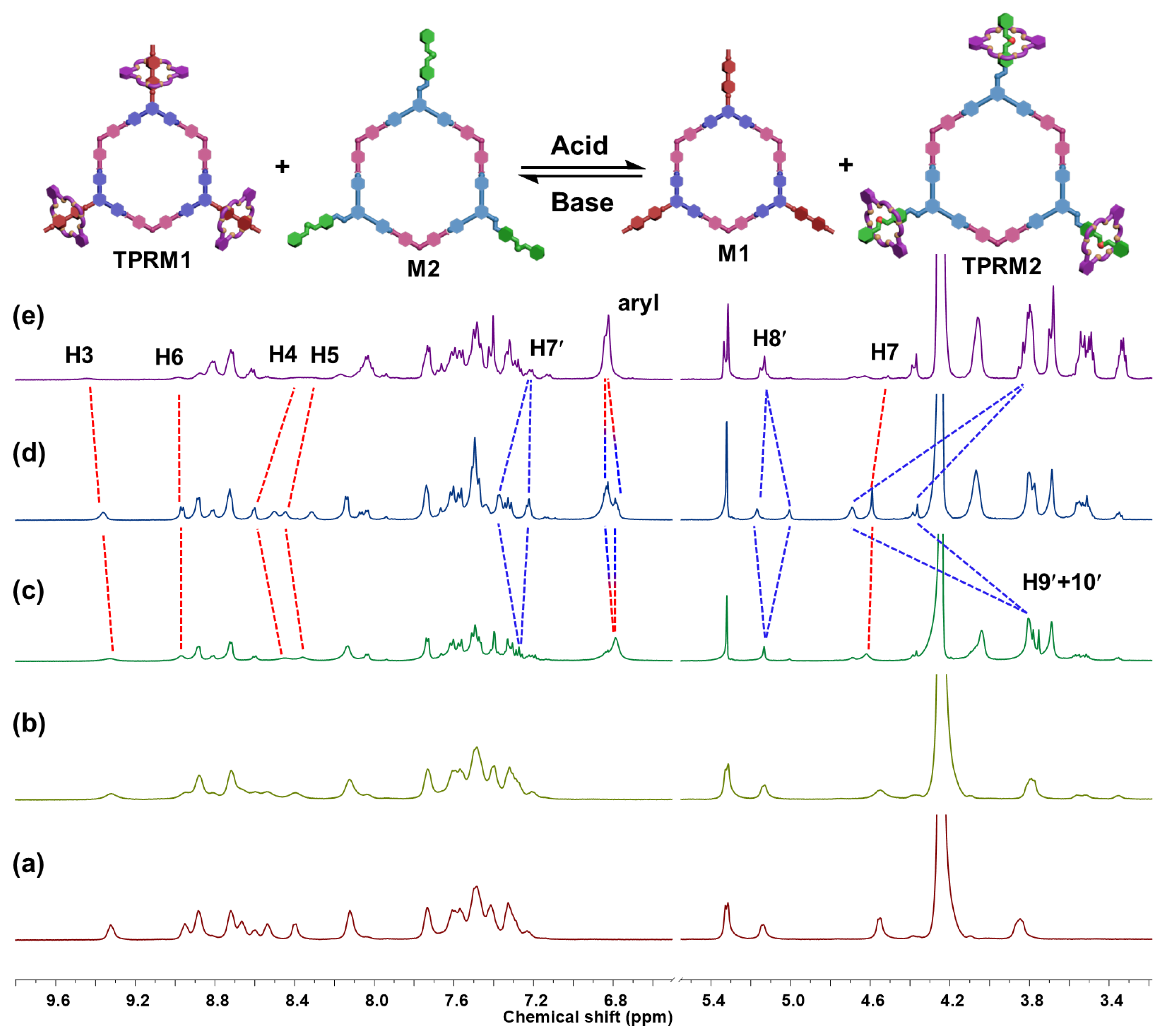
2.2. Self-Sorting Process of Metallacycles M1 and M2
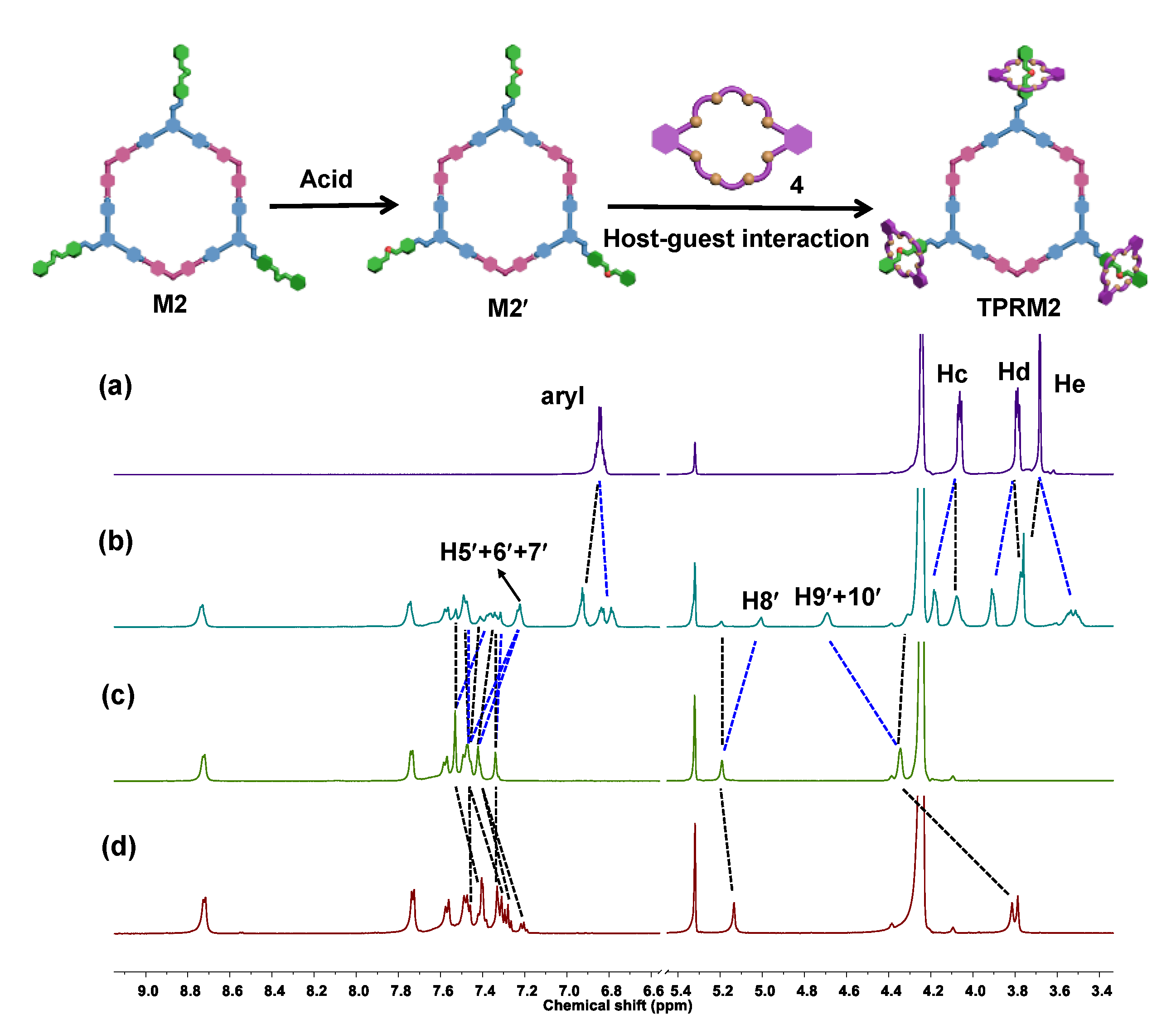
2.3. Tris[2]pseudorotaxanes Metallacycles TPRM1 and TPRM2
2.4. Acid-Activated Motion Switching of DB24C8
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemsitry, 5th ed.; W.H. Freeman: New York, NY, USA, 2003; Volume 34. [Google Scholar]
- Karagiannis, P.; Ishii, Y.; Yanagida, T. Molecular machines like myosin use randomness to behave predictably. Chem. Rev. 2014, 114, 3318–3334. [Google Scholar] [CrossRef] [PubMed]
- Kinbara, K.; Aida, T. Toward intelligent molecular machines: Directed motions of biological and artificial molecules and assemblies. Chem. Rev. 2005, 105, 1377–1400. [Google Scholar] [CrossRef]
- Sluysmans, D.; Stoddart, J.F. Growing community of artificial molecular machinists. Proc. Natl. Acad. Sci. USA 2018, 115, 9359–9361. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Lubbe, A.S.; Štacko, P.; Wezenberg, S.J.; Feringa, B.L. Dynamic control of function by light-driven molecular motors. Nat. Rev. Chem. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Dietrich-Buchecker, C.; Jimenez-Molero, M.C.; Sartor, V.; Sauvage, J.P. Rotaxanes and catenanes as prototypes of molecular machines and motors. Pure Appl. Chem. 2003, 75, 1383–1393. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef]
- Cheng, C.; McGonigal, P.R.; Schneebeli, S.T.; Li, H.; Vermeulen, N.A.; Ke, C.; Stoddart, J.F. An artificial molecular pump. Nat. Nanotechnol. 2015, 10, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, L.; Pezzato, C.; Feng, Y.; Li, W.; Nguyen, M.T.; Cheng, C.; Shen, D.; Guo, Q.-H.; Shi, Y.; et al. A molecular dual pump. J. Am. Chem. Soc. 2019, 141, 17472–17476. [Google Scholar] [CrossRef]
- Collin, J.-P.; Dietrich-Buchecker, C.; Gaviña, P.; Jimenez-Molero, M.C.; Sauvage, J.-P. Shuttles and muscles: Linear molecular machines based on transition metals. Acc. Chem. Res. 2001, 34, 477–487. [Google Scholar] [CrossRef]
- Fang, L.; Hmadeh, M.; Wu, J.; Olson, M.A.; Spruell, J.M.; Trabolsi, A.; Yang, Y.-W.; Elhabiri, M.; Albrecht-Gary, A.-M.; Stoddart, J.F. Acid-base actuation of [c2]daisy chains. J. Am. Chem. Soc. 2009, 131, 7126–7134. [Google Scholar] [CrossRef]
- Iwaso, K.; Takashima, Y.; Harada, A. Fast response dry-type artificial molecular muscles with [c2]daisy chains. Nat. Chem. 2016, 8, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Wang, W.; Wang, X.-Q.; Li, M.; Ke, Y.; Yao, R.; Wen, J.; Yin, G.-Q.; Jiang, B.; Li, X.; et al. Daisy chain dendrimers: Integrated mechanically interlocked molecules with stimuli-induced dimension modulation feature. J. Am. Chem. Soc. 2020, 142, 8473–8482. [Google Scholar] [CrossRef] [PubMed]
- Anelli, P.L.; Spencer, N.; Stoddart, J.F. A molecular shuttle. J. Am. Chem. Soc. 1991, 113, 5131–5133. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, V.N.; Zhu, K.; Baggi, G.; Loeb, S.J. Optical distinction between ″slow″ and ″fast″ translational motion in degenerate molecular shuttles. Angew. Chem. Int. Ed. 2017, 56, 6136–6141. [Google Scholar] [CrossRef]
- Goldup, S.M. Molecular machines swap rings. Nature 2018, 557, 39–40. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, W.-J.; Wang, W.; Wen, J.; Zhang, Y.; Tan, H.; Yang, H.-B. Construction of type III-C rotaxane-branched dendrimers and their anion-induced dimension modulation feature. J. Am. Chem. Soc. 2019, 141, 13923–13930. [Google Scholar] [CrossRef]
- Lewandowski, B.; De Bo, G.; Ward, J.W.; Papmeyer, M.; Kuschel, S.; Aldegunde, M.J.; Gramlich, P.M.E.; Heckmann, D.; Goldup, S.M.; D’Souza, D.M.; et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 2013, 339, 189–193. [Google Scholar] [CrossRef]
- Badjic, J.D.; Balzani, V.; Credi, A.; Silvi, S.; Stoddart, J.F. A molecular elevator. Science 2004, 303, 1845–1849. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.-J.; Wang, X.-Q.; Sun, B.; Li, X.; Zhang, Y.; Shi, J.; Yu, Y.; Zhang, L.; Liu, M.; et al. Organometallic rotaxane dendrimers with fourth-generation mechanically interlocked branches. Proc. Natl. Acad. Sci. USA 2015, 112, 5597–5601. [Google Scholar] [CrossRef]
- Stoddart, J.F. The chemistry of the mechanical bond. Chem. Soc. Rev. 2009, 38, 1802–1820. [Google Scholar] [CrossRef]
- Fang, L.; Olson, M.A.; Benitez, D.; Tkatchouk, E.; Goddard, W.A., III; Stoddart, J.F. Mechanically bonded macromolecules. Chem. Soc. Rev. 2010, 39, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H.W. Stimuli-responsive host-guest systems based on the recognition of cryptands by organic guests. Acc. Chem. Res. 2014, 47, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.Y.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive host-guest functional systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jiang, W.; Schalley, C. Integrative self-sorting: A versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 2015, 44, 779–789. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Han, Y.; Chen, C.-F. pH-Controlled motions in mechanically interlocked molecules. Mater. Chem. Front. 2020, 4, 12–28. [Google Scholar] [CrossRef]
- Kim, K. Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies. Chem. Soc. Rev. 2002, 31, 96–107. [Google Scholar] [CrossRef]
- Pazos, E.; Novo, P.; Peinador, C.; Kaifer, A.E.; García, M.D. Cucurbit[8]uril (CB[8])-based supramolecular switches. Angew. Chem. Int. Ed. 2019, 58, 403–416. [Google Scholar] [CrossRef]
- Yang, H.-B.; Ghosh, K.; Northrop, B.H.; Zheng, Y.-R.; Lyndon, M.M.; Muddiman, D.C.; Stang, P.J. A highly efficient approach to the self-assembly of hexagonal cavity-cored tris[2]pseudorotaxanes from several components via multiple noncovalent interactions. J. Am. Chem. Soc. 2007, 129, 14187–14189. [Google Scholar] [CrossRef][Green Version]
- Price, T.L.; Gibson, H.W. Supramolecular pseudorotaxane polymers from biscryptands and bisparaquats. J. Am. Chem. Soc. 2018, 140, 4455–4465. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.-Y.; Zhang, X.-J.; Song, Z.-H.; Zhao, X.-J.; Liu, Y. Light-controlled reversible formation and dissociation of nanorods via interconversion of pseudorotaxanes. Chem. Commun. 2015, 51, 7329–7332. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Chen, Y.; Huo, X.F.; Wu, X.; Gu, B.H.; Liu, Y. Sulfonato-β-cyclodextrin mediated supramolecular nanoparticle for controlled release of berberine. ACS Appl. Mater Interfaces 2018, 10, 24987–24992. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of pseudorotaxanes and rotaxanes: From synthesis to stimuliresponsive motions to applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Li, W.-J.; Wang, W.; Yang, H.-B. Heterorotaxanes. Chem. Commun. 2018, 54, 13303–13308. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nameki, R.; Sogawa, H.; Takata, T. Macrocyclic dinuclear palladium complex as a novel doubly threaded [3]rotaxane scaffold and its application as a rotaxane cross-linker. Angew. Chem. Int. Ed. 2020, 59, 18023–18028. [Google Scholar] [CrossRef]
- Hwang, W.; Yoo, J.; Hwang, I.-C.; Lee, J.; Ko, Y.H.; Kim, H.W.; Kim, Y.; Lee, Y.; Hur, M.Y.; Parl, K.M.; et al. Hierarchical self-assembly of poly-pseudorotaxanes into artificial microtubules. Angew. Chem. Int. Ed. 2020, 59, 3460–3464. [Google Scholar] [CrossRef]
- Meng, Z.; Xiang, J.-F.; Chen, C.-F. Directional molecular transportation based on a catalytic stopper-leaving rotaxane system. J. Am. Chem. Soc. 2016, 138, 5652–5658. [Google Scholar] [CrossRef]
- Shi, Z.-T.; Yu, J.-J.; Zhang, Q.; Li, M.-M.; Liang, W.-J.; Zhao, C.-X.; Qu, D.-H. Controlling interfacial interactions of supramolecular assemblies by light-responsive overcrowded alkenes. Chem. Commun. 2019, 55, 10292–10295. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Shi, X.L.; Phan, H.; Qu, H.; Hu, Y.-X.; Yin, G.-Q.; Zhao, X.-L.Z.; Li, X.; Xu, L.; Yu, Q.L.; et al. Efficient self-assembly of heterometallic triangular necklace with strong antibacterial activity. Nat. Commun. 2020, 11, 3178. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 2005, 38, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newkome, G.R. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-responsive metal−ligand assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, H.-B. Construction of stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Mukherjee, P.S. Self-assembled discrete molecules for sensing nitroaromatics. Chem. Eur. J. 2015, 21, 6656–6666. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, Z.; Shang, C.; Wang, G.; Wang, Z.; Qi, Y.; Li, Z.-Y.; Wang, H.; Cao, L.; Li, X.; et al. Coordination-driven self-assembled metallacycles incorporating pyrene: Fluorescence mutability, tunability, and aromatic amine sensing. J. Am. Chem. Soc. 2019, 141, 1757–1765. [Google Scholar] [CrossRef]
- Datta, S.; Misra, S.K.; Saha, M.L.; Lahiri, N.; Louie, J.; Pan, D.; Stang, P.J. Orthogonal self-assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8087–8092. [Google Scholar] [CrossRef]
- Li, R.-J.; Holstein, J.J.; Hiller, W.G.; Andréasson, J.; Clever, G.H. Mechanistic interplay between light switching and guest binding in photochromic [Pd2Dithienylethene4] coordination cages. J. Am. Chem. Soc. 2019, 141, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-B.; Xu, L.; Zhu, J.-L.; Wang, Y.-X.; Sun, B.; Li, X.; Yang, H.-B. Real-time monitoring the dynamics of coordination-driven self-assembly by fluorescence-resonance energy transfer. J. Am. Chem. Soc. 2017, 139, 9459–9462. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, M.; Sartin, M.M.; Sei, Y.; Akita, M.; Takeuchi, S.; Tahara, T.; Yoshizawa, M. Preparation of highly fluorescent host-guest complexes with tunable color upon encapsulation. J. Am. Chem. Soc. 2015, 137, 9266–9269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Shi, B.; Xing, H.; Sun, Y.; Lu, S.; Shangguan, L.; Li, X.; Huang, F.; Stang, P.J. Formation of planar chiral platinum triangles via pillar[5]arene for circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 17340–17345. [Google Scholar] [CrossRef]
- Hong, C.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Self-assembled tetrahedral hosts as supramolecular catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. [Google Scholar] [CrossRef]
- Chen, L.-J.; Chen, S.; Qin, Y.; Xu, L.; Yin, G.-Q.; Zhu, J.-L.; Zhu, F.-F.; Zheng, W.; Li, X.; Yang, H.-B. Construction of porphyrin-containing metallacycle with improved stability and activity within mesoporous carbon. J. Am. Chem. Soc. 2018, 140, 5049–5052. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Hao, X.; Xu, L.; Xie, X.; Xiong, B.; Hu, Z.; Sun, H.; Yin, G.-Q.; Li, X.; Peng, H.; et al. Construction of supramolecular liquid-crystalline metallacycles for holographic storage of colored images. J. Am. Chem. Soc. 2020, 142, 6285–6294. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Sun, Y.; Lu, S.; Huo, H.; Tan, T.; Li, A.; Li, X.; Ungar, G.; Liu, F.; et al. Luminescent metallacycle-cored liquid crystals induced by metal coordination. Angew. Chem. Int. Ed. 2020, 59, 10143–10150. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Sun, B.; Chen, L.-J.; Xu, X.-D.; Wang, M.; Li, X.; Yu, Y.; Jiang, W.; Yang, H.-B. The construction of complex multicomponent supramolecular systems via the combination of orthogonal self-assembly and the self-sorting approach. Chem. Sci. 2014, 5, 4554–4560. [Google Scholar] [CrossRef]
- Yin, H.; Rosas, R.; Gigmes, D.; Ouari, O.; Wang, R.; Kermagoret, A.; Bardelang, D. Metal actuated ring translocation switches in water. Org. Lett. 2018, 20, 3187–3191. [Google Scholar] [CrossRef]
- Samanta, S.K.; Moncelet, D.; Briken, V.; Isaacs, L. Metal–organic polyhedron capped with cucurbit[8]uril delivers doxorubicin to cancer cells J. Am. Chem. Soc. 2016, 138, 14488–14496. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-B.; Hawkridge, A.M.; Huang, S.D.; Das, N.; Bunge, S.D.; Muddiman, D.C.; Stang, P.J. Coordination-driven self-assembly of metallodendrimers possessing well-defined and controllable cavities as cores J. Am. Chem. Soc. 2007, 129, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Amirsakis, D.G.; Elizarov, A.M.; Garcia-Garibay, M.A.; Glink, P.T.; Stoddart, J.F.; White, A.J.; Williams, D.J.; Amirsakis, D.G.; Elizarov, A.M.; Garcia-Garibay, M.A.; et al. Diastereospecific Photochemical Dimerization of a Stilbene-Containing Daisy Chain Monomer in Solution as well as in the Solid State. Angew. Chem. Int. Ed. 2003, 42, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Yang, H.-B.; Northrop, B.H.; Lyndon, M.M.; Zheng, Y.-R.; Muddiman, D.C.; Stang, P.J. Coordination-driven self-assembly of cavity-cored multiple crown ether derivatives and poly[2]pseudorotaxanes. J. Am. Chem. Soc. 2008, 130, 5320–5334. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-X.; Wu, G.-Y.; Wang, X.-Q.; Yin, G.-Q.; Zhang, C.-W.; Li, X.; Xu, L.; Yang, H.-B. Acid-Activated Motion Switching of DB24C8 between Two Discrete Platinum(II) Metallacycles. Molecules 2021, 26, 716. https://doi.org/10.3390/molecules26030716
Hu Y-X, Wu G-Y, Wang X-Q, Yin G-Q, Zhang C-W, Li X, Xu L, Yang H-B. Acid-Activated Motion Switching of DB24C8 between Two Discrete Platinum(II) Metallacycles. Molecules. 2021; 26(3):716. https://doi.org/10.3390/molecules26030716
Chicago/Turabian StyleHu, Yi-Xiong, Gui-Yuan Wu, Xu-Qing Wang, Guang-Qiang Yin, Chang-Wei Zhang, Xiaopeng Li, Lin Xu, and Hai-Bo Yang. 2021. "Acid-Activated Motion Switching of DB24C8 between Two Discrete Platinum(II) Metallacycles" Molecules 26, no. 3: 716. https://doi.org/10.3390/molecules26030716
APA StyleHu, Y.-X., Wu, G.-Y., Wang, X.-Q., Yin, G.-Q., Zhang, C.-W., Li, X., Xu, L., & Yang, H.-B. (2021). Acid-Activated Motion Switching of DB24C8 between Two Discrete Platinum(II) Metallacycles. Molecules, 26(3), 716. https://doi.org/10.3390/molecules26030716




