Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases
Abstract
1. Introduction
2. Cholinesterase Inhibitory Potential of Natural-Product-Derived Alkaloids
3. Multi-Target Modulation Potential of Alkaloids in Neurodegenerative Diseases
3.1. Neuroprotection
3.2. Neuroinflammation
3.3. Neurogenesis
3.4. Aggregation of Amyloid Beta
3.5. Tau Hyperphosphorylation
4. Physicochemical Analysis of the Alkaloids
5. Method
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Srivastava, A.; Srivastava, P.; Dhuriya, Y.K.; Pandey, A.; Kumar, D.; Rajpurohit, C.S. Advances in Stem Cell Research- A Ray of Hope in Better Diagnosis and Prognosis in Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. EPIDEMIOLOGY OFNEURODEGENERATION. Annu. Rev. Neurosci. 2003, 26, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Nichols, E.; E I Szoeke, C.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; O Akinyemi, R.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialog Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Tarawneh, R.; Holtzman, D.M. The Clinical Problem of Symptomatic Alzheimer Disease and Mild Cognitive Impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef]
- Moneim, A.E.A. Oxidant/Antioxidant Imbalance and the Risk of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson s disease: A review. Front. Biosci. 2014, S6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.R. Etiology and pathogenesis of Alzheimer’s disease. Am. J. Heal. Pharm. 1998, 55, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J.-W. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kanhar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef] [PubMed]
- Grieg, N.H.; Kamal, M.A.; Jabir, N.R.; Tabrez, S.; Nasim, F.H.; Abuzenadah, A.M.; Aliev, G. Chapter 6—Specific Cholinesterase Inhibitors: A Potential Tool to Assist in Management of Alzheimer Disease. In Drug Design and Discovery in Alzheimer’s Disease; Atta ur, R., Choudhary, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 366–386. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain 1999, 122, 383–404. [Google Scholar] [CrossRef]
- Martorana, A.; Esposito, Z.; Koch, G. Beyond the Cholinergic Hypothesis: Do Current Drugs Work in Alzheimer’s Disease? CNS Neurosci. Ther. 2010, 16, 235–245. [Google Scholar] [CrossRef]
- Fadaeinasab, M.; Basiri, A.; Kia, Y.; Karimian, H.; Ali, H.M.; Murugaiyah, V. New Indole Alkaloids from the Bark of Rauvolfia Reflexa and their Cholinesterase Inhibitory Activity. Cell. Physiol. Biochem. 2015, 37, 1997–2011. [Google Scholar] [CrossRef]
- Liew, S.Y.; Khaw, K.; Murugaiyah, V.; Looi, C.Y.; Wong, Y.L.; Mustafa, M.R.; Litaudon, M.; Awang, K. Natural indole butyrylcholinesterase inhibitors from Nauclea officinalis. Phytomedicine 2015, 22, 45–48. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Liu, W.; Chou, G.; Wang, Z.; Wang, C. Potent AChE and BChE inhibitors isolated from seeds of Peganum harmala Linn by a bioassay-guided fractionation. J. Ethnopharmacol. 2015, 168, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.-X.; Song, X.-J.; Li, S.-; Fan, C.-L.; Chen, G.-D.; Hu, D.; Yao, X.-S.; Gao, H. A new cinnamamide derivative and two new β-carboline alkaloids from the stems of Picrasma quassioides. Fitoterapia 2019, 139, 104375. [Google Scholar] [CrossRef] [PubMed]
- Plazas, E.; Hagenow, S.; Murillo, M.A.; Stark, H.; Cuca, L.E.; Plazas, E.; Suarez, L.C. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorganic Chem. 2020, 98, 103722. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.Q.; Ngo, Q.-M.T.; Seong, S.H.; Youn, U.J.; Kim, J.A.; Kim, J.; Kim, J.-C.; Woo, M.H.; Choi, J.S.; Min, B.S. Cholinesterase inhibitory alkaloids from the rhizomes of Coptis chinensis. Bioorganic Chem. 2018, 77, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-W.; Cai, L.; Fang, Y.-S.; Xiao, H.; Li, Z.-J.; Ding, Z. Proaporphine and aporphine alkaloids with acetylcholinesterase inhibitory activity from Stephania epigaea. Fitoterapia 2015, 104, 102–107. [Google Scholar] [CrossRef]
- Mollataghi, A.; Coudiere, E.; Hadi, A.H.A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef]
- Zhan, G.; Zhou, J.; Liu, J.; Huang, J.; Zhang, H.; Liu, R.; Yao, G. Acetylcholinesterase Inhibitory Alkaloids from the Whole Plants of Zephyranthes carinata. J. Nat. Prod. 2017, 80, 2462–2471. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Jitonnom, J.; Komek, M.; Ritthiwigrom, T.; Laphookhieo, S. Acetylcholinesterase inhibitory activity and molecular docking study of steroidal alkaloids from Holarrhena pubescens barks. Steroids 2016, 108, 92–98. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Feng, Y.-D.; Lu, X.; Nie, J.-B.; Li, W.; Wang, L.-N.; Tian, L.-J.; Liu, Q.-H. Isosteroidal alkaloids as potent dual-binding site inhibitors of both acetylcholinesterase and butyrylcholinesterase from the bulbs of Fritillaria walujewii. Eur. J. Med. Chem. 2017, 137, 280–291. [Google Scholar] [CrossRef]
- Botić, T.; Defant, A.; Zanini, P.; Žužek, M.C.; Frangež, R.; Janussen, D.; Kersken, D.; Knez, Ž.; Mancini, I.; Sepčić, K. Discorhabdin alkaloids from Antarctic Latrunculia spp. sponges as a new class of cholinesterase inhibitors. Eur. J. Med. Chem. 2017, 136, 294–304. [Google Scholar] [CrossRef]
- Nilsu, T.; Thorroad, S.; Ruchirawat, S.; Thasana, N. Squarrosine A and Pyrrolhuperzine A, New Lycopodium Alkaloids from Thai and Philippine Huperzia squarrosa. Planta Med. 2016, 82, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Nilsu, T.; Thaisaeng, W.; Thamnarak, W.; Eurtivong, C.; Jumraksa, A.; Thorroad, S.; Khunnawutmanotham, N.; Ruchirawat, S.; Thasana, N. Three Lycopodium alkaloids from Thai club mosses. Phytochemistry 2018, 156, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, P.-S.; Ren, Q.; Chen, X.; Zhou, G.; Li, D.; Li, X.-M.; Xu, K.-P.; Yu, X.; Tan, G.-S. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their cholinesterase inhibitory activities. Fitoterapia 2018, 130, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Ke, J.-P.; Zhang, P.; Yang, Z.; Bao, G.-H. Novel Cinnamoylated Flavoalkaloids Identified in Tea with Acetylcholinesterase Inhibition Effect. J. Agric. Food Chem. 2020, 68, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.A.; Ramli, A.N.M.; Yusoff, M. Indole Alkaloids from Plants as Potential Leads for Antidepressant Drugs: A Mini Review. Front. Pharmacol. 2017, 8, 96. [Google Scholar] [CrossRef]
- Passos, C.D.S.; Simoes-Pires, C.; Henriques, A.; Cuendet, M.; Carrupt, P.-A.; Christen, P. Chapter 4—Alkaloids as Inhibitors of Monoamine Oxidases and Their Role in the Central Nervous System. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 123–144. [Google Scholar]
- Kukula-Koch, W.; Widelski, J. Chapter 9—Alkaloids. Pharmacognosy 2017, 163–198. [Google Scholar] [CrossRef]
- Shamma, M.; Slusarchyk, W.A. The Aporphine Alkaloids. Chem. Rev. 1964, 64, 59–79. [Google Scholar] [CrossRef]
- Dong, J.-W.; Cai, L.; Li, X.-J.; Wang, J.-P.; Mei, R.-F.; Ding, Z. Monoterpene esters and aporphine alkaloids from Illigera aromatica with inhibitory effects against cholinesterase and NO production in LPS-stimulated RAW264.7 macrophages. Arch. Pharmacal Res. 2017, 40, 1394–1402. [Google Scholar] [CrossRef]
- Kostelnik, A.; Pohanka, M. Inhibition of Acetylcholinesterase and Butyrylcholinesterase by a Plant Secondary Metabolite Boldine. BioMed Res. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2013, 30, 849–868. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, M.-W.; Cheng, K.-J.; Yu, P.-Z.; Wei, X.; Shi, Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 2015, 36, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, H.; Kita, Y. Marine Pyrroloiminoquinone Alkaloids, Makaluvamines and Discorhabdins, and Marine Pyrrole-Imidazole Alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 251–283. [Google Scholar] [CrossRef]
- Kobayashi, J.; Morita, H. The Lycopodium Alkaloids. Alkaloids Chem. Biol. 2005, 61, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gang, D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004, 21, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.M.; Calvert, M.B.; Sperry, J. Flavoalkaloids—Isolation, Biological Activity, and Total Synthesis. Alkaloids Chem. Biol. 2017, 77, 85–115. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Seidl, S.E.; Potashkin, J.A. The Promise of Neuroprotective Agents in Parkinson’s Disease. Front. Neurol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Li, X.; Yu, H.-Y.; Xiong, Y.-F.; Zhang, P.; Pi, H.-F.; Ruan, H. Alkaloids from the bulbs of Lycoris longituba and their neuroprotective and acetylcholinesterase inhibitory activities. Arch. Pharmacal. Res. 2015, 38, 604–613. [Google Scholar] [CrossRef]
- Park, T.H.; Kwon, O.; Park, S.Y.; Han, E.S.; Lee, C.S. N-methylated β-carbolines protect PC12 cells from cytotoxic effect of MPP+ by attenuation of mitochondrial membrane permeability change. Neurosci. Res. 2003, 46, 349–358. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, Y.Y.; Han, E.S.; Lee, C.S. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur. J. Neurosci. 2001, 13, 1861–1872. [Google Scholar] [CrossRef]
- Lee, C.S.; Han, E.S.; Jang, Y.Y.; Han, J.H.; Ha, H.W.; Kim, D.E. Protective Effect of Harmalol and Harmaline on MPTP Neurotoxicity in the Mouse and Dopamine-Induced Damage of Brain Mitochondria and PC12 Cells. J. Neurochem. 2002, 75, 521–531. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Pezzella, A.; Prota, G. New Reaction Pathways of Dopamine under Oxidative Stress Conditions: Nonenzymatic Iron-Assisted Conversion to Norepinephrine and the Neurotoxins 6-Hydroxydopamine and 6,7-Dihydroxytetrahydroisoquinoline. Chem. Res. Toxicol. 1999, 12, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, C.S.; Ko, H.H.; Song, J.H.; Han, E.S. Effect of R-(-)-deprenyl and harmaline on dopamine- and peroxynitrite-induced membrane permeability transition in brain mitochondria. Neurochem. Res. 2002, 27, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Othman, W.N.N.W.; Sivasothy, Y.; Liew, S.Y.; Bin Mohamad, J.; Nafiah, M.A.; Ahmad, K.; Litaudon, M.; Awang, K. Alkaloids from Cryptocarya densiflora Blume (Lauraceae) and their cholinesterase inhibitory activity. Phytochem. Lett. 2017, 21, 230–236. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Yu, X.; Na, S.; Li, K.; Yang, Z.; Xie, X.; Yang, J.; Yue, J. The Protective Role of Brain CYP2J in Parkinson’s Disease Models. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Sattler, R.; Yang, E.J.; Nunes, A.; Ayukawa, Y.; Akhtar, S.; Ji, G.; Zhang, P.-W.; Rothstein, J.D. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology 2011, 60, 1168–1175. [Google Scholar] [CrossRef]
- Ouyang, Y.-B.; Xu, L.; Liu, S.; Giffard, R.G. Role of Astrocytes in Delayed Neuronal Death: GLT-1 and its Novel Regulation by MicroRNAs. In Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain; Parpura, V., Schousboe, A., Verkhratsky, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 171–188. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Hallak, J.E.C. Effects of the Naturalβ-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies. J. Psychoact. Drugs 2017, 49, 1–10. [Google Scholar] [CrossRef]
- Cai, C.-Z.; Zhou, H.-F.; Yuan, N.-N.; Wu, M.-Y.; Lee, S.M.-Y.; Ren, J.-Y.; Su, H.-X.; Lu, J.-J.; Chen, X.; Li, M.; et al. Natural alkaloid harmine promotes degradation of alpha-synuclein via PKA-mediated ubiquitin-proteasome system activation. Phytomedicine 2019, 61, 152842. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef]
- Han, Y.-S.; Kim, J.-M.; Cho, J.-S.; Lee, C.S.; Kim, D.-E. Comparison of the Protective Effect of Indole β-carbolines and R-(-)-deprenyl Against Nitrogen Species-Induced Cell Death in Experimental Culture Model of Parkinson’s Disease. J. Clin. Neurol. 2005, 1, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Negahdar, F.; Mehdizadeh, M.; Joghataei, M.T.; Roghani, M.; Mehraeen, F.; Poorghayoomi, E. Berberine chloride pretreatment exhibits neuroprotective effect against 6-hydroxydopamine-induced neuronal insult in rat. Iran. J. Pharm. Res. IJPR 2015, 14, 1145–1152. [Google Scholar]
- Azam, S.; Al Mamun, A.; Kabir, T.; Ahmad, J.; Jeandet, P.; Sarwar, S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef]
- Deng, H.; Jia, Y.; Pan, D.; Ma, Z. Berberine alleviates rotenone-induced cytotoxicity by antioxidation and activa-tion of PI3K/Akt signaling pathway in SH-SY5Y cells. Neuroreport 2020, 31, 41–47. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, T.; Lu, Q.; Xu, K.; He, J.; Xie, L.; Chen, Z.; Zheng, Z.; Ye, L.; Xu, K.; et al. Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cell. Mol. Neurobiol. 2019, 40, 587–602. [Google Scholar] [CrossRef]
- Krauss, R.; Bosanac, T.; Devraj, R.; Engber, T.; Hughes, R.O. Axons Matter: The Promise of Treating Neurodegenerative Disorders by Targeting SARM1-Mediated Axonal Degeneration. Trends Pharmacol. Sci. 2020, 41, 281–293. [Google Scholar] [CrossRef]
- Loring, H.S.; Parelkar, S.S.; Mondal, S.; Thompson, P.R. Identification of the first noncompetitive SARM1 inhibitors. Bioorganic Med. Chem. 2020, 28, 115644. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Wang, J.; Gong, J.; Shi, J.; Yu, S. Berberine Induces CYP2J2 Expression in Human U251 Glioma Cells via Regulation of Peroxisome Proliferator-Activated Receptor Alpha. Pharmacology 2019, 105, 360–368. [Google Scholar] [CrossRef]
- Bonan, C. Ectonucleotidases and nucleotide/nucleoside transporters as pharmacological targets for neurological disorders. CNS Neurol Disord Drug Targets 2012, 11, 739–750. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Abdalla, F.H.; Dornelles, G.L.; Palma, T.V.; Signor, C.; Bernardi, J.D.S.; Baldissarelli, J.; Lenz, L.S.; De Oliveira, V.A.; Schetinger, M.R.C.; et al. Neuroprotective effects of berberine on recognition memory impairment, oxidative stress, and damage to the purinergic system in rats submitted to intracerebroventricular injection of streptozotocin. Psychopharmacology 2019, 236, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, Q.; Zhou, X.; He, W.; Yi, H.; Yin, X.; Gearing, M.; Levey, A.; Yan, R. Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 2007, 26, 2755–2767. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Von Bernhardi, R.; Giaume, C.; Sáez, J.C. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev. Neurosci. 2012, 23, 163–177. [Google Scholar] [CrossRef]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer’s disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.C.; Kwon, O.; Han, E.S.; Song, J.H.; Shin, Y.K.; Lee, C.S. Protective effect of boldine on dopamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells. Biochem. Pharmacol. 2002, 63, 495–505. [Google Scholar] [CrossRef]
- Deng, G.; Wu, C.; Rong, X.; Li, S.; Ju, Z.; Wang, Y.; Ma, C.; Ding, W.; Guan, H.; Cheng, X.; et al. Ameliorative effect of deoxyvasicine on scopolamine-induced cognitive dysfunction by restoration of cholinergic function in mice. Phytomedicine 2019, 63, 153007. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Kinney, J.W.; BeMiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.; Wang, Y.; Su, X.; Li, Y.; Yan, M.; Ma, L.; Che, H. Harmine Ameliorates Cognitive Impairment by Inhibiting NLRP3 Inflammasome Activation and Enhancing the BDNF/TrkB Signaling Pathway in STZ-Induced Diabetic Rats. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Wei, Y.; Liu, W.; Huang, F.; Shi, H.; Zhang, B.; Wu, X.; Wang, C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015, 768, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Hussien, H.M.; Abd, E.-M.N.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.-J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; SedighNejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult Neurogenesis in Neurodegenerative Diseases: Figure 1. Cold Spring Harb. Perspect. Biol. 2015, 7, a021287. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Revenga, M.D.L.F.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis In Vitro. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Yano, M.; Nakashima, S.; Oda, Y.; Nakamura, S.; Matsuda, H. BBB-permeable aporphine-type alkaloids in Nelumbo nucifera flowers with accelerative effects on neurite outgrowth in PC-12 cells. J. Nat. Med. 2020, 74, 212–218. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Tencomnao, T. Amyloidosis in Alzheimer’s Disease: The Toxicity of Amyloid Beta (Aβ), Mechanisms of Its Accumulation and Implications of Medicinal Plants for Therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Panahi, N.; Mahmoudian, M.; Mortazavi, P.; Hashjin, G.S. Experimental research Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med Sci. 2013, 1, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; He, W.; Chen, Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting β/γ-Secretases Activity and Enhancing α-Secretases. Curr. Alzheimer Res. 2018, 15, 1045–1052. [Google Scholar] [CrossRef]
- Fawver, J.N.; Duong, K.T.; Wise-Scira, O.; Chapa, R.P.; Schall, H.E.; Coskuner, O.; Zhu, X.; Colom, L.V.; Murray, I.V.; Coskuner-Weber, O. Probing and Trapping a Sensitive Conformation: Amyloid-β Fibrils, Oligomers, and Dimers. J. Alzheimer Dis. 2012, 32, 197–215. [Google Scholar] [CrossRef]
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2020, 81, 165–183. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Abid, M.D.N.; Yan, H.; Huang, H.; Wan, L.; Feng, Z.; Chen, J. Berberine Attenuates Axonal Transport Impairment and Axonopathy Induced by Calyculin A in N2a Cells. PLoS ONE 2014, 9, e93974. [Google Scholar] [CrossRef]
- Yu, G.; Li, Y.; Tian, Q.; Liu, R.; Wang, Q.; Wang, J.-Z.; Wang, X. Berberine Attenuates Calyculin A-Induced Cytotoxicity and Tau Hyperphosphorylation in HEK293 Cells. J. Alzheimer Dis. 2011, 24, 525–535. [Google Scholar] [CrossRef]

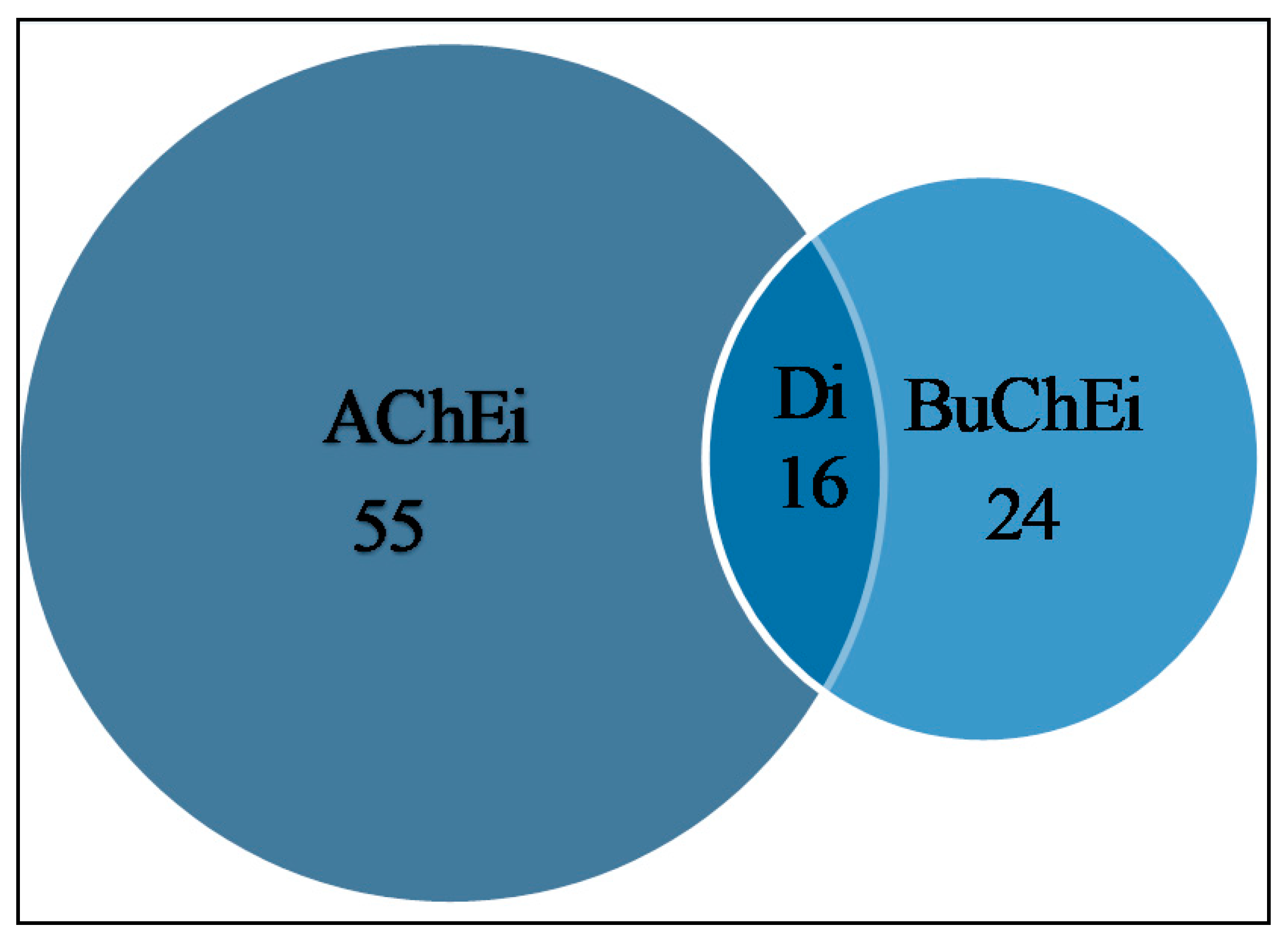
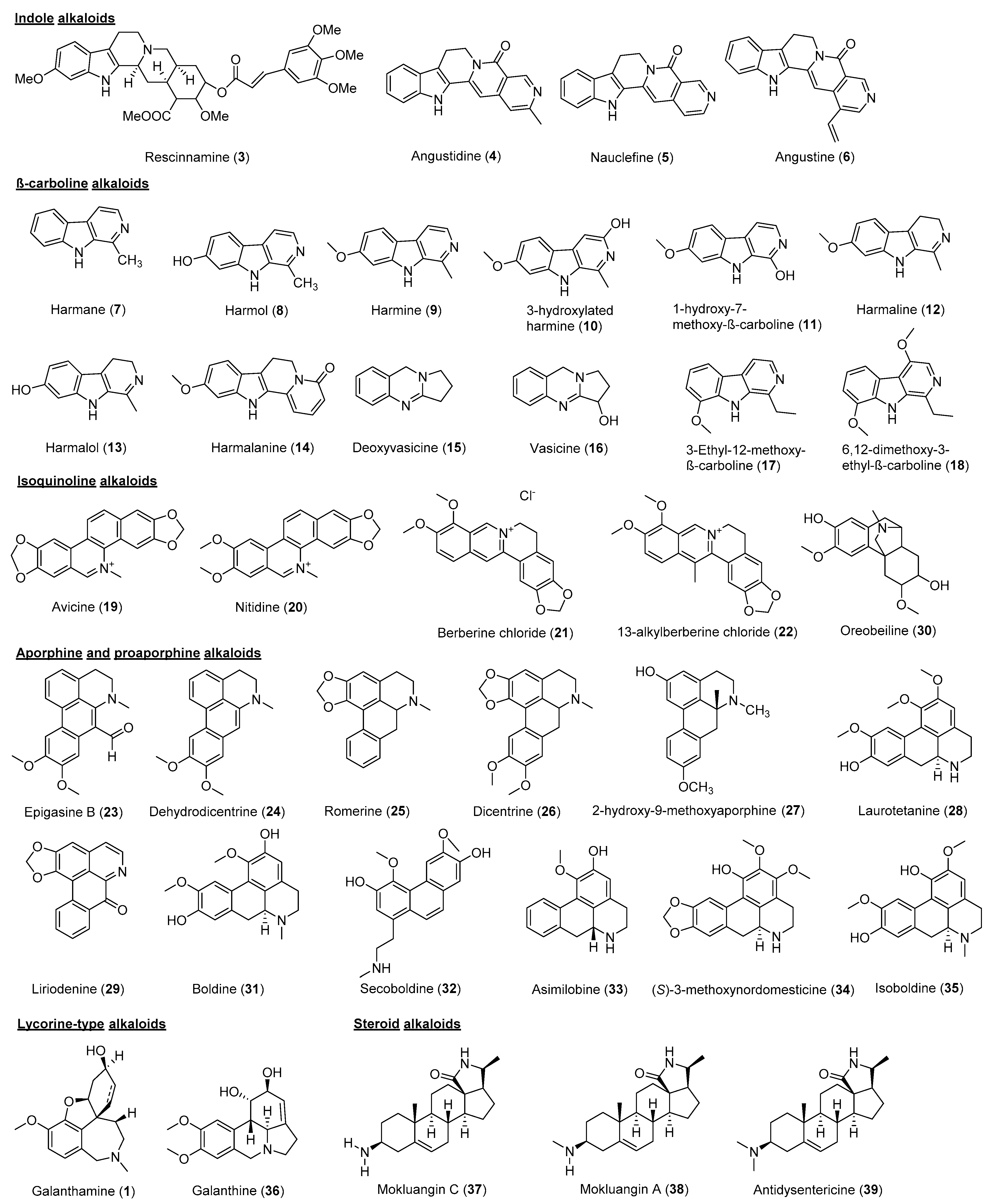
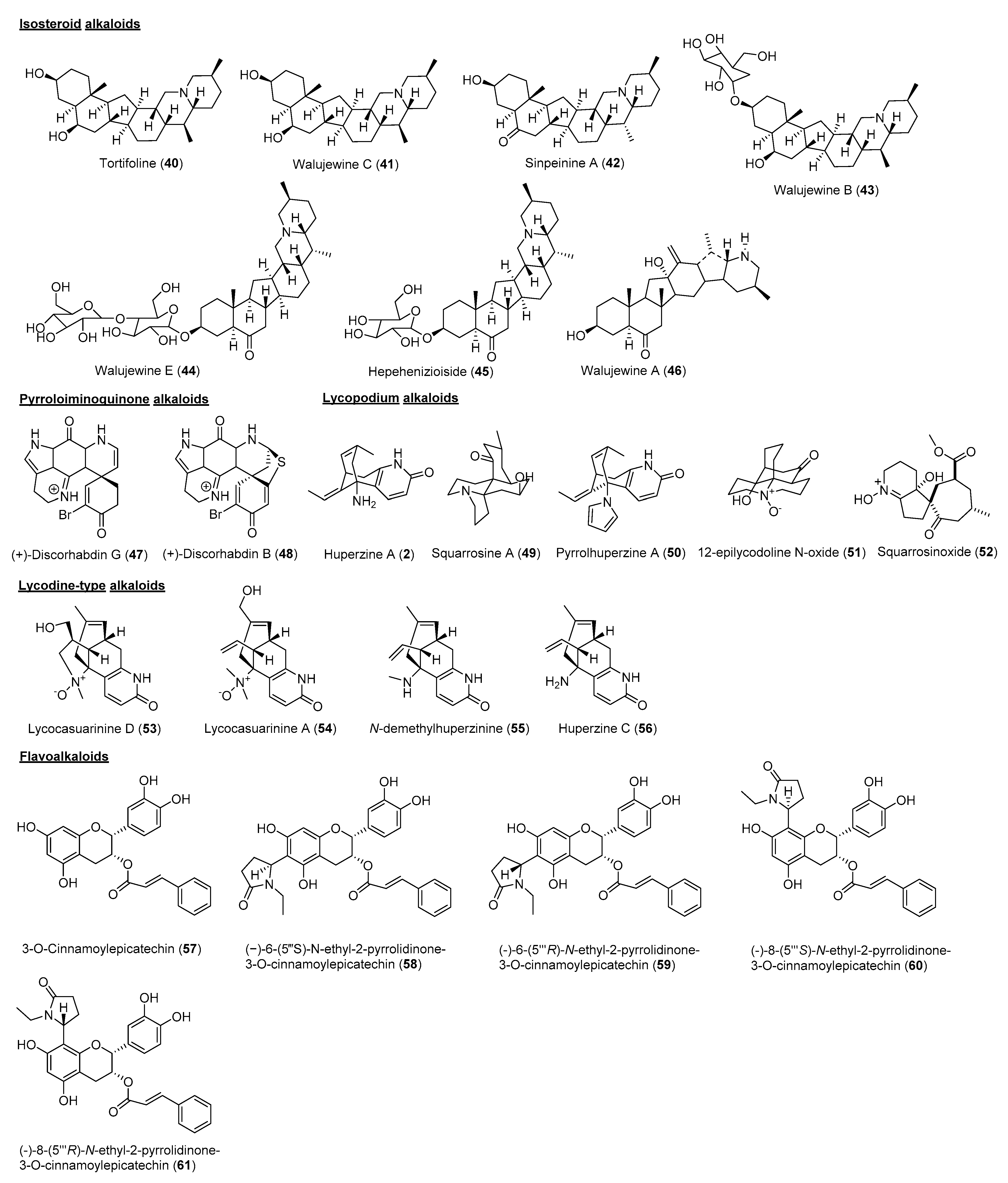

| Species | Part | Alkaloid Class | Chemical Constituent | IC50 (μM) | References | |
|---|---|---|---|---|---|---|
| AChE | BuChE | |||||
| Rauvolfia reflexa | Bark | Indole | Rescinnamine (3) | >10 | 8.06 | [20] |
| Nauclea officinalis | Bark | Indole β-carboline | Angustidine (4) | >10 | 1.03 | [21] |
| Nauclefine (5) | - | 7.70 | ||||
| Angustine (6) | - | 4.98 | ||||
| Peganum harmala | Seed | Indole β-carboline | Harmane (7) | 3.64 | 1.04 | [22] |
| Harmol (8) | 1.90 | 0.35 | ||||
| Harmine (9) | 1.21 | 2.79 | ||||
| 3-Hydroxylated harmine (10) | >10 | 3.25 | ||||
| 1-Hydroxy-7-methoxy-β-carboline (11) | 7.19 | 5.15 | ||||
| Harmaline (12) | 1.95 | 5.38 | ||||
| Harmalol (13) | 3.45 | 0.66 | ||||
| Harmalanine (14) | >10 | 3.24 | ||||
| β-carboline | Deoxyvasicine (15) | 2.37 | 0.04 | |||
| Vasicine (16) | 3.38 | 0.10 | ||||
| Picrasma quassioide | Stem | β-carboline | 3-Ethyl-12-methoxy-β-carboline (17) | 6.37 | - | [23] |
| 6,12-Dimethoxy-3-ethyl-β-carboline (18) | 9.01 | - | ||||
| Zanthoxylum rigidum | Root | Isoquinoline | Avicine (19) | 0.15 | 0.88 | [24] |
| Nitidine (20) | 0.65 | 5.73 | ||||
| Coptis chinensis | Rhizome | Isoquinoline | Berberine chloride (21) | 1.1 | > 10 | [25] |
| 13-alkylberberine (22) | 5.6 | > 10 | ||||
| Stephania epigaea | Root | Proaporphine Aporphine | Epigasine B (23) | 4.36 | - | [26] |
| Dehydrodicentrine (24) | 2.98 | - | ||||
| Romerine (25) | 8.32 | - | ||||
| Dicentrine (26) | 6.6 | - | ||||
| Beilschmiedia alloiophylla Beilschmiedia kunstleri | Bark | Aporphine | 2-Hydroxy-9-methoxyaporphine (27) | 2.0 | - | [27] |
| Laurotetanine (28) | 3.2 | - | ||||
| Oxoaporphine | Liriodenine (29) | 3.5 | - | |||
| Morphinan (isoquinoline) | Oreobeiline (30) | 5.0 | - | |||
| Aporphine | Boldine (31) | 8.5 | - | |||
| Secoboldine (32) | 10.0 | - | ||||
| Asimilobine (33) | 8.7 | - | ||||
| (S)-3-Methoxynordomesticine (34) | 10.0 | - | ||||
| Isoboldine (35) | 9.4 | - | ||||
| Zephyranthes carinata | Whole plant | Lycorine-type | Galanthine (36) | 6.10 | - | [28] |
| Galanthamine (1) | 1.27 | - | ||||
| Holarrhena pubescens | Bark | Steroid | Mokluangin C (37) | 1.44 | - | [29] |
| Mokluangin A (38) | 2.12 | - | ||||
| Antidysentericine (39) | 4.09 | - | ||||
| Fritillaria walujewii | Bulb | Isosteroid | Tortifoline (40) | 5.8 | 2.08 | [30] |
| Walujewine C (41) | 7.2 | 2.58 | ||||
| Sinpeinine A (42) | 8.3 | 3.05 | ||||
| Walujewine B (43) | >10 | 3.89 | ||||
| Walujewine E (44) | 9.8 | 5.71 | ||||
| Hepehenizioiside (45) | >10 | 6.80 | ||||
| Walujewine A (46) | 7.6 | >10 | ||||
| Antarctic Latrunculia spp. | Sponge | Pyrroloiminoquinone | (+)-Discorhabdin G (47) | 1.3 | - | [31] |
| (+)-Discorhabdin B (48) | 5.7 | - | ||||
| Huperzia squarrosa | Aerial | Lycopodium | Squarrosine A (49) | 7.3 | - | [32] |
| Pyrrolhuperzine A (50) | 8.91 | - | ||||
| 12-epilycodoline N-oxide (51) | 0.59 | - | ||||
| Huperzine A (2) | 0.01 | - | ||||
| Huperzia squarrosa | Whole plant | Lycopodium | Squarrosinoxide (52) | 3.12 | - | [33] |
| Huperzine A (2) | 0.034 | - | ||||
| Lycopodiastrum casuarinoides | Aerial part | Lycodine-type | Lycocasuarinine D (53) | 0.22 | >10 | [34] |
| Lycocasuarinine A (54) | 4.74 | >10 | ||||
| N-demethylhuperzinine (55) | 0.89 | 1.86 | ||||
| Huperzine C (56) | 0.37 | 7.33 | ||||
| Camellia sinensisvar | Leaf | Flavoalkaloid | 3-O-Cinnamoylepicatechin (57) | 1.0 | - | [35] |
| (−)-6-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (58) | 0.14 | - | ||||
| (−)-6-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (59) | 0.13 | - | ||||
| (−)-8-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (60) | 0.18 | - | ||||
| (−)-8-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (61) | 0.21 | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. https://doi.org/10.3390/molecules26030728
Kong YR, Tay KC, Su YX, Wong CK, Tan WN, Khaw KY. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules. 2021; 26(3):728. https://doi.org/10.3390/molecules26030728
Chicago/Turabian StyleKong, Yew Rong, Kai Ching Tay, Yi Xiang Su, Choon Kwang Wong, Wen Nee Tan, and Kooi Yeong Khaw. 2021. "Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases" Molecules 26, no. 3: 728. https://doi.org/10.3390/molecules26030728
APA StyleKong, Y. R., Tay, K. C., Su, Y. X., Wong, C. K., Tan, W. N., & Khaw, K. Y. (2021). Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules, 26(3), 728. https://doi.org/10.3390/molecules26030728







