Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01
Abstract
:1. Introduction
2. Results
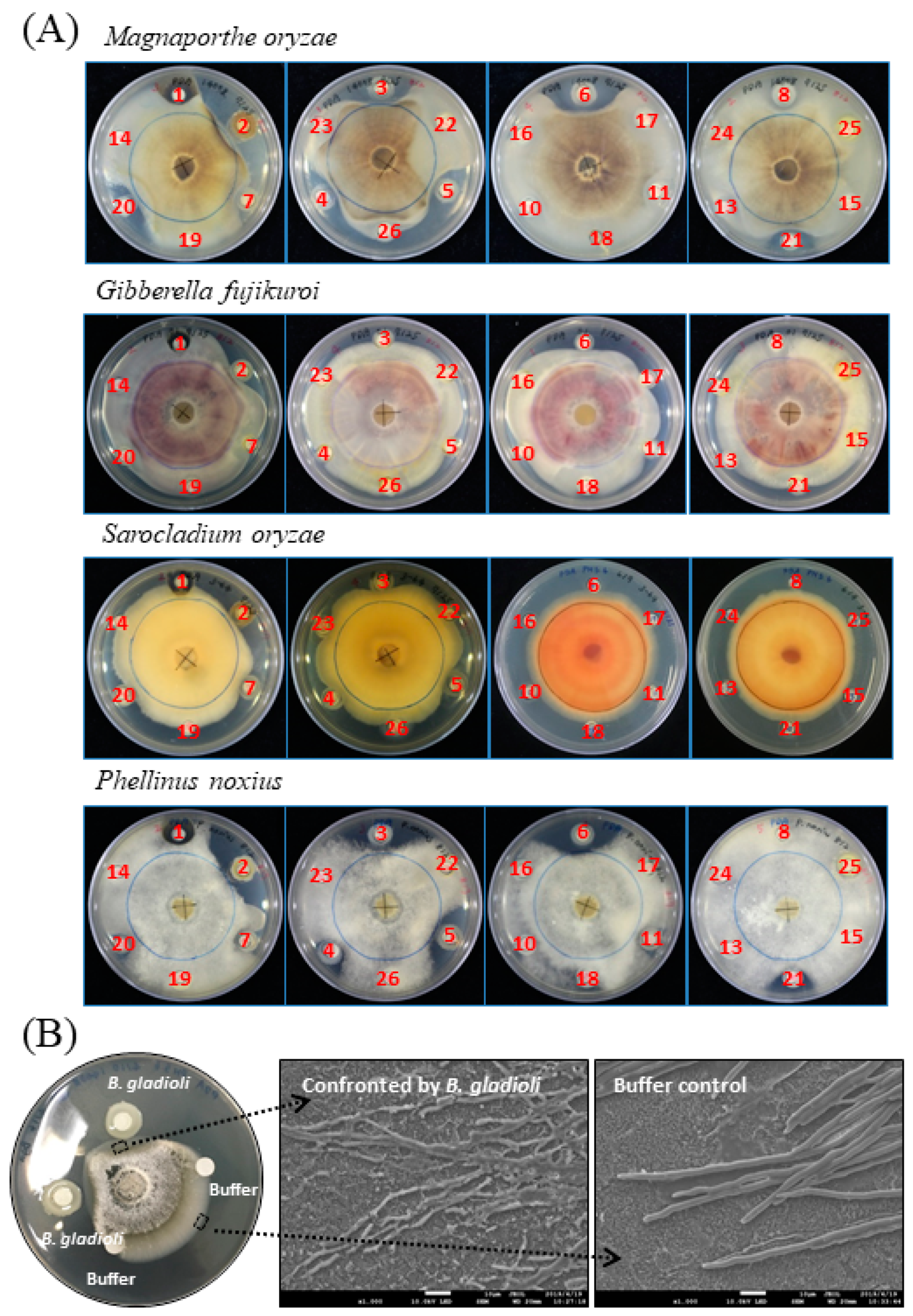
2.1. Bacteria Exhibiting Antifungal Activity
2.2. Phylogenetic Relationship of B. gladioli BBB-01 with Its Kin
2.3. B. gladioli BBB-01 Being Capable of Producing Antifungal Vapor
2.4. Chemical Identification of VOCs Produced by B. gladioli BBB-01
2.5. Antifungal Activity of 2,5-Dimethylfuran and Dimethyl Disulfide via Fumigation
3. Discussion
4. Materials and Methods
4.1. Media and Chemicals
4.2. The Screen of Antifungal Bacteria
4.3. Pangenomic Analysis of B. gladioli Isolates
4.4. Assay of Bacterial Antifungal VOCs
4.5. Assay of Bacterium-Emitted Hydrogen Cyanide
4.6. Chemical Identification of Bacterial VOCs by GC-MS
4.7. Antifungal Activity Assay of Chemically Synthesized Fumigants
4.8. Scanning Electron Microscopy (SEM)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Worldwide pesticide use. In Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: Berlin, Germany, 2014; pp. 5–6. [Google Scholar]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a killer: Microbial volatilome and its role in the biological control of plant pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, A.S.; Filippi, M.C.; Silva, G.B.; Lobo, V.L.S.; Moraes, O.P. An unprecedented outbreak of rice blast on a newly released cultivar BRS Colosso in Brazil. In Advances in Genetics, Genomics and Control of Rice Blast Disease; Wang, G.L., Valente, B., Eds.; Springer: New York, NY, USA, 2009; pp. 257–266. [Google Scholar]
- Nalley, L.; Tsiboe, F.; Durand-Morat, A.; Shew, A.; Thoma, G. Economic and environmental impact of rice blast pathogen (Magnaporthe oryzae) alleviation in the United States. PLoS ONE 2016, 11, e0167295. [Google Scholar] [CrossRef] [PubMed]
- Bakanae-IRRI Rice Knowledge Bank. Available online: www.knowledgebank.irri.org/index.php?option=com_zoo&task=item&item_id=924&Itemid=739 (accessed on 31 January 2021).
- Ghosh, M.K.; Amudha, R.; Jayachandran, S.; Sakthivel, N. Detection and quantification of phytotoxic metabolites of Sarocladium oryzae in sheath rot-infected grains of rice. Lett. Appl. Microbiol. 2002, 34, 398–401. [Google Scholar] [CrossRef] [Green Version]
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Webster, G.; Mullins, A.J.; Jenner, M.; Bull, M.J.; Dashti, Y.; Spilker, T.; Parkhill, J.; Connor, T.R.; LiPuma, J.J.; et al. Kill and cure: Genomic phylogeny and bioactivity of a diverse collection of Burkholderia gladioli bacteria capable of pathogenic and beneficial lifestyles. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J.R. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Blumer, C.; Haas, D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 2000, 173, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Sponring, A.; Bauer, M.; Filipiak, A.; Ager, C.; Wiesenhofer, H.; Nagl, M.; Troppmair, J.; Amann, A. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol. 2012, 12, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, L.D.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, W.; Lenney, W.; Wang, T.; Spanel, P.; Alcock, A.; Smith, D. Detection of volatile compounds emitted by Pseudomonas aeruginosa using selected ion flow tube mass spectrometry. Pediatr. Pulmonol. 2005, 39, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Labows, J.N.; McGinley, K.J.; Webster, G.F.; Leyden, J.J. Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography-mass spectrometry. J. Clin. Microbiol. 1980, 12, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 5, F1000 Faculty Rev-1007. [Google Scholar] [CrossRef]
- Chiou, A.L.; Wu, W.S. Isolation, identification and evaluation of bacterial antagonists against Botrytis ellipticaon on lily. J. Phytopathol. 2001, 149, 319–324. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Racioppi, R.; Scrano, L.; Iacobellis, N.S.; Bufo, S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 2012, 13, 16291–16302. [Google Scholar] [CrossRef] [Green Version]
- Shehata, H.R.; Lyons, E.M.; Jordan, K.S.; Raizada, M.N. Bacterial endophytes from wild and ancient maize are able to suppress the fungal pathogen Sclerotinia homoeocarpa. J. Appl. Microbiol. 2016, 120, 756–769. [Google Scholar] [CrossRef] [Green Version]
- Swain, D.M.; Yadav, S.K.; Tyagi, I.; Kumar, R.; Kumar, R.; Ghosh, S.; Das, J.; Jha, G. A prophage tail-like protein is deployed by Burkholderia bacteria to feed on fungi. Nat. Commun. 2017, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Salgado, S.; Tinoco, R.; Vazquez-Duhalt, R.; Caballero-Mellado, J.; Perez-Rueda, E. Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered 2013, 4, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarita, F.; Yvon, M.; Nardi, M.; Chambellon, E.; Delettre, J.; Bonnarme, P. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microbiol. 2004, 70, 7348–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laville, J.; Blumer, C.; von Schroetter, C.; Gaia, V.; Défago, G.; Keel, C.; Haas, D. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J. Bacteriol. 1998, 180, 3187–3196. [Google Scholar] [CrossRef] [Green Version]
- Gross, H.; Stockwell, V.O.; Henkels, M.D.; Nowak-Thompson, B.; Loper, J.E.; Gerwick, W.H. The genomisotopic approach: A systematic method to isolate products of orphan biosynthetic gene clusters. Chem. Biol. 2007, 14, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Yang, S.Y.; Kim, Y.C.; Lee, C.W.; Park, M.S.; Kim, J.C.; Kim, I.S. Identification of orfamide A as an insecticidal metabolite produced by Pseudomonas protegens F6. J. Agric. Food Chem. 2013, 61, 6786–6791. [Google Scholar] [CrossRef]
- Ma, Z.; Geudens, N.; Kieu, N.P.; Sinnaeve, D.; Ongena, M.; Martins, J.C.; Höfte, M. Biosynthesis, chemical structure, and structure-activity relationship of orfamide lipopeptides produced by Pseudomonas protegens and related species. Front. Microbiol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Olorunleke, F.E.; Hua, G.K.H.; Kieu, N.P.; Ma, Z.; Höfte, M. Interplay between orfamides, sessilins and phenazines in the control of Rhizoctonia diseases by Pseudomonas sp.CMR12a. Environ. Microbiol. Rep. 2015, 7, 774–781. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.-Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leimbach, A. Bac-Genomics-Scripts: Bovine E. coli Mastitis Comparative Genomics Edition. 2016. Available online: zenodo.org/record/215824#.YBZ4_-hKg2w (accessed on 31 January 2021).
- Katoh, K.; Misawa, K.; Kuma, K.-I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]


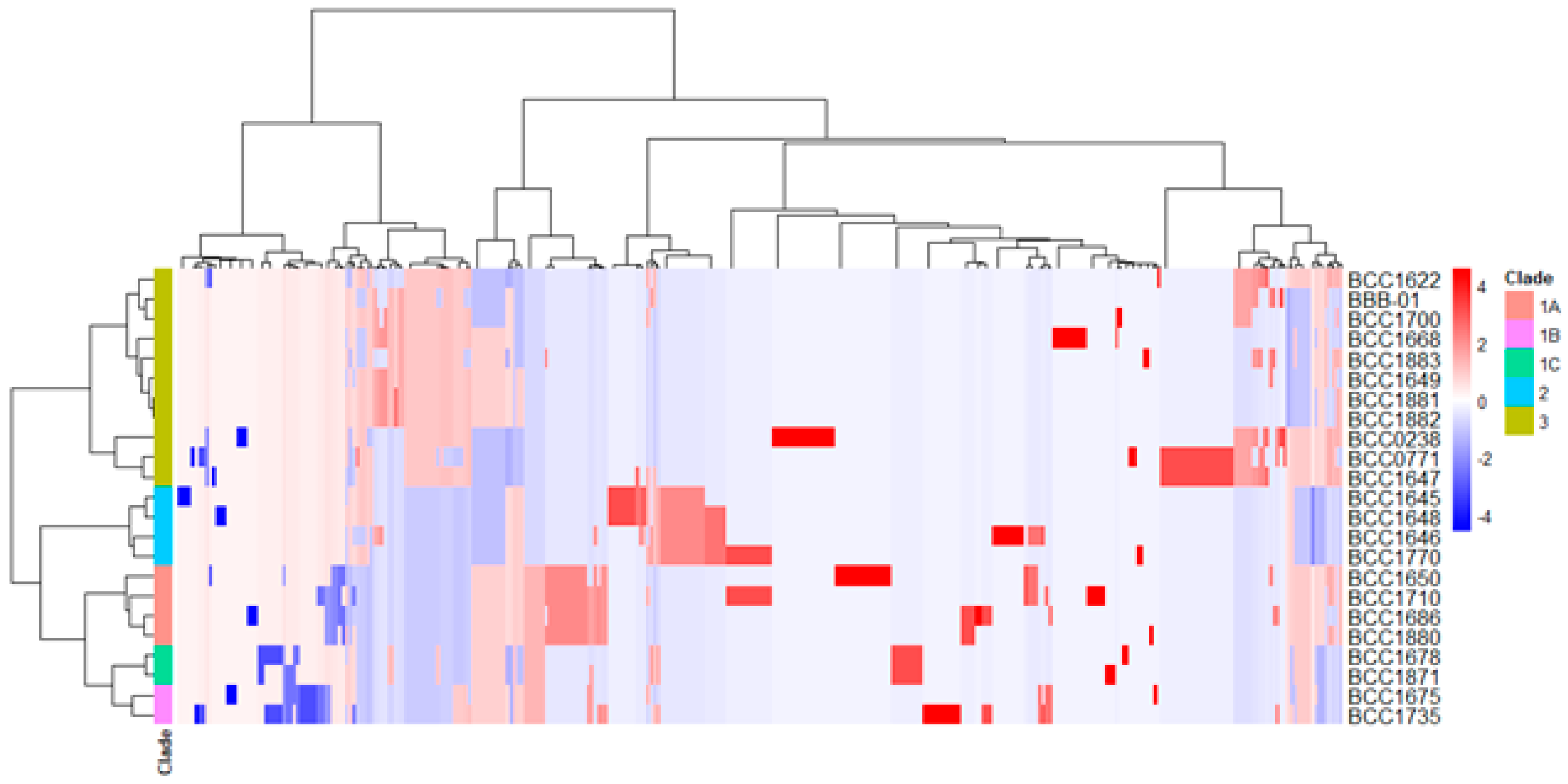



| Clade | Strain Name | Accession Number 1 | Synonym | Bongkrekic Acid Production | Remark |
|---|---|---|---|---|---|
| 1A | BCC1710 | ERS785039 | + | CF isolate 2 | |
| BCC1650 | ERS784828 | ENV; B. gladioli pv cocovenenans, LMG 11626, Indonesia | + | ||
| BCC1880 | ERS1371628 | ENV; B. gladioli pv cocovenenans, LMG 18113, China | + | ||
| BCC1686 | ERS784816 | + | CF isolate | ||
| 1B | BCC1675 | ERS785062 | + | CF isolate | |
| BCC1735 | ERS785008 | + | CF isolate | ||
| 1C | BCC1678 | ERS784880 | + | CF isolate | |
| BCC1871 | ERS1371626 | CF isolate | |||
| 2 | BCC1645 | ERS784911 | ENV; B. gladioli pv. alliicola, LMG 6954, Australia | ||
| BCC1648 | ERS784957 | ENV; B. gladioli pv. alliicola, LMG 2121, USA | |||
| BCC1770 | ERS1328772 | CF isolate | |||
| BCC1646 | ERS784928 | ENV; B. gladioli pv. alliicola, LMG 6878, India | |||
| 3 | BCC1622 | ERS784864 | CF isolate | ||
| BCC1647 | ERS784943 | B. gladioli pv. gladioli, LMG 6882, USA | |||
| BCC0238 | ERS784907 | CF isolate | |||
| BCC0771 | ERS784806 | B. gladioli pv. gladioli, LMG 2216 (ATCC 10248), USA | |||
| BCC1668 | ERS784974 | CF isolate | |||
| BCC1700 | ERS784851 | CF isolate | |||
| BCC1649 | ERS784812 | ENV; B. gladioli pv. gladioli, LMG 6880t4, Zimbabwe | |||
| BCC1881 | ERS1371629 | ENV; B. gladioli pv. agaricicola, NCPPB 3580, UK | |||
| BCC1882 | ERS1371630 | ENV; B. gladioli pv. agaricicola, NCPPB 3632, UK | |||
| BCC1883 | ERS1371630 | ENV; B. gladioli pv. agaricicola, NCPPB 3852, New Zealand | |||
| BBB-01 | B. gladioli BBB-01 | Isolated in this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Lee, C.-C.; Leu, W.-M.; Wu, J.-J.; Huang, Y.-C.; Meng, M. Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01. Molecules 2021, 26, 745. https://doi.org/10.3390/molecules26030745
Lin Y-T, Lee C-C, Leu W-M, Wu J-J, Huang Y-C, Meng M. Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01. Molecules. 2021; 26(3):745. https://doi.org/10.3390/molecules26030745
Chicago/Turabian StyleLin, Ying-Tong, Cheng-Cheng Lee, Wei-Ming Leu, Je-Jia Wu, Yu-Cheng Huang, and Menghsiao Meng. 2021. "Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01" Molecules 26, no. 3: 745. https://doi.org/10.3390/molecules26030745
APA StyleLin, Y.-T., Lee, C.-C., Leu, W.-M., Wu, J.-J., Huang, Y.-C., & Meng, M. (2021). Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01. Molecules, 26(3), 745. https://doi.org/10.3390/molecules26030745







